Abstract
Topically applied ingenol mebutate (IngMeb) is approved for field-treatment of actinic keratosis and is currently being investigated for treatment of non-melanoma skin cancer (NMSC). Ablative fractional lasers (AFXLs) generate microscopic ablation zones (MAZs) in the skin, which may help induce a deep penetration needed for effective treatment of NMSC. Using Franz diffusion cells, uptake and bio-distribution were investigated over 21 h in intact (n = 9) and AFXL-exposed porcine skin (n = 58). A 2940-nm fractional Er:YAG laser generated intraepidermal (11.2 mJ/MAZ; 66 μm deep, 177 μm wide) and intradermal (128 mJ/MAZ; 570 μm deep, 262 wide) MAZ’s with 16, 97, and 195 MAZs/cm2. Surface ablation densities corresponded to 0.5, 2.5, and 5 % for intraepidermal MAZs, and corresponded to 1, 5, and 10.5 % for intradermal MAZs. Liquid-chromatography–mass-spectrometry quantified deposition of IngMeb in stratum corneum, epidermis, dermis, and receiver chamber. In intact skin, IngMeb readily penetrated to the epidermal layer (1,314 ng, 41 % of the applied IngMeb), while dermal deposition was limited (508 ng, 16 %). In AFXL-exposed skin, a profound dermal deposition of IngMeb was achieved, while less accumulated in SC and epidermis. Uptake depended entirely on laser density; increasing coverage from 0 % to 0.5 %, 1 %, 2.5 %, 5 %, and 10.5 % enhanced dermal uptake 1.6-, 2.1-, 3.1-, 3.4-, and 3.9-fold, respectively (p < 0.0001). Channel depth did not influence drug uptake; at 5 % density, dermal deposition with intraepidermal and intradermal MAZs was analogous (1801 vs. 1744; p = 0.447). In conclusion, IngMeb readily distributes to superficial layers of intact skin, whereas dermal uptake is limited. Independent of channel depth, AFXL enhances dermal drug deposition, providing for customized topical delivery and potential use of IngMeb for treatment of NMSC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ingenol Mebutate (IngMeb) gel is a new topical drug approved for field-treatment of actinic keratosis (AK) [14]. IngMeb induces cell death through necrosis and apoptosis, and generates an inflammatory response through stimulation of immune regulatory pathways [13, 24]. The cytotoxicity in combination with the immune-activation is believed to result in the effective eradication of atypical cells in the skin.
The induction of cell death has been observed in human keratinocyte-derived cancer cells and is achieved at high micromolar concentrations. Application of a clinical dose of IngMeb (1.15 mM) primarily results in epidermal accumulation, and cytotoxic levels of 300 μM are reached within 2 h of application, while dermal deposition is restricted (<40 μM) [24]. For the purpose of AK treatment, epidermal predilection is desired, as IngMeb is retained around the target lesions and systemic toxicity is avoided [18]. However, for the treatment of skin cancer, a deeper penetration is required, as cutaneous tumors invade the dermal compartment. IngMeb is currently being investigated for treatment of non-melanoma skin cancer (NMSC), and Phase II trials on superficial basal cell carcinomas (BCCs) have found tumor clearances below the desired standard of care [23]. The limited dermal penetration is likely to result in sub-therapeutic concentrations in deeper parts of the tumors. Thus, ensuring sufficient dermal penetration may improve treatment responses when treating NMSC with IngMeb.
Several physical drug delivery techniques have been developed to increase uptake of topical agents [5, 18]. Physically disruptive strategies include tape-stripping, electroporation, iontophoresis, microneedling, and sonophoresis [4]. In 2010, ablative fractional laser (AFXL) was introduced as a physical delivery-enhancement technique. AFXL generates microscopic ablation zones (MAZs) consisting of vertical channels of ablated tissue [12, 20, 22]. The MAZs temporarily interrupt the skin-barrier and provide an alternative pathway for uptake of topically applied drugs [1, 11, 15–17, 20, 26]. Unlike many other physical drug delivery techniques, AFXL can generate MAZs of specific depths and densities. Adjusting these parameters may allow for controlled delivery of drug amount and depth within the skin [3, 9, 25].
