Abstract
Topical microemulsion systems for the antifungal drug, butenafine hydrochloride (BTF) were designed and developed to overcome the problems associated with the cutaneous delivery due to poor water solubility. The solubility of BTF in oils, surfactants and co-surfactants was evaluated to screen the components of the microemulsion. Isopropyl palmitate was used as the oil phase, aerosol OT as the surfactant and sorbitan monooleate as co-surfactant. The pseudoternary diagrams were constructed to identify the area of microemulsion existence and optimum systems were designed. The systems were assessed for drug-loading efficiency and characterized for pH, robustness to dilution, globule size, drug content and stability. Viscosity analysis, spreadability, drug content assay, ex vivo skin permeation study and antifungal activity assay were performed for the optimized microemulsion-loaded hydrogel. The optimized BTF microemulsion had a small and uniform globule size. The incorporation of microemulsion system into Carbopol 940 gel was found to be better as compared to sodium alginate or hydroxyl propyl methyl cellulose (HPMC K4 M) gel. The developed gel has shown better ex vivo skin permeation and antifungal activity when compared to marketed BTF cream. Thus, the results provide a basis for the successful delivery of BTF from microemulsion-loaded hydrogel formulation, which resulted in improved penetration of drug and antifungal activity in comparison with commercial formulation of BTF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effective treatment of increasing number of fungal infections caused by fungi, such as those belonging to the genus Candida, Aspergillus, and Cryptococcus is a big challenge for current healthcare sector. This is a particular complication encountered in transplant patients; those administered a large quantity of antibiotics, anticancer drugs, or steroidal agents over a long period, AIDS patients, or those suffering from cancer in the terminal stage, and those with haematological malignancies undergoing intensive chemotherapy and/or bone marrow transplantation [3]. Invasive fungal infections are a major cause of morbidity and mortality in patients receiving bone marrow transplants for leukaemia as well as in immuno-compromised cancer patients [1].
Butenafine hydrochloride (BTF) is one of the antifungal drugs whose structure resembles that of allylamine antifungals, with a butylbenzyl group in place of the allyl group. Like the allylamines, butenafine is fungicidal by inhibition of squalene epoxidase enzyme which is responsible for the epoxidation of squalene [18]. This inhibition suppresses the biosynthesis of ergosterol, an essential lipid component of fungal cell membranes, and causes rapid accumulation of squalene [15]. Because allylamines and butenafine block ergosterol synthesis independent of cytochrome P-450-dependent steroidogenesis, they are unlikely to cause adverse reactions that are related to the impaired production of adrenal or testicular steroids, which has been associated with administration of azoles [7, 16]. BTF exhibits potent fungicidal activity particularly against dermatophytes, aspergilli, dimorphic and dematiaceous fungi. Topical butenafine cream (1 %) has been reported to be effective for the treatment of Tinea pedis, Tinea corporis and Tinea cruris for short-term therapy [17].
The log partition coefficient of BTF is 4.65 in a system of n-octanol–water, which indicates a high lipophilicity of the drug. Nevertheless, the drug has been used successfully in the treatment of candidiasis and onychomycosis with low toxicity, indicating satisfactory therapeutic index. However, the extent of penetration of butenafine from the existing market formulation (i.e. cream) is very low. To increase the penetration and overall efficacy, it can be incorporated into a microemulsion, which could overcome the existing drawbacks associated with its conventional delivery. Microemulsion-loaded drug delivery system opens up a number of opportunities with regard to efficient drug therapy for fungal infection and would be more effective for patients. A topical application may be helpful for many patients who would have difficulty in swallowing the drug orally. The potential advantages of topical administration route include site-targeted delivery, which can obviate the need for oral and other systemic treatments, and can reduce the total drug dose, thereby reducing the non-target site toxicities.
