Abstract
The primary cause of non-melanoma skin cancer is ultraviolet (UV) light from the sun. Many studies have demonstrated that cutaneous inflammation resulting from UV exposure is important for the development of skin cancer. In fact, anti-inflammatory drugs have been shown to be effective in preventing skin cancer in animal models and in clinical trials. One new class of inflammatory mediators that could regulate UV-induced inflammation and skin carcinogenesis is alarmins. Alarmins are endogenous molecules that act as potent pro-inflammatory mediators when they are released by cells or accumulate extracellularly. The purpose of the current studies was to examine the expression and release of the alarmin high mobility group box 1 (HMGB1) after acute and chronic UV irradiation. Acute UV exposure stimulated the release of HMGB1 in cultured human keratinocytes and epidermal keratinocytes in murine skin. HMGB1 release correlated with pro-inflammatory cytokine production in vitro and inflammatory cell infiltration in vivo. HMGB1 was also examined in tumors arising in chronically irradiated murine skin. HMGB1 protein expression in low grade, benign papillomas was similar to adjacent skin. However, HMGB1 staining was more widespread with a higher number of HMGB1-positive cells observed in high grade papillomas and malignant tumors. Overall, the data suggest that HMGB1 may be an important regulator of UV-induced cutaneous inflammation and tumor formation. Additional studies are needed to assess whether targeting HMGB1 would be a useful strategy to prevent tumors from developing in response to chronic UV exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to ultraviolet light is believed to be the most important risk factor for developing non-melanoma skin cancer (NMSC). NMSC, which includes both basal cell carcinoma and squamous cell carcinoma, is the most common form of cancer [36]. Ultraviolet (UV) light, particularly UVB, induces a classic inflammatory response in the skin characterized by redness, swelling, and the activation and infiltration of inflammatory cells [49]. This inflammatory response, along with reactive oxygen species and DNA damage resulting from UV exposure, contributes to tumor formation in the skin [34]. The importance of UV-induced inflammation in the development and progression of skin tumors has been demonstrated in various animal models, and is highlighted by studies which show that anti-inflammatory drugs can act as potent chemopreventive agents [14, 35, 40, 48].
Inflammation is most commonly initiated when the body detects a potential pathogen, but inflammation can also be initiated in the absence of pathogen-associated signals. Exposure to UV light, for example, induces inflammation independently of microbial recognition. One class of mediators that act as endogenous danger signals to stimulate ‘sterile inflammation’, or inflammation that occurs in the absence of microbes, is alarmins [5]. Alarmins are sometimes referred to as damage-associated molecular patterns (DAMPs) because these endogenous molecules act in a similar way to exogenous pathogen-associated molecular patterns (PAMPs), which stimulate inflammation in response to microbes [5, 12]. Many alarmins reside inside the cell or within cell nuclei under normal conditions, but are released from the cell upon damage. When alarmins are released or build up in the extracellular space, they bind pattern recognition receptors (PRRs) or other receptors on the surface of inflammatory cells resulting in NF-κB activation and subsequent pro-inflammatory cytokine production [5]. Some alarmins can also activate inflammasomes, leading to caspase-1 activation and the release of mature IL-1β [38]. For example, adenosine triphosphate (ATP) causes inflammasome activation and results in IL-1β production [24, 29, 42].
High mobility group box-1 (HMGB1) is one well-studied alarmin [5, 22]. HMGB1 is a non-histone DNA binding protein that is localized to the nucleus under normal conditions [12]. However, in response to cell damage or exposure to certain cytokines, HMGB1 moves from the nucleus to the cytoplasm and is eventually released from the cell. Extracellular HMGB1 stimulates inflammation by binding to the receptor of advanced glycation end products (RAGE) or toll-like receptors (TLRs) on the surface of inflammatory cells [30]. Based on the importance of HMGB1 in sterile inflammation, this alarmin likely helps regulate the cutaneous inflammatory response initiated by exposure to UV light.
In the current study, HMGB1 expression and release was characterized in keratinocytes after acute UV exposure. HMGB1 expression was also examined in skin and tumors after chronic UV irradiation. The studies indicate that HMGB1 release correlates with acute inflammation and show that HMGB1 is expressed in murine skin tumors. Overall, the results suggest that this alarmin could be important for the development and progression of UV-induced skin tumors.