Methods
Study set-up
In Franz diffusion cells (FC), bio-distribution of IngMeb was investigated in a total of 67 skin samples [7]. Seven intervention groups represented FCs with intact control skin (n = 9) and skin exposed to AFXL at different depths and densities (n = 58; Table 1). Liquid Chromatography Mass Spectrometry (LC–MS) was used to quantify deposition of IngMeb within the skin in stratum corneum (SC), epidermis, and dermis, as well as IngMeb traversing the skin into the receiver chamber.
Skin preparation and AFXL settings
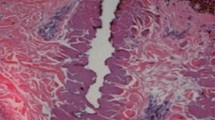
Porcine skin was collected from the flank of female Danish Yorkshire/Landrace pigs immediately after euthanasia and stored at −20 °C for a maximum of 12 weeks. Before laser exposure, the skin was thawed for 24 h. Excessive hair was trimmed and subcutaneous fat was removed. A 2940-nm ablative fractional Er:YAG prototype (P.L.E.A.S.E. Professional prototype, Pantec Biosolutions AG, Ruggell, Liechtenstein) delivered energies of 11.2 mJ/MAZ (125 μs, 300 Hz, 2 stacked pulses) and 128 mJ/MAZ (225 μs, 100 Hz, 10 stacked pulses) [25]. Laser channel dimensions for the specific settings were previously established; 11.2 mJ/MAZ generated intraepidermal MAZs with a median ablation depth of 66 μm, width of 177 μm, and a coagulation zone of 6 μm [25]. At 128 mJ/MAZ, ablation depth was 570 μm, width 262 μm, and coagulation zone 42 μm. MAZ counts of 16, 97, and 195 MAZs/cm2 were examined for both intraepidermal and indtradermal MAZs. Density, which refers to the aggregate area of MAZs per unit skin surface area, was calculated for by multiplying MAZ counts (n) by MAZ surface ablation area (n × π(width/2))2. Single samples of both MAZ types were visualized on 5 μm formalin fixated, paraffin embedded, and hematoxylin-eosin stained slides (Fig. 1).
Histological hematoxylin-eosin stained samples depicting cross sections of microscopic ablation zones (MAZs) generated by laser treatment. a Laser-treatment delivered with 11.2 mJ/MAZ resulted in superficial MAZs measuring 120 μm in width and 49 μm in depth with minimal thermal damage. b Energy level of 128 mJ/MAZ generated mid-dermal, cone-shaped MAZ measuring 387 μm in width and 524 μm in depth with a 73 μm coagulation zone
Franz cells
Franz cells with a receptor volume of 5 ml and a permeation area of 0.64 cm2 were filled with phosphate buffered saline (pH 7.4) and mounted with skin samples. IngMeb 0.05 % gel (Picato®, LEO Pharma A/S, Ballerup, Denmark) was applied at 10 μl/cm2 directly on the skin, corresponding to a total of 3200 ng IngMeb per FC. Water at 37 °C surrounded the FC-chambers and kept the skin temperature constant at 32 °C, while magnetic stir bars stirred the receptor fluid. Parafilm® (Bemis, Oshkosh, WI, USA) covered the donor chambers to avoid desiccation of the skin and at 21 h, skin was dismounted from the FC and padded with dry gauze. Tape-stripping using D-squame® (Cuderm, Dallas, TX, USA) removed SC from cellular skin. To separate epidermis from dermis, the skin sample was placed in a humidified chamber at 0.7 kg H2O per 1.0 kg air and heated to 60 °C for 15 min, whereafter the two layers were separated with forceps.