Microemulsions are colloidal dispersions composed of an oil phase, aqueous phase, surfactant, and co-surfactant in appropriate ratios. Unlike coarse emulsions micronized with external energy, microemulsions are based on low interfacial tension. This is achieved by adding a co-surfactant, which leads to spontaneous formation of a thermodynamically stable microemulsion. The droplet size in the dispersed phase is very small, usually in a range from 10 to 200 nm in diameter, which makes the microemulsions a transparent liquid [19]. In principle, microemulsions can be used to deliver drugs to the patients via several routes, but the topical application of microemulsions has gained increasing interest. The three main factors determining the transdermal permeation of drugs are the mobility of a drug in the vehicle, release of a drug from the vehicle, and permeation of a drug into the skin. These factors affect either the thermodynamic activity that drives the drug into the skin or the permeability of drug in the skin, particularly stratum corneum. It has been reported that microemulsions improve the transdermal delivery of several drugs over the conventional topical preparations, such as emulsions and gels [11, 12].
The aim of the present study was to investigate the potential of microemulsion-loaded gel formulation of BTF to be delivered by transdermal route. Such topical delivery may serve as an adjunct to existing treatment and bypass the gastrointestinal adverse effects and improve the patient compliance.
Materials and methods
Materials
BTF was a generous gift from Glenmark Pharmaceuticals Limited (Mumbai, India). Aerosol OT (sodium bis-2-ethylhexyl-sulfosuccinate) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Sorbitan monooleate and Carbopol 940 were purchased from Loba Chemie (Mumbai, India). HPMC K4 M was purchased from Himedia Laboratories Pvt. Ltd., Mumbai, India. Sodium alginate was purchased from Otto Chemie Pvt. Ltd., Mumbai, India. Isopropyl palmitate was purchased from Nice Chemicals (Kochi, Kerala, India). All other chemicals were of analytical grade and used as such without further purification.
Selection of oil, surfactant and co-surfactant of the microemulsion
For selecting different components for formulating microemulsion of BTF, the solubility of butenafine was investigated in different oils and surfactants, such as isopropyl palmitate, aerosol OT and sorbitan monooleate. An excess amount of BTF was added to 10 mL of each selected oils and surfactants and was shaken at 20 °C for 24 h. The supernatant portion was carefully poured off, filtered and analysed by spectrophotometry at 223 nm.
Construction of pseudoternary phase diagram
The pseudoternary phase diagrams were constructed using water dilution method [5]. Isopropyl palmitate was used as the oil phase, aerosol OT as the surfactant and sorbitan monooleate as co-surfactant. Phase diagrams were constructed with 9:1 to 5:5 v/v ratio of oil to surfactant. Surfactant to co-surfactant ratio was kept as 1:1 in each case. For each phase diagram, oil, surfactant and co-surfactant mixtures were prepared and then diluted with water by sequential addition of water drop by drop using a micropipette. The samples were classified as being optically clear or turbid. The microemulsion regions were identified as translucent and isotropic mixtures. The percentage of the three different phases, i.e. oil, water and the mixture of surfactant and co-surfactant was calculated.
Preparation of O/W microemulsion loaded with BTF
Appropriate quantities of aerosol OT (surfactant), sorbitan monooleate (co-surfactant) and isopropyl palmitate (oil) were weighed. BTF (0.5 g) was dissolved in isopropyl palmitate and then the mixture of surfactant and co-surfactant was added. The mixture was stirred with a magnetic bar on magnetic stirrer, at room temperature with continuous addition of weighed amount of water, until the formation of a translucent system.
Characterization of microemulsion systems
Stability studies
Dispersion stability tests were performed by centrifuging the microemulsion formulations at 4000 g for 30 min. The formulations that showed no phase separations were taken for the freeze–thaw cycle (heating and cooling cycle). Three cycles between 4 and 45 °C with storage at each temperature for 24 h were done. The formulations which were stable at these temperatures and survived the stability tests were selected for further studies.
Measurement of droplet size and zeta potential
The average size and polydispersity index of the microemulsion droplets were determined by Dynamic Light Scattering (DLS) method using a particle size analyzer (NICOMP 380 ZLS, Particles Sizing Systems, Santa Barbara, CA). 100 mg of microemulsion was diluted with 10 ml of methanol by manual shaking. The instrumental settings were fixed at temperature 20°C, viscosity 0.01 poise and refractive index 1.333. The zeta potential was also recorded.
Scanning electron microscopy
The microemulsion formulation was mounted on an aluminium stub with double-sided adhesive carbon tape and examined with the scanning electron microscope (Model: JSM-6390LV; JEOL, USA).