Materials and methods
Cell culture
Primary normal human epidermal keratinocytes (NHEK) from pooled neonatal donors were cultured in keratinocyte growth medium-2 (KGM-2; Lonza, Walkersville, MD). Cells were maintained at 37 °C and 5 % CO2 and subcultured according to manufacturer’s guidelines. Cells were passaged no more than three times. NHEK were grown to approximately 70 % confluence in 100 mm tissue culture dishes. Media were removed and cells were washed twice with phosphate buffered saline (PBS). Cells were irradiated in PBS at various doses of UVB. Unirradiated cells were treated in the same way except they were not exposed to UV. UVB light was emitted by Philips FS25 UVB lamps (American Ultraviolet Company, Lebanon, IN) and the dose was determined using a UVX meter (UVP Inc., Upland, CA). After irradiation, PBS was replaced with KGM-2. Cells were irradiated with a dose of 300 J/m2 UVB and supernatants and cell lysates were harvested at 6, 12, or 24 h post-irradiation. Alternately, cells were irradiated with 75, 150, or 300 J/m2 UVB then cells and supernatants were harvested at 24 h post-irradiation.
Assessment of cell death
Cell death was estimated by measuring release of the intracellular enzyme lactate dehydrogenase into supernatants using the CytoTox 96 assay (Promega, Madison, WI) as described by Krysko et al. [23]. Briefly, cell supernatants were added to 96 well plates and incubated with substrate solution for 30 min, then stop buffer was added and the absorbance was measured at 490 nm.
To analyze apoptosis, adherent cells were harvested from tissue culture dishes using 0.25 % trypsin/EDTA solution (Lonza) and combined with floating cells from the same dish that had been separated from the supernatants by centrifugation. Cells were washed two times with PBS. Staining with APC-conjugated annexin V and SYTOX green (Molecular Probes, Eugene, OR) was used to analyze apoptosis by fluorescence activated cell sorting (FACS). Briefly, cells were resuspended at a concentration of 1 × 106 cells/ml in annexin binding buffer (Molecular Probes) and 1 × 105 cells were stained with 5 μl annexin V-APC and 1 μl of 1M SYTOX green for 15 min at 37 °C in the dark. Staining was detected within 1 h by FACS. Apoptosis was confirmed through cell cycle analysis in propidium iodide-stained cells. Cells (1 × 105) were fixed overnight in cold 70 % ethanol then permeabilized for 15 min on ice with 0.25 % Triton X-100 in PBS. Following permeabilization, cells were stained with PBS containing 20 μM propidium iodide (BD Biosciences, San Jose, CA) and 10 μg/ml RNase (Life Technologies, Carlsbad, CA). Annexin/SYTOX staining and propidium iodide staining were detected using a FACSCalibur flow cytometer and analysis was performed using CellQuest Pro software (BD Biosystems). Cells staining positive for Annexin V but negative for SYTOX green or propidium iodide-stained cells in the sub-G1 peak were considered apoptotic.
Animals
Female SKH-1 hairless mice (6–8 weeks old, Charles River, Wilmington, MA) were housed in the vivarium at the Ohio State University according to the requirements established by the American Association for Accreditation of Laboratory Animal Care. All procedures were approved by the Institutional Animal Care and Use Committee prior to beginning the studies. For irradiations, mice were exposed dorsally to 2,240 J/m2 of UVB light (approximately one minimal erythemic dose) from Phillips FS40 UVB lamps (American Ultraviolet Company). The lamps were fitted with Kodacel filters (Eastman Kodak, Rochester, NY) to ensure the emission of only UVB light (290–320 nm). For acute studies, mice were euthanized 6, 12, 24 or 48 h after a single exposure, with unirradiated mice serving as controls. For long-term studies, mice were irradiated three times weekly for 15 weeks then maintained without UV exposure for an additional 9 weeks. This procedure has been previously shown to generate multiple tumors of various grades [50]. At this point, skin and tumors were harvested from irradiated mice. Skin from age-matched, unirradiated mice was used as controls. Samples were either frozen in liquid nitrogen, frozen in TBS tissue freezing media (Triangle Biomedical Sciences, Durham, NC), or fixed in 10 % neutral buffered formalin and embedded in paraffin. For acute samples, a portion of the skin was reserved for separation of the epidermis from the dermis by submersion in 60 °C water followed immediately by submersion in ice water [15]. For tumor samples, grades were assigned as described previously [44] using histological criteria by a board-certified veterinary pathologist. Clear evidence of stromal invasion was required for a tumor to be considered a microinvasive SCC.