Liquid chromatography mass spectrometry
Epidermal and dermal samples were dissolved in protein kinase K and digestion-buffer followed by homogenization. Acetonitrile (20 ml) was added to all sample tubes containing SC, epidermis or dermis, whereafter the samples were centrifuged and the supernatant was isolated for LC–MS analyses. LC–MS quantified IngMeb on a Shimadzu LC system (SIL-20 ACXR Autosampler and LC-20ADXR solvent delivery module, Shimadzu Corporation, Tokyo, Japan) with a Zorbax SB-C18 5 µm, 2.1 × 50 mm column (Agilent Technologies, Santa Clara, CA) coupled to an AB SCIEX QTRAP® 5500 (AB SCIEX, Framingham, MA). Concentrations were converted to absolute mass and presented as ng/0.64 cm2 present in SC, epidermis, dermis, and receiver chamber.
Statistics
Nonparametric statistics compared uptake in different skin compartments using Mann–Whitney U tests for two-group comparisons. Data were presented as medians with interquartile ranges (IQR). p values were 2-sided and considered statistically significant when less than 0.05. SPSS version 20 (IBM Corporation, Armonk, NY, USA) performed the statistical analyses.
Results
Microscopic ablation zones and calculated densities
Single histology samples depicted MAZs penetrating into epidermis or mid-dermis, depending on delivered energy. At 11.2 mJ/MAZ, the channel appeared as an intraepidermal pit with minimal coagulation zone (Fig. 1a). At 128 mJ/MAZ, intradermal, cone-shaped MAZs were found with substantial coagulation zones lining the laser channel (Fig. 1b). MAZ counts of 16, 97, and 195 MAZs/cm2 corresponded to surface ablation densities of 0.5, 2.5, and 5 % for intraepidermal MAZs, and corresponded to 1, 5, and 10.5 % for intradermal MAZs (Table 1).
Biodistribution of ingenol mebutate in intact skin
Uptake of IngMeb is presented in Table 1 and Fig. 2. The intracutaneous deposition of IngMeb in intact skin was 1822 ng, corresponding to 57 % of applied dose. IngMeb was primarily deposited in the epidermis, 1314 ng (41 % of applied dose), of which 708 ng was located in SC (22 %) and 606 ng in cellular epidermis (19 %). Dermal deposition was 508 ng (16 % of applied dose), and IngMeb was undetected (0 ng, 0 %) in the receiver chamber.
Biodistribution of ingenol mebutate in intact and laser-exposed skin. Ablative fractional laser (AFXL)-exposure altered the distribution of ingenol mebutate (IngMeb) in the different skin compartments. The accumulation of IngMeb in stratum corneum was reduced from AFXL-exposure and decreased gradually with increasing density. Deposition in cellular epidermis persisted when applying intraepidermal Microscopic Ablation Zones (MAZs) at low laser densities but decreased with intraepidermal MAZs at 5 % density and with intradermal MAZs at 1–10.5 % laser densities. The maximal dermal deposition was achieved with intradermal MAZ at 10.5 % density resulting in 62 % deposition of applied dose (p < 0.001)
Biodistribution of ingenol mebutate in AFXL-exposed skin
Ablative fractional laser-exposure altered the biodistribution of IngMeb in the different skin compartments, resulting in an overall less superficial and deeper deposition (Table 1; Fig. 2). The dermal uptake of IngMeb depended entirely on laser density; increasing coverage from 0 % to 0.5 %, 1 %, 2.5 %, 5 %, and 10.5 % enhanced the dermal uptake 1.6-, 2.1-, 3.1-, 3.4-, and 3.9-fold, respectively (Table 1, p < 0.0001). Unlike density, dermal deposition was not influenced by MAZ depth. At 5 % laser density, dermal deposition with intraepidermal and intradermal MAZs was analogous, 1801 vs. 1744 ng (p = 0.447; Fig. 3). The maximal dermal deposition was achieved at 10.5 % laser density where deposition was 1986 ng, corresponding to 62 % of the applied IngMeb (p < 0.001).