Preparation of microemulsion-loaded hydrogel
The microemulsion formulation was incorporated into gels to make it convenient for application on skin. Gels were prepared using three different polymers: sodium alginate (1 % w/w; F1), HPMC-K4 M (1 % w/w; F2) and Carbopol 940 (1 % w/w; F3). Required quantities of gelling agents were dispersed in the aqueous phase under continuous stirring. In case of Carbopol 940, neutralization was performed using triethanolamine to attain gelling.
Evaluation of microemulsion-loaded gel
Determination of pH
1 g of gel was accurately weighed and dispersed in 10 ml of purified water and pH value of the dispersion was measured by a digital pH meter (Infra Instruments Pvt. Ltd., Chennai, India) at 20 ± 2 °C. The pH meter was calibrated before each use with buffered solutions of different pH.
Estimation of drug content
Microemulsion-loaded gel equivalent to 5 mg of BTF was taken in a volumetric flask containing 15 ml methanol and stirred for 30 min. The volume was made up to 50 ml with methanol. From the above solution, 2 mL was further diluted with 3 ml of methanol and 5 ml of 0.1 N HCl to get a final concentration of 20 µg/ml. The resultant solution was filtered through 0.45 μm membrane filter and the absorbance of the solution was measured spectrophotometrically at 223 nm (UV-1800 UV–Vis Spectrophotometer, Shimadzu Corporation, Japan).
Spreadability study
Spreadability of the gel was determined using a method suggested by Multimer et al. [13], suitably modified by us. The apparatus consists of a wooden block having a pulley at one end. Spreadability was measured on the basis of “slip” and “drag” characteristics of gels. A ground glass slide was fixed on the wooden block. An excess of gel (about 2.5 g) was placed on this ground slide. The gel was then sandwiched between this slide and another glass slide having the dimension of fixed slide and provided with a hook. A weight of 300 g was placed on the top of the two slides for 5 min to expel air and to provide a uniform film of the gel between the slides. Excess of the gel was scrapped off from the edges. The top plate was then subjected to pull off 100 g weight with the help of a string attached to the hook and the time (in seconds) required by the top slide to cover a distance of 7.5 cm was noted. A shorter time interval indicates better spreadability.
The spreadability (S) was calculated using the following formula:
where M is the weight tied to the upper slide, L is the length of glass slides and T is the time taken to separate the slides. The experiment was repeated three times for each gel.
Viscosity measurement
The viscosity of all the gels was determined at 25 ± 2 °C using Brookfield viscometer fitted with spindle S64 (Model: DV-II Pro; Brookfield Engineering Laboratories, MA, USA). Viscosity of each gel was determined three times.
Primary skin irritation test
The primary skin irritation test of developed microemulsion-loaded gels was carried out using Draize patch test on rabbits [6]. The experimental protocol was approved by the Institutional Animal Ethics Committee, Amrita Institute of Medical Sciences & Research Centre, Kochi. Twelve New Zealand white rabbits weighing 2–2.5 kg were acclimatized for 1 week before beginning of the study. Animals were divided into four groups (n = 3) as follows:
-
Group 1: Ethanol (control)
-
Group 2: Microemulsion-loaded alginate gel (F1)
-
Group 3: Microemulsion-loaded HPMC gel (F2)
-
Group 4: Microemulsion-loaded Carbopol 940 gel (F3)
Twenty-four hours prior to application of the formulations, the back of the rabbits was clipped of hair. 0.5 g of test samples was applied on the hair-free skin of rabbits by uniform spreading within an area of 6 cm2. The skin was observed for any visible change, such as erythema or edema after 1, 24, 48 and 72 h. Scores between 0 and 4 were used to grade erythema and edema, which range from no response to a severe response.
Release kinetic studies of microemulsion-loaded gels
0.5 g of microemulsion gel was placed in an open ended tube tied at one end with a cellophane membrane immersed into 30 ml of dissolution medium of phosphate buffer of pH 5.5. To simulate the human skin condition during the experiment, temperature was maintained at 37 °C. 3 ml samples were withdrawn at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, and 24 h and replaced with equal volume of the buffer. The withdrawn samples were analysed spectrophotometrically at 223 nm. The amount of drug released was calculated and were fitted into various kinetic models, i.e. zero order, first order, Higuchi’s diffusion model and Korsmeyer Peppas model. The plots were drawn using Microsoft Excel 2007 and the regression equations were obtained for each plot. The linearity of the plots was estimated from the value of regression coefficient. The model with highest linearity was chosen as the best fit kinetic model.