Protein analysis
Total protein was isolated from NHEK lysates or epidermal tissue using RIPA (radioimmunoprecipitation assay) buffer containing protease inhibitor cocktail (Thermo Scientific, Rockford, IL). Cultured cells were lysed directly in tissue culture dishes and epidermal samples were homogenized in RIPA buffer and sonicated. After centrifugation, total protein concentrations were determined using the BCA (bicinchoninic acid) protein assay (Thermo).
HMGB1 and β-actin levels were determined by Western blot using standard methods. Briefly, total protein was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). Membranes were blocked and probed using anti-HMGB1 (Abcam, Cambridge, MA) and anti-β-actin (Cell Signaling, Danvers, MA) antibodies. Secondary horseradish peroxidase (HRP)-conjugated anti-rabbit antibodies (Cell Signaling) were used in conjunction with Immun-Star WesternC chemiluminescence reagents (Bio-Rad) for protein detection. Digital images were captured using the Chemidoc XRS imaging system (Bio-Rad). Densitometry was performed using Quantity One software (Bio-Rad).
Commercially available enzyme-linked immunosorbent assays (ELISAs) were used to quantify the amount of HMGB1 (IBL International, Toronto, ON) or TNF-α, IL-1β, and IL-6 (R&D Systems, Minneapolis, MN) in NHEK supernatants according to manufacturer’s instructions.
Immunohistochemistry
Immediately following euthanization, skin sections were either fixed in 10 % neutral buffered formalin, processed, and embedded in paraffin, or frozen in TBS tissue freezing media. Immunohistochemistry was used to detect HMGB1, Ly-6G (neutrophils), and MOMA-2 (macrophages). After each step described below, slides were washed in PBS, except after serum blocking steps.
HMGB1 immunostaining was performed in 4 μm paraffin sections. After deparaffinization and rehydration, antigen retrieval was performed by steaming slides in target retrieval solution (DAKO, Carpinteria, CA) for 15 min. After cooling slides in the target retrieval solution for 10 min, sections were blocked with 3 % hydrogen peroxide in water for 10 min, then 10 % normal goat serum for 30 min. Samples were then incubated with rabbit anti-HMGB1 antibodies (Abcam, Cambridge, MA) overnight at 4 °C in a humid chamber. For negative staining controls, some slides were incubated with serum only (primary antibody omitted) or with rabbit IgG (Vector Laboratories, Burlingame, CA) at the same final concentration as the HMGB1 antibody (data not shown). The following day, samples were incubated with biotinylated goat anti-rabbit secondary antibodies (Vector Laboratories) and then avidin/biotinylated horseradish peroxidase complex (ABC-HRP complex; Vector Laboratories) for 30 min each. The samples were then incubated with DAB solution (KPL, Gaithersburg, MD) for 8 min in the dark to visualize the staining. After rinsing with distilled water, sections were counterstained with hematoxylin 2 (Richard-Allan Scientific, Kalamazoo, MI). Slides were then dehydrated and coverslips were mounted before samples were viewed and photographed.
Neutrophils were identified in paraffin sections by immunohistochemical detection of the neutrophil marker Ly-6G. Sections were treated as described above for HMGB1 staining, except that the antigen retrieval and hydrogen peroxide blocking steps were omitted and normal rabbit serum, rat anti-mouse Ly-6G (BD Biosciences, San Jose, CA) primary antibodies, and biotinylated rabbit anti-rat secondary antibodies were used. The number of Ly-6G-positive cells in the dermis was counted in at least five 400X fields per section.
To detect macrophages, 10 μm cryosections were air dried and fixed in acetone for 15 min. Slides were treated with 0.3 % hydrogen peroxide in methanol for 30 min then blocked with 10 % normal rabbit serum (Vector Laboratories) for 30 min. Sections were incubated with rat anti-mouse MOMA-2 antibodies (Abcam) in serum overnight at 4 °C. After incubation with biotinylated rabbit anti-rat secondary antibodies and ABC-HRP complex, DAB solution was used to visualize staining as described above.
Statistical analysis
Prism software (Graphpad, La Jolla, CA) was used for statistical analysis. One-way or two-way ANOVA with Bonferroni’s post hoc testing were used where appropriate. p-values <0.05 were considered statistically significant.