Dermal deposition of ingenol mebutate—intraepidermal vs. intradermal microscopic ablation zones (MAZs). Dermal deposition was not influenced by laser channel depth, but depended entirely on surface ablation. Increasing coverage from 0 % to 0.5, 1.0, 2.5, 5.0, and 10.5 % increased the uptake of IngMeb from 508 ng to 843 ng, 1584 ng, 1744/1801 ng (intraepidermal/intradermal), and 1986 ng, respectively
While dermal uptake increased from AFXL-exposure, accumulation in epidermis (SC and cellular epidermis) either persisted or decreased, depending on the laser settings. In SC, the deposition gradually decreased with increasing density (p < 0.001; Fig. 2). In cellular epidermis, the deposition was similar to intact skin when applying intraepidermal MAZs at low laser densities (0 %, 606 ng; 0.5 %, 558 ng; 2.5 %, 568 ng; p > 0.400), but decreased with intraepidermal MAZs at 5 % density and with intradermal MAZs at 1, 5, and 10.5 % densities (p < 0.008; Fig. 2). The maximal epidermal deposition after AFXL-exposure was achieved with intraepidermal MAZs at 2.5 % density where uptake remained at 568, corresponding to 18 % of applied dose (p < 0.001).
Transdermal permeation
Permeation of IngMeb through the skin was seen after AFXL-exposure (Table 1). Accumulation of IngMeb in receiver chamber increased from 0 ng in intact skin up to 547 ng (17 % of applied dose) and 677 ng (21 % of applied dose) with intraepidermal and intradermal MAZs, respectively (p < 0.001). At a fixed density of 5 %, transcutaneous delivery did not differ between intraepidermal and intradermal MAZs (547 vs. 677 ng; p = 0.113).
Discussion
In this study, we investigated bio-distribution of IngMeb in intact and AFXL-exposed skin. Unlike previous studies, drug deposition was examined in different skin compartments allowing us to understand the impact of laser settings, not only on the total cutaneous accumulation of the drug, but also on distribution within the skin. AFXL was found to notably alter the biodistribution of IngMeb, inducing a more profound dermal deposition while less was accumulated in the superficial skin layers. Laser channel depth had no effect on uptake or bio-distribution, while dermal deposition increased with increasing laser density. These findings indicate that density can be adjusted to facilitate delivery of IngMeb into different skin compartments, providing for customized topical delivery and use of IngMeb for dermally located, keratinocyte-derived tumors.
Ingenol mebutate is derived from the sap of Euphorbia peblus, which has bioactive properties mediated through activation of the PKC-delta receptor. IngMeb has been found to exert a pro-apoptotic and cytotoxic effect in several human cancer cell lines, including keratinocyte-derived cancer cells [6, 24]. Although different immunological conditions may apply in epidermis and dermis, cytotoxicity is observed at concentrations of 200–300 μM, and to secure effective treatment of cutaneous dysplasia and dermal tumors, these levels need to be reached around the dysplastic/neoplastic cells. IngMeb is a relatively small and lipophilic drug (432 Da; logP octanol/water 2.7). Such molecules tend to diffuse well across SC, the outer-most barrier layer of the skin, consistent with the findings in our study. However, cutaneous delivery of 57 % of an applied drug is exceptionally high when compared with most other topical agents, which rarely exceed 5 % [21]. The high penetration of IngMeb into intact skin in this study may be attributed in part to occlusion of the skin in FC-chambers during diffusion. Occlusion results in hydration and swelling of the corneocytes, which increases uptake across the SC. Even without occlusion, IngMeb has been confirmed to penetrate well into the epidermis of intact skin, while dermal penetration is limited [24].