Ex vivo skin permeation study
For the skin permeation study, pig ear skin was obtained from the local slaughter house. The skin hair was removed by shaving and the subcutaneous fatty tissue was removed using a scalpel and surgical scissors. The surface of the skin was cleaned with Ringer’s solution and the skin was allowed to dry (exposed to ambient air conditions for 20 min). After drying, the skin was packed in aluminium foil and stored in polyethylene bag at −20 °C. Franz diffusion cells presenting an effective diffusional area of 3.14 cm2 and approximately 11 mL of receptor cell capacity were used. The frozen skin was allowed to thaw, cleaned with Ringer’s solution and transferred onto Franz diffusion cells. The receptor compartment was filled with phosphate buffer pH 5.5. The epidermal side of the skin was exposed to the donor compartment, while the dermal side was bathed with receptor solution. To mimic the body conditions during experiment, the temperature was maintained as 37 °C with an external constant water circulator and the receiver medium was continuously stirred with a magnetic bar to prevent any boundary layer effects. The skin in the Franz diffusion cell was allowed to equilibrate at the above-mentioned temperature for 30 min.
Permeation studies of microemulsion-loaded gels (F1, F2 and F3), Carbopol 940 gel with plain BTF, BTF solution and marketed formulation containing BTF (Butop cream, purchased locally) were carried out. The test gel/cream/drug solution was applied to the donor compartment of Franz diffusion cells. At a predetermined time intervals, 3 ml of samples were withdrawn and the compartment was refilled with the same volume of fresh receptor solution. The samples were analysed spectrophotometrically at 223 nm. Three replicates of each experiment were performed.
The steady-state flux (J ss, μg/cm2/h) of BTF was calculated from the slope of the linear portion of the plots of cumulative amount of drug permeated versus time in steady-state conditions. Permeability coefficient (K p, cm/h) was calculated by dividing the flux with initial concentration of the drug in the donor compartment and enhancement ratios were calculated by dividing the flux (J ss) of the formulation with the flux of the drug solution (control).
Histopathological examination of the skin
After performing the above-mentioned skin permeation study, the skin samples were washed thoroughly and kept in 10 % buffered formalin solution. Paraffin sections were prepared and cut into 5-µm-thick sections in a rotary microtome. The sections were then stained with haematoxylin and eosin dye, observed under light microscope and compared with control samples. The changes in skin after treatment were evaluated.
Microbiological assay of BTF
In vitro antifungal studies were performed against Candida albicans in Yeast Peptone Dextrose (YPD) Agar media by agar cup method. Sterile YPD plates were prepared and 0.1 ml of the inoculums of test organism was spread uniformly. Wells were prepared using a sterile borer of diameter 8 mm and 1 g each of developed optimized gel (microemulsion-loaded Carbopol gel; F3) and the marketed BTF cream (Butop) were poured into the wells. These plates were incubated at 37 ± 1 °C for 48 h. The mean zone of inhibition of BTF released was calculated in millimetres.
Results
Solubility of BTF in oils and surfactants
The physicochemical properties of BTF suggest that it has good potential for topical drug delivery. Among the selected oil and surfactants, maximum solubility of BTF was found in isopropyl palmitate (32.25 mg/ml) followed by aerosol OT (26.18 mg/ml) and sorbitan monooleate (22.74 mg/ml).
Construction of pseudoternary phase diagram
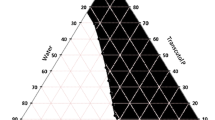
The construction of pseudoternary phase diagrams is used to determine the concentration range of components in the microemulsion existence range. The pseudoternary phase diagrams with various weight ratios of aerosol OT, sorbitan monooleate and isopropyl palmitate are depicted in Fig. 1. The translucent microemulsion region is presented in phase diagrams with no distinct conversion from water in oil to oil in water. The rest of the region on the phase diagram represents the turbid and conventional emulsions based on visual observation. The area of microemulsion isotropic region changed slightly in size with the increasing ratio of surfactant to co-surfactant.