Results
UV irradiation stimulates HMGB1 release and pro-inflammatory cytokine secretion in cultured keratinocytes
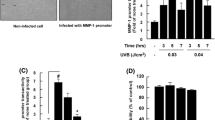
To determine whether acute UV exposure alters HMGB1 levels in cultured keratinocytes, NHEK lysates were analyzed by Western blot. HMGB1 protein levels were similar to unirradiated cells (No UV) at 6 and 12 h after UV irradiation, with HMGB1 expression ratios near 1 for irradiated/unirradiated cells (Fig. 1a). HMGB1 levels declined significantly in irradiated cells 24 h after UV exposure. To determine whether the reduction in cellular HMGB1 was due to release of the protein after irradiation, HMGB1 was analyzed in supernatants by ELISA (Fig. 1b). HMGB1 levels were low in supernatants from unirradiated cells, but increased significantly in response to UV. HMGB1 began to increase in supernatants 12 h after irradiation and was highest at 24 h. Together, the loss of HMGB1 protein in cell lysates and the concomitant increase in HMGB1 in supernatants after irradiation demonstrate that HMGB1 is released by cultured keratinocytes in response to UV.
HMGB1 and pro-inflammatory cytokine levels in cultured NHEKs after acute UV irradiation. NHEK cells were grown to 70 % confluence and exposed to 300 J/m2 of UV light. Unirradiated cells (No UV) served as controls. Cell lysates and supernatants were collected at 6, 12, or 24 h after irradiation. HMGB1 was detected in protein isolated from adherent cells by Western blot (a). Densitometry was used to quantify HMGB1 expression and the data were normalized to β-actin levels. The ratio of normalized HMGB1 in irradiated cells to unirradiated cells at each timepoint is shown graphically. Bars represent the mean ratio (UV:NoUV) of normalized HMGB1 ± S.E.M. (n = 3–4 samples per timepoint; *p < 0.05 by one-way ANOVA with Bonferroni post hoc testing). A representative blot is shown below the graph. HMGB1 (b), IL-1β (c), TNF-α (d), and IL-6 (e) release were assessed by measuring the concentrations of each mediator in supernatants by ELISA. Bars represent mean cytokine level ± S.E.M. (n = 3–6 samples per timepoint; *p < 0.05, **p < 0.01 and ***p < 0.001 by two-way ANOVA with Bonferroni post hoc testing)
To assess whether HMGB1 release corresponded to the production of pro-inflammatory cytokines, the levels of classic pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) were determined in NHEK supernatants by ELISA (Fig. 1c–e). The timing and pattern of pro-inflammatory cytokine secretion was similar to HMGB1 release, as the levels of all three cytokines began to rise in supernatants from irradiated cells 12 h after UV exposure and were the greatest at 24 h. These results show that HMGB1 release correlates with UV-induced cytokine production by keratinocytes.
UV-induced HMGB1 release correlates with cell death in cultured keratinocytes
Studies have shown that HMGB1 release often occurs as a result of cell death, including release by necrotic cells and cells undergoing apoptosis or pyroptosis [3, 18, 26, 27, 39]. Given that UV exposure causes a predictable cell death response keratinocytes [7, 33], NHEKs were exposed to multiple doses of UV to determine whether HMGB1 release correlated with keratinocyte cell death. HMGB1 and pro-inflammatory cytokine levels increased significantly at 24 h only with the highest dose (300 J/m2) of UV (Fig. 2a–c). Similarly, significant increases in cell death, estimated by measuring lactate dehydrogenase release (Fig. 2d), and apoptosis (Fig. 2e–f) were also only observed after irradiation with 300 J/m2 of UV. These data indicate that cell death is likely important for HMGB1 release by cultured keratinocytes after UV exposure.