Assisting delivery of IngMeb with AFXL notably altered the biodistribution of IngMeb in the skin. The impact of density was investigated for intraepidermal and intradermal MAZ with 16, 97, and 195 MAZ/cm2. As MAZ-width varied between intraepidermal and intradermal MAZs (262 vs. 177 μm), the actual surface ablation density was distinctly different for the two settings and corresponded to 0.5, 2.5, and 5.0 % for intraepidermal MAZs and corresponded to 1.0, 5.0, and 10.5 % for intradermal MAZs. These calculated surface ablation densities deviate from manufacture-stated densities of 1 % (195 MAZ/cm2), 5 % (195 MAZ/cm2), and 10 % (195 MAZ/cm2). Caution should therefore be exercised in the clinic when adjusting fluences with fixed MAZ counts, as density may vary considerably and result in over-, or under-estimating your treatment or induction of unexpected complications.
When actual surface ablation densities are considered, dermal uptake was found to depend entirely on laser density, increasing up to 4-fold with 10.5 % coverage. Deposition in SC decreased drastically with increasing laser density, while epidermal uptake was maintained at low laser densities and decreased at higher. The decrease in SC-deposition and increase in dermal delivery could in theory be explained by loss of SC from laser ablation, or by drug depletion near the skin surface as the drug distributes to deeper dermal compartments. Intraepidermal MAZs were approximately 66 μm, deep enough to remove some SC (Fig. 1). At densities of 0.5, 2.5, and 5 %, a corresponding percentage of SC-tissue is ablated, while 99.5, 97.5, and 95 % of SC remain intact. If the observed decrease of IngMeb in the SC was due only to SC-ablation, the decrease would correspond to laser density. Instead, we found a considerably greater decrease than expected; at 0.5, 2.5, and 5 % laser density, the decrease in IngMeb deposition was 15 % (708–603 ng), 81 % (708–135 ng), and 91 % (708–59 ng), respectively. Thus, the decrease in SC-deposition is most likely a result of drug redistribution controlled by laser density.
In addition to laser density, we investigated the impact of laser channel depth. Contrary to expectations, applying deeper MAZs did not result in deeper deposition of IngMeb. Previous in vitro studies investigating AFXL-assisted delivery of similarly sized drugs (<500 Da) have demonstrated equivalent results [2, 10]. Bachhav et al. [1, 2] investigated deposition of lidocaine (MW 234 Da; logD 7.4 1.2) and diclofenac (MW 296 Da; logD 7.4 0.84) as a function of MAZ depth. At fixed MAZ counts of 400 and 900 MAZs per cm2, the total intracutaneous deposition of lidocaine and diclofenac was not enhanced when MAZ depth increased from superficial to deep intraepidermal depths [1, 2]. Correspondingly, Haak et al. [10] showed that superficial-dermal and deep-dermal MAZs generated similar methyl aminolevulinate (MAL)-induced porphyrin fluorescence in superficial and deep-dermal layers (MW 145 Da; logD 7.4 −0.76). The depth-independence found in our and previous studies may have several explanations. Cellular epidermis and dermis do not exert notable diffusion resistance for small drugs and once the SC is surpassed, the drugs may readily diffuse throughout the cellular cutis. Other explanations may include obstruction of the channels with debris or air, or increased diffusion resistance over the often thicker coagulation zone lining deeper laser channels. Another factor may be changes in the magnitude and spatial distribution of concentration gradients within the tissue; saturation of a drug within the epidermis or dermis between MAZs would decrease the local concentration gradient that drives diffusion. The mechanism(s) by which drugs are prevented from entering MAZ channels, or diffusing beyond them, need further investigation.