The phase study revealed that the maximum proportions of oil were incorporated in microemulsion systems when the surfactant to co-surfactant ratio was 1:1. The composition of optimized microemulsion system is shown in Table 1.
Characterization of microemulsion
The microemulsion appeared bright against dark surroundings (Fig. 2). The droplet size ranged between 100 and 175 nm. The average droplet size of microemulsion was found to be 142.15 nm with low value of polydispersity index of 1.39. Zeta potential of the optimized formulation was found to be −45 mV and hence the formulation was found to be stable.
Evaluation of microemulsion-loaded gel
The pH of the microemulsion-loaded gel formulations were found to be in the range of 6.8–7.1 (Table 2), which was within the acceptable limits for topical application. The drug content was in the range of 89.8–94.9 % for the developed gel formulations. The spreadability plays an important role in the patient compliance and helps in uniform application of gel to the skin. The spreadability of formulated gels indicates (Table 2) that the gels will take less time to spread at the site of application. The viscosity of the microemulsion-loaded gels was found to be 3287.76–5134.68 cps (Table 2).
The gel prepared with sodium alginate gave high viscosity with sticky texture and the gel was unclear. It was observed that sodium alginate affected the structure of the microemulsion and resulted in separation of the oily phase. On the other hand, HPMC K4 M yielded turbid gel due to agglomeration of microemulsion. Carbopol 940 at the concentration of 1 % was able to produce microemulsion-loaded gel with desired rheological property. Carbopol could yield a gel without disturbing the microstructure of the BTF microemulsion.
Primary skin irritation test
The results of skin irritation test at 1, 24, 48 and 72 h are shown in Table 3. The scores given in the table indicate that there is no serious sensitivity reaction in neither of the developed microemulsion-loaded gels.
Release kinetic studies of microemulsion-loaded gels
To develop an ideal kinetic model to interpret the drug release data, various kinetic models, including zero order, first order, Higuchi’s diffusion and Korsmeyer Peppas model were applied. As shown in Table 4, the in vitro release of BTF from alginate gel (F1) was best described by the zero-order model. HPMC gel (F2) and Carbopol 940 gel (F3) followed first-order release pattern. Formulation F3 also followed Higuchi’s diffusion model. This indicates that the rate controlling step in the release process was the diffusion of the dissolved drug through the gel network of Carbopol 940 to the external medium, which in turn, explains the prolonged release of the drug.
Ex vivo skin permeation study
As shown in Fig. 3, among the three microemulsion-loaded gels, Carbopol 940 gel (F3) showed the highest drug release followed by HPMC and sodium alginate gels.
The skin permeation study was also performed with a marketed cream formulation (Butop) containing BTF. The obtained results clearly indicate that the microemulsion-loaded Carbopol 940 gel is showing maximum ex vivo release of the medicament. To evaluate the superiority of microemulsion-loaded gel, a Carbopol gel containing plain BTF (i.e. without microemulsion form) was also studied and the obtained results indicate that skin permeation of BTF from microemulsion-loaded gel is better than Carbopol gel containing plain BTF. The calculated (mean value of three replicates) permeability coefficient, flux and enhancement ratio is shown in Table 5.
Histopathology of skin
The histology of skin treated with normal saline, drug (BTF) solution, marketed BTF cream and microemulsion-loaded Carbopol 940 gel (optimized formulation) is represented in the Figs. 4, 5, 6, 7. In case of control (normal saline), it is clear that the stratum corneum, epidermis and dermis are closely packed. In case of marketed BTF cream, this are less orderly arranged, and this infers the enhanced permeation of marketed cream over the drug solution. However, in case of microemulsion gel, the lipid layer of stratum corneum is highly disrupted. It indicates rapid permeation of the BTF through the skin, thereby easily bypassing the skin barrier which leads to enhanced skin permeation.
Microbiological assay of BTF
The results of microbiological assay are presented in Table 6. The result revealed that the developed microemulsion-loaded gel with Carbopol 940 showed large zone of inhibition (the antifungal activity) as compared to marketed BTF cream.