HMGB1 release correlates with keratinocyte death in cultured NHEKs after acute irradiation. NHEK cells were grown to 70 % confluence and exposed to 75, 150 or 300 J/m2 of UV light. Unirradiated cells (No UV) served as controls. Supernatants and cells (adherent and detached) were collected at 24 h after irradiation. HMGB1 (a), IL-1β (b), and TNF-α (c) levels were measured in supernatants by ELISA. Cell death was assessed by measuring lactate dehydrogenase (LDH) in supernatants (d) and apoptosis was assessed by FACS in cells stained with annexin V-APC and SYTOX green (e) or propidium iodide (f). Bars represent mean ± S.E.M. (n = 3–6 samples per timepoint; *p < 0.05, and ***p < 0.001 by one-way ANOVA with Bonferroni post hoc testing)
Acute UV exposure induces HMGB1 release and dermal inflammation in murine skin
In addition to assessing the effects of UV on HMGB1 in cultured keratinocytes, the effects of irradiation on HMGB1 levels were also examined in murine skin in vivo. Immunohistochemical analysis of HMGB1 showed strong staining in the nuclei of epidermal keratinocytes in unirradiated skin (Fig. 3a). A similar pattern of staining was seen 6 (Fig. 3b) and 12 h (data not shown) after irradiation. However, there appeared to be less epidermal staining at 24 h (Fig. 3c), and reduced HMGB1 staining became even more pronounced at 48 h (Fig. 3d). Western blot analysis of epidermal protein was used to confirm the reduced HMGB1 levels observed by immunohistochemistry (Fig. 3e). Significantly lower epidermal HMGB1 levels were observed 48 h after irradiation. Based on the results shown in Fig. 1, the reduction in epidermal HMGB1 staining is likely due to the release of HMGB1 by keratinocytes in response of UV. While HMGB1 staining declined in the epidermis, dermal staining for HMGB1 increased after UV exposure. The dermal cells are likely to be HMGB1-positive immune cells recruited to the skin as part of the acute UV-induced inflammatory response.
HMGB1 expression in murine skin after acute UV irradiation. Immunohistochemical staining was used to examine HMGB1 protein expression in murine skin after acute irradiation. SKH-1 mice were exposed to 2,240 J/m2 UV light (n = 4 mice per timepoint). Representative photomicrographs are shown for unirradiated skin (a) and skin harvested 6 (b), 24 (c), or 48 (d) hours after irradiation. Scale bars 100 μm; brown positive staining. HMGB1 protein levels were also examined by Western blot in epidermal tissue that had been separated from the dermis (e). Densitometry was used to quantify HMGB1 expression and data were normalized to β-actin levels. Bars represent the mean values of normalized HMGB1 ± S.E.M. (n = 3–4 samples from separate mice per timepoint; *p < 0.05 by one-way ANOVA with Bonferroni post hoc testing). A representative blot is shown below the graph
Similar to what was observed in cultured NHEK, reduced cellular HMGB1 protein levels in epidermal keratinocytes (HMGB1 release) corresponded to inflammation in the skin (Fig. 4). As expected, UV exposure resulted in dermal inflammation, with an increase in the number of neutrophils and macrophages in irradiated skin. The greatest numbers of both types of inflammatory cells were present 48 h after irradiation when HMGB1 release was the highest, suggesting that keratinocyte-derived HMGB1 may contribute to UV-induced inflammation.
Inflammatory cell infiltration in murine skin after acute UV irradiation. Immunohistochemical staining was used to detect neutrophils (Ly-6G+ cells) and macrophages (MOMA-2+ cells) in SKH-1 murine skin after exposure to 2,240 J/m2 UV light (n = 4 mice per timepoint). The number of neutrophils was counted per high power field (HPF), which is represented graphically (a). Bars represent the mean number of Ly-6G+ cells/HPF ± S.E.M. (n = 4 samples from separate mice per timepoint; **p < 0.01, ***p < 0.001 by one-way ANOVA with Bonferroni post hoc testing). Representative photomicrographs are shown for Ly-6G staining in unirradiated skin (b) and skin harvested 24 h after irradiation (c). Representative images are also shown for MOMA-2 staining in unirradiated skin (d) and skin harvested 24 h after irradiation (e). Scale bars 50 μm; brown positive staining
HMGB1 is expressed in skin tumors after chronic irradiation
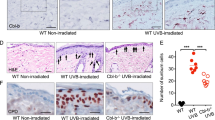
Because inflammation plays an important role in UV-induced tumor formation, HMGB1 was also examined in chronically irradiated skin and tumors. Immunohistochemical staining showed that HMGB1 was expressed primarily in the nucleus of epidermal keratinocytes in unirradiated skin (Fig. 5a) and irradiated skin (Fig. 5b). The presence of nuclear HMGB1 even in irradiated skin is likely due to the 9 week period in which the mice were not exposed to UV that followed 15 weeks of chronic irradiation. HMGB1 was expressed in low grade papillomas, with the most intense staining observed in tumor cells near the tumor-stroma interface (Fig. 5c, grade 1 papilloma; Fig. 5d, grade 2 papilloma). The percentage of tumor cells expressing HMGB1 increased in grade 3 papillomas (Fig. 5e) and microinvasive squamous cell carcinomas (MISCC) (Fig. 5f), with HMGB1-positive tumor cells present throughout the tumor. In addition to intense HMGB1 staining within the tumor in MISCC, a large number of stromal cells also displayed positive staining.