The Franz Cell in vitro drug diffusion model is widely accepted for investigation of cutaneous drug delivery. Diffusion over 24 h is recommended to appreciate transcutaneous absorption in intact skin, while in AFXL-exposed skin a shorter diffusion time may suffice [8]. We allowed diffusion to run for 21 h after applying a finite dose to mimic topical treatments. The kinetics of IngMeb uptake was not investigated, and equilibrium between donor and skin is likely to have been reached at 21 h. A limitation of the study is that the FC model only investigates passive diffusion, which does not consider effects from the in vivo microvasculatory systems, in vivo drug metabolism, or active drug transportation that are believed to play a role in in vivo delivery of IngMeb [19]. In addition, IngMeb induces local immunological responses that may become adverse or systemic with AFXL. However, these effects cannot be monitored in the in vitro FC model and caution has to be taken when extrapolating the in vitro data for use in human patients.
Ablative fractional laser-assisted delivery of IngMeb has several clinical implications. With AFXL pre-treatment, dermal deposition of IngMeb is greatly increased, which may enable IngMeb to reach therapeutic concentrations within the dermis for locally invasive tumors. For topical treatment of NMSC, ensuring a deep drug penetration is pivotal for an effective outcome, and AFXL-assisted delivery of IngMeb may ensure such conditions and come to serve as an effective treatment option for tumors not suitable for surgical intervention. In addition, AFXL may be used to alter the treatment regime of IngMeb. Currently, IngMeb is a home-based treatment, prescribed to patients with actinic damage and self-administered with a daily application for 2–3 days. It could be further investigated whether a one-time AFXL-assisted treatment would rival or exceed the efficacy of self-applications. This might have implications for non-compliant patients who are unable to complete serial treatments at home. Such candidates would be able to undergo single treatments at a medical clinic, and the concerns regarding patient compliance would be avoided.
In conclusion, IngMeb successfully distributes to superficial layers of intact skin, whereas dermal uptake is limited. AFXL-exposure alters the biodistribution and increases the dermal deposition. These findings indicate that laser settings can be adjusted to facilitate delivery of IngMeb into different skin compartments, providing for customized topical delivery and use of IngMeb in dermally located, keratinocyte-derived tumors.
References
Bachhav YG, Heinrich A, Kalia YN (2011) Using laser microporation to improve transdermal delivery of diclofenac: increasing bioavailability and the range of therapeutic applications. Eur J Pharm Biopharm 78:408–414
Bachhav YG, Summer S, Heinrich A, Bragagna T, Böhler C, Kalia YN (2010) Effect of controlled laser microporation on drug transport kinetics into and across the skin. J Control Release 146:31–36
Bloom BS, Brauer JA, Geronemus RG (2013) Ablative fractional resurfacing in topical drug delivery: an update and outlook. Dermatol Surg 39:839–848
Brown MB, Martin GP, Jones SA, Akomeah FK (2006) Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv 13:175–187
Elias PM, Menon GK (1991) Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res 24:1–26
Ersvaer E, Kittang AO, Hampson P, Sand K, Gjertsen BT, Lord JM, Bruserud O (2010) The protein kinase C agonist PEP005 (ingenol 3-angelate) in the treatment of human cancer: a balance between efficacy and toxicity. Toxins (Basel) 2:174–194
Franz TJ (1975) Percutaneous absorption on the relevance of in vitro data. J Invest Dermatol 64:190–195
Franz TJ (1983) Kinetics of cutaneous drug penetration. Int J Dermatol 22:499–505
Haak CS, Bhayana B, Farinelli WA, Anderson RR, Haedersdal M (2012) The impact of treatment density and molecular weight for fractional laser-assisted drug delivery. J Control Release 163:335–341