Discussion
BTF is a hydrophobic drug having low penetration and poor absorption through skin. The present study was aimed to develop a suitable carrier for the topical delivery of BTF. The preformulation studies and partition coefficient of the BTF confirmed that the drug is lipid soluble and microemulsion may be an efficient carrier for its topical delivery. The microemulsion formulation was developed and tested for stability. The stable formulation was characterized for droplet size, polydispersity index, zeta potential, etc.
Polydispersity is the ratio of standard deviation to the mean droplet size. This is proportional with the uniformity of the droplet size in the formulation. The polydispersity index value obtained by us for the microemulsion formulation indicates the uniformity of droplet size within the formulation. The magnitude of the zeta potential gives an indication of the potential stability of the colloidal system. If all the globules have a large negative or positive zeta potential, they will repel each other and there is dispersion stability. A dividing line between stable and unstable aqueous dispersion is generally taken at either +30 or −30 mV. The developed microemulsions showed zeta potential as −45 mV and hence was found stable.
As compared to liquid state microemulsion, the addition of gel matrix into microemulsion results in a more suitable formulation for dermal application. Three kinds of gelling agents, i.e. sodium alginate, HPMC K4 M and Carbopol 940 were selected to develop microemulsion-loaded gels. Spreadability and viscosity data confirmed that the gels are suitable for application on skin and Carbopol 940 gel was found best among the three gels.
Skin irritation test on rabbits confirmed that Carbopol 940 gel is not producing any redness or inflammation as evaluated on Draize scale. Release kinetics further confirmed the suitability of gels for topical application as the gels followed a zero/first-order release. The Carbopol 940 gel also followed Higuchi’s diffusion pattern.
Carbopol 940 gel was considered to be the optimized one as it gave better release of drug and better permeation in a steady manner over a desired period of time through pig skin. Comparative skin permeation study of the optimized Carbopol gel and the marketed cream was performed and permeation of BTF from the developed gel was found better than that from the marketed cream.
Conventional creams have a mean droplet size ranging from 10 to 100 μm. Such formulations have demonstrated poor penetration of drug-loaded oil droplets into deep skin layers. Gupta and Garg [8] have reported that microparticles with diameters ranging from 3 to 10 μm selectively penetrate follicular ducts, whereas particles >10 μm remain on the skin surface, and those <3 μm are distributed randomly into hair follicles and stratum corneum. The histological examination of the skin after permeation experiment confirmed that BTF has a better permeation from the Carbopol gel as compared to the marketed cream. This could be due to the presence of the surfactant and co-surfactant in microemulsion which may affect the stratum corneum structure and reduce the diffusional barrier by acting as a permeation enhancer [14]. The microemulsion is expected to penetrate the stratum corneum and exist intact in the whole horny layer. Once it enters into the stratum corneum, the microemulsion may simultaneously alter both the lipid and the polar pathways [2, 10]. The lipophilic domain of the microemulsion can interact with the stratum corneum in many ways. BTF dissolved in the lipid domain of a microemulsion can directly partition into the lipids of the stratum corneum or the lipid vesicles themselves can intercalate between the lipid chains of the stratum corneum, thereby destabilizing its bilayer structure. In effect, these interactions would lead to an increase in the permeability of the lipid pathway to BTF. On the other hand, the hydrophilic domain of the microemulsion can hydrate the stratum corneum to a great extent [4]. There is a general experience that hydration of the skin plays an important role in the percutaneous uptake of poorly soluble drug. When the aqueous fluid of the microemulsion enters the polar pathways, it increases the interlamellar volume of stratum corneum lipid bilayers, resulting in disruption of the interfacial structure. Since some lipid chains are covalently attached to corneocytes, hydration of these proteins will also lead to the disorder of lipid bilayers. Similarly, swelling of the intercellular proteins may also disturb the lipid bilayers; a lipophilic drug like BTF can then permeate more easily through the lipid pathway of the stratum corneum. The greater drug penetration-enhancing activity of microemulsions may be attributed to the combined effects of both the lipophilic and hydrophilic domains of microemulsions [9].