HMGB1 expression in murine skin and tumors after chronic UV irradiation. SKH-1 mice were exposed three times weekly to 2,240 J/m2 UVB for 15 weeks, after which mice were maintained without UV exposure for an additional 9 weeks (24 weeks total). Age-matched, unirradiated mice served as controls. Representative images of HMGB1 immunostaining are shown for unirradiated skin (a), chronically irradiated skin (b), grade 1 papilloma (c), grade 2 papilloma (d), grade 3 papilloma (e), and microinvasive squamous cell carcinoma (f). Scale bars 100 μm; brown positive staining
Discussion
Inflammation is a significant component of the cutaneous response to UV irradiation. After acute UV exposure, inflammation contributes to the classic sunburn reaction, which is characterized by redness, swelling, and the recruitment and activation of inflammatory cells [49]. Chronic UV exposure causes persistent inflammation, which promotes skin carcinogenesis by causing oxidative DNA damage and increasing growth factors and cytokines that promote the growth of mutated keratinocytes [37]. Studies indicating that prominent inflammation is associated with skin tumors and experiments showing that anti-inflammatory drugs can prevent the formation of skin tumors demonstrate the significant role of inflammation in skin carcinogenesis [14, 35, 48]. Based on the importance of inflammation in the development and progression of skin cancer, anti-inflammatory drugs, such as COX-2 inhibitors, have become a viable strategy to combat this disease [11, 19]. However, cardiac events and other side effects associated with the use of many non-steroidal anti-inflammatory drugs [45] highlight the importance of identifying new targets to reduce UV-induced inflammation and potentially prevent skin cancer.
One relatively new class of inflammatory mediators beginning to be recognized for their importance in cutaneous inflammation is alarmins. Alarmins are endogenous molecules that act as pro-inflammatory mediators, which often occurs when they are released or accumulate outside of the cell [5, 12]. Because alarmins mediate ‘sterile inflammation’, or inflammation that occurs in the absence of infection, they could be particularly important for regulating skin inflammation triggered by UV light. In the current study, alterations in the expression of HMGB1, a classic alarmin, were examined after acute and chronic UV irradiation.
In acute studies using a single UV exposure, irradiation resulted in HMGB1 release by keratinocytes. This was demonstrated by a decrease in cellular HMGB1 protein levels with a concomitant increase in HMGB1 over time in supernatants from irradiated primary keratinocytes in culture. UV exposure is known to cause cell death in keratinocytes [7, 33], and HMGB 1 is released by damaged or dying cells. HMGB1 release was originally described in necrotic cells [39], but subsequent studies showed that HMGB1 is also released during apoptosis [3, 18] and pyroptosis [26, 27]. Therefore, cell death was also examined in keratinocytes after exposure to various doses of UV. Significant HMGB1 release was only detected at UV doses for which lactate dehydrogenase release (a marker of cell death) and apoptosis were prominent. Thus, it appears that UV-induced cell death is important for HMGB1 release in cultured keratinocytes. Interestingly, UV has been shown to activate caspase-1 [13, 21], which could be contributing to HMGB1 release in the current studies. HMGB1 can undergo unconventional secretion [6, 16], a process that is mediated by caspase-1 [21, 32]. HMGB1 can also be released during pyroptosis [26, 27], which is also caspase-1-dependent [4].
Similar to keratinocyte lysates, reduced HMGB1 levels were also detected by immunohistochemistry in keratinocytes after a single irradiation in murine skin. Reduced nuclear HMGB1 staining is commonly interpreted as a sign of HMGB1 release in vivo in other models of acute injury [2, 10, 25, 43, 51]; therefore, the decrease in staining is likely due to HMGB1 release. Together, the data from cultured cells and murine skin provide strong evidence that UV light stimulates the release of HMGB1 by keratinocytes. Like the results presented here with UV light, HMGB1 release by keratinocytes has been reported in other studies examining acute skin damage [2, 10, 25, 43, 51].