Haak CS, Farinelli WA, Tam J, Doukas AG, Anderson RR, Haedersdal M (2012) Fractional laser-assisted delivery of methyl aminolevulinate: impact of laser channel depth and incubation time. Lasers Surg Med 44:787–795
Haedersdal M, Katsnelson J, Sakamoto FH, Farinelli WA, Doukas AG, Tam J, Anderson RR (2011) Enhanced uptake and photoactivation of topical methyl aminolevulinate after fractional CO2 laser pretreatment. Lasers Surg Med 43:804–813
Haedersdal M, Sakamoto FH, Farinelli WA, Doukas AG, Tam J, Anderson RR (2010) Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med 42:113–122
Kedei N, Lundberg DJ, Toth A, Welburn P, Garfield SH, Blumberg PM (2004) Characterization of the interaction of ingenol 3-angelate with protein kinase C. Cancer Res 64:3243–3255
Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman B (2012) Ingenol mebutate gel for actinic keratosis. N Engl J Med 366:1010–1019
Lee W-R, Shen S-C, Al-Suwayeh SA, Yang H-H, Li Y-C, Fang J-Y (2013) Skin permeation of small-molecule drugs, macromolecules, and nanoparticles mediated by a fractional carbon dioxide laser: the role of hair follicles. Pharm Res 30:792–802
Lee W-R, Shen S-C, Al-Suwayeh SA, Yang H-H, Yuan C-Y, Fang J-Y (2011) Laser-assisted topical drug delivery by using a low-fluence fractional laser: imiquimod and macromolecules. J Control Release 153:240–248. doi:10.1016/j.jconrel.2011.03.015
Lee W-R, Shen S-C, Pai M-H, Yang H-H, Yuan C-Y, Fang J-Y (2010) Fractional laser as a tool to enhance the skin permeation of 5-aminolevulinic acid with minimal skin disruption: a comparison with conventional erbium:YAG laser. J Control Release 14:124–133
LEO Pharma A/S (2012) Product information picato gel. 1–4
Li L, Shukla S, Lee A, Garfield SH, Maloney DJ, Ambudkar SV, Yuspa SH (2010) The skin cancer chemotherapeutic agent ingenol-3-angelate (PEP005) is a substrate for the epidermal multidrug transporter (ABCB1) and targets tumor vasculature. Cancer Res 70:4509–4519
Manstein D, Herron GS, Sink RK, Tanner H, Anderson RR (2004) Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med 34:426–438
Nino M, Calabrò G, Santoianni P (2010) Topical delivery of active principles : the field of dermatological research. Dermatol Online J 16(1):4
Paasch U, Haedersdal M (2011) Laser systems for ablative fractional resurfacing. Expert Rev Med Devices 8:67–83
Siller G, Rosen R, Freeman M, Welburn P, Katsamas J, Ogbourne SM (2010) PEP005 (ingenol mebutate) gel for the topical treatment of superficial basal cell carcinoma: results of a randomized phase IIa trial. Australas J Dermatol 51:99–105
Stahlhut M, Bertelsen M, Høyer-Hansen M, Svendsen N, Eriksson AH, Lord JM, Scheel-Toellner D, Young SP, Zibert JR (2012) Ingenol mebutate-induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol 11:1181–1192
Taudorf EH, Haak CS, Erlendsson AM, Philipsen PA, Anderson RR, Paasch U, Haedersdal M (2014) Fractional ablative erbium YAG laser: histological characterization of relationships between laser settings and micropore dimensions. Lasers Surg Med 46:281–289
Togsverd-Bo K, Haak CS, Thaysen-Petersen D, Wulf HC, Anderson RR, Hædersdal M (2012) Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol 166:1262–1269
Acknowledgments
The authors wish to thank Dina Wennike for expert technical assistance during the study.
Conflicts of interest
AM Erlendsson received PhD Scholarship from LEO Pharma A/S. M Haedersdal received research grants from LEO Pharma A/S and Pantec Biosolutions. AH Eriksson and JR Zibert are employees at LEO Pharma A/S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erlendsson, A.M., Taudorf, E.H., Eriksson, A.H. et al. Ablative fractional laser alters biodistribution of ingenol mebutate in the skin. Arch Dermatol Res 307, 515–522 (2015). https://doi.org/10.1007/s00403-015-1561-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-015-1561-3