In vitro antifungal activity study against Candida albicans revealed that the zone of inhibition is higher for microemulsion-loaded Carbopol gel as compared to marketed cream. This may be due to the enhanced permeation of microemulsion oil globules containing drug through the fungal cell wall, thereby inhibiting the ergosterol synthesis, causing the fungal cell death.
Conclusion
The results indicate that under optimized conditions, BTF can be successfully incorporated in a microemulsion system using topically acceptable surfactants, co-surfactants and oily phase. The results provide a basis for the successful design of BTF microemulsion-loaded gel which resulted in improved penetration and antifungal activity of the drug in comparison with commercial formulation of BTF.
References
Anaissie E (1992) Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin Infect Dis 14:S43–S53
Azeem A, Khan ZI, Aqil M, Ahmad FJ, Khar RK, Talegaonkar S (2009) Microemulsions as a surrogate carrier for dermal drug delivery. Drug Dev Ind Pharm 35:525–547
Bodey GP (1992) Azole antifungal agents. Clin Infect Dis 14:S161–S169
Chen H, Chang X, Du D, Li J, Xu H, Yang X (2006) Microemulsion-based hydrogel formulation of ibuprofen for topical delivery. Int J Pharm 315:52–58
Djordjevic L, Primorac M, Stupar M, Krajisnik D (2004) Characterization of caprylocaproyl macrogolglycerides based microemulsion drug delivery vehicles for an amphiphilic drug. Int J Pharm 271:11–19
Draize JH, Woodard G, Calvery HO (1944) Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharm Exp Therap 82:377–390
Elewski BE (1993) Mechanisms of action of systemic antifungal agents. J Am Acad Dermatol 28:S28–S34
Gupta P, Garg S (2002) Recent advances in semisolid dosage forms for dermatological application. Pharm Technol 26:144–162
Junyaprasert VB, Boonme P, Songkro S, Krauel K, Rades T (2007) Transdermal delivery of hydrophobic and hydrophilic local anesthetics from o/w and w/o Brij 97-based microemulsions. J Pharm Pharm Sci 10:288–298
Kreilgaard M (2002) Influence of microemulsions on cutaneous drug delivery. Adv Drug Deliv Rev 54:S77–S98
Kreilgaard M, Pedersen EJ, Jaroszewski JW (2000) NMR characterization and transdermal drug delivery potential of microemulsion systems. J Control Release 69:421–433
Ktistis G, Niopas I (1998) A study on the in vitro percutaneous absorption of propranolol from disperse systems. J Pharm Pharmacol 50:413–418
Mutimer MN, Riffkin C, Hill JA, Glickman ME, Cyr GN (1956) Modern ointment bases technology-II-comparative evaluation of bases. J Am Pharm Assoc Am Pharm Assoc (Baltim) 45:212–218
Peltola S, Saarinen-Savolainen P, Kiesvaara J, Suhonen TM, Urtti A (2003) Microemulsions for topical delivery of estradiol. Int J Pharm 254:99–107
Ryder NS (1992) Terbinafine: mode of action and properties of the squalene epoxidase inhibition. Br J Dermatol 126:2–7
Schuster I (1985) The interaction of representative members from two classes of antimycotics—the azoles and the allylamines—with cytochrome P-450 in steroidogenic tissues and liver. Xenobiotica 15:529–546
Singal A (2008) Butenafine and superficial mycoses: current status. Expert Opin Drug Metab Toxicol 4:999–1005
Stutz A (1988) Synthesis and structure-activity correlations within allylamine antimycotics. Ann N Y Acad Sci 544:46–62
Tenjarla S (1999) Microemulsions: an overview and pharmaceutical applications. Crit Rev Ther Drug Carrier Syst 16:461–521
Acknowledgments
The authors thank Glenmark Pharmaceuticals Limited (Mumbai, India) for providing the gift sample of butenafine HCl. The authors also acknowledge the Kerala State Council for Science, Technology and Environment, Kerala, India, for providing financial assistance (Grant no. 129/SPS/2013/CSET).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pillai, A.B., Nair, J.V., Gupta, N.K. et al. Microemulsion-loaded hydrogel formulation of butenafine hydrochloride for improved topical delivery. Arch Dermatol Res 307, 625–633 (2015). https://doi.org/10.1007/s00403-015-1573-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-015-1573-z