Many studies have shown that extracellular HMGB1 promotes inflammation [1, 10, 31, 39, 46]. A correlation between HMGB1 release and inflammation was also observed in the current studies. The timing and pattern of UV-induced HMGB1 release by keratinocytes mimicked the release of pro-inflammatory cytokines in vitro and inflammatory cell infiltration in vivo, suggesting that HMGB1 release contributes to the cutaneous inflammatory response following UV exposure. The idea that HMGB1 release could stimulate inflammation after acute skin injury is supported by other studies linking HMGB1 with cutaneous inflammation. For example, HMGB1 stimulates inflammatory cell recruitment in wounded skin [10], HMGB1 release correlates with inflammation and redness in cutaneous lupus erythematosus lesions induced by UV exposure [2], and neutralizing HMGB1 activity reduces pro-inflammatory cytokine production and inflammatory cell recruitment in the skin after topical treatment with chemical tumor promotors [31]. Interestingly, HMGB1 was not released immediately after UV exposure, suggesting that this protein may be more important for enhancing or maintaining inflammation, rather than acting as an initial stimulus.
Due to the importance of inflammation in the development, growth and progression of UV-induced skin tumors, HMGB1 expression was also examined in murine skin and tumors after long-term UV irradiation. In contrast to the loss of HMGB1 staining in keratinocytes observed after acute irradiation, epidermal HMGB1 expression and localization in chronically irradiated skin appeared to be similar to unirradiated skin, with positive nuclear staining observed in keratinocytes. It should be noted that for chronically irradiated mice, animals were exposed to UV three times per week for 15 weeks followed by 9 weeks with no UV exposure. The strong nuclear HMGB1 expression is likely explained by the fact that the samples were harvested after an extended recovery period and not immediately after UV exposure. As expected, the chronic irradiation protocol resulted in the formation of multiple skin tumors per mouse which varied in grade. HMGB1 expression was examined in UV-induced tumors ranging from grade 1 papillomas to MISCC. Expression in low grade papillomas was similar to adjacent, chronically irradiated skin, with positive cells localized to the tumor margin. A larger number of tumor cells stained positively for HMGB1 and positive cells were more widely distributed throughout the tumors in grade 3 papillomas and MISCCs. Compared to benign papillomas, more intense staining for HMGB1 was observed in MISCC, both in tumor cells and in cells within the surrounding stromal tissue. Unfortunately, it is difficult to determine whether HMGB1 is being released by the tumor cells based on immunohistochemistry. In acute injury models, reduced nuclear staining is often interpreted as release; however, it is possible that over-expression of HMGB1 in tumor cells is obscuring the detection of HMGB1 release, as the production of new HMGB1 protein could be continually replacing any secreted HMGB1. To our knowledge, this is the first study to examine HMGB1 expression in UV-induced skin tumors. However, increased levels of HMGB1 in skin tumors have been shown by immunohistochemistry, Western blot and RT-PCR in a chemical carcinogenesis model [41]. HMGB1 over-expression has also been described for other tumor types and studies have linked high HMGB1 levels with enhanced tumor growth and metastasis [8, 9, 20, 28, 47]. A functional role for HMGB1 in skin tumors has been suggested by studies in which mice lacking RAGE or TLR-4, two receptors for HMGB1, displayed reduced inflammation and tumor susceptibility in a chemical carcinogenesis model [17, 31].
Overall, the results described here indicate that UV light, the most important etiologic agent in the development of skin cancer, causes the release of HMGB1 by keratinocytes. Together, the correlation between HMGB1 release and inflammation combined with the over-expression of HMGB1 in skin tumors described in the current study suggest that HMGB1 could contribute to inflammation and tumor formation in response to UV exposure. More detailed studies will be needed to define the functional significance of HMGB1 over-expression in skin cancer and to determine whether HMGB1 could be targeted to minimize inflammation and prevent tumor formation.
References
Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ (2000) High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 192(4):565–570
Barkauskaite V, Ek M, Popovic K, Harris HE, Wahren-Herlenius M, Nyberg F (2007) Translocation of the novel cytokine HMGB1 to the cytoplasm and extracellular space coincides with the peak of clinical activity in experimentally UV-induced lesions of cutaneous lupus erythematosus. Lupus 16(10):794–802
Bell CW, Jiang W, Reich CF 3rd, Pisetsky DS (2006) The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol 291(6):C1318–C1325
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7(2):99–109
Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81(1):1–5
Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME (2003) Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 22(20):5551–5560
Caricchio R, McPhie L, Cohen PL (2003) Ultraviolet B radiation-induced cell death: critical role of ultraviolet dose in inflammation and lupus autoantigen redistribution. J Immunol 171(11):5778–5786
Chen J, Liu X, Zhang J, Zhao Y (2012) Targeting HMGB1 inhibits ovarian cancer growth and metastasis by lentivirus-mediated RNA interference. J Cell Physiol 227(11):3629–3638
Chuangui C, Peng T, Zhentao Y (2012) The expression of high mobility group box 1 is associated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. Pathol Oncol Res 18(4):1021–1027
Dardenne AD, Wulff BC, Wilgus TA (2013) The alarmin HMGB-1 influences healing outcomes in fetal skin wounds. Wound Repair Regen 21(2):282–291
Elmets CA, Viner JL, Pentland AP, Cantrell W, Lin HY, Bailey H, Kang S, Linden KG, Heffernan M, Duvic M, Richmond E, Elewski BE, Umar A, Bell W, Gordon GB (2010) Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst 102(24):1835–1844
Erlandsson Harris H, Andersson U (2004) Mini-review: the nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol 34(6):1503–1512
Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer HD (2007) The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol 17(13):1140–1145
Fischer SM, Lo HH, Gordon GB, Seibert K, Kelloff G, Lubet RA, Conti CJ (1999) Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinog 25(4):231–240
Fort JJ, Mitra AK (1994) Effects of epidermal/dermal separation methods and ester chain configuration on the bioconversion of a homologous series of methotrexate dialkyl esters in dermal and epidermal homogenates of hairless mouse skin. Int J Pharm 102(1–3):241–247
Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A (2002) The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 3(10):995–1001
Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K, Enk A, Arnold B, Bierhaus A, Nawroth PP, Hess J, Angel P (2008) RAGE signaling sustains inflammation and promotes tumor development. J Exp Med 205(2):275–285
Jiang W, Bell CW, Pisetsky DS (2007) The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid. J Immunol 178(10):6495–6503
Johannesdottir SA, Chang ET, Mehnert F, Schmidt M, Olesen AB, Sorensen HT (2012) Nonsteroidal anti-inflammatory drugs and the risk of skin cancer: a population-based case-control study. Cancer 118(19):4768–4776
Kang R, Tang D, Schapiro NE, Loux T, Livesey KM, Billiar TR, Wang H, Van Houten B, Lotze MT, Zeh HJ (2013) The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene
Keller M, Ruegg A, Werner S, Beer HD (2008) Active caspase-1 is a regulator of unconventional protein secretion. Cell 132(5):818–831
Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A (2008) HMGB1: endogenous danger signaling. Mol Med 14(7–8):476–484
Krysko DV, Vanden Berghe T, D’Herde K, Vandenabeele P (2008) Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods 44(3):205–221
Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA (2002) Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol 168(12):6436–6445
Lanier ST, McClain SA, Lin F, Singer AJ, Clark RA (2011) Spatiotemporal progression of cell death in the zone of ischemia surrounding burns. Wound Repair Regen 19(5):622–632
Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JP, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, Tracey KJ (2012) Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488(7413):670–674
Lu B, Wang H, Andersson U, Tracey KJ (2013) Regulation of HMGB1 release by inflammasomes. Protein Cell 4(3):163–167
Luo Y, Chihara Y, Fujimoto K, Sasahira T, Kuwada M, Fujiwara R, Fujii K, Ohmori H, Kuniyasu H (2013) High mobility group box 1 released from necrotic cells enhances regrowth and metastasis of cancer cells that have survived chemotherapy. Eur J Cancer 49(3):741–751
Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440(7081):228–232
Mazarati A, Maroso M, Iori V, Vezzani A, Carli M (2011) High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and receptor for advanced glycation end products. Exp Neurol 232(2):143–148
Mittal D, Saccheri F, Venereau E, Pusterla T, Bianchi ME, Rescigno M (2010) TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J 29(13):2242–2252
Nickel W, Rabouille C (2009) Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol 10(2):148–155
Nickoloff BJ, Qin JZ, Chaturvedi V, Bacon P, Panella J, Denning MF (2002) Life and death signaling pathways contributing to skin cancer. J Investig Dermatol Symp Proc 7(1):27–35
Nishigori C (2006) Cellular aspects of photocarcinogenesis. Photochem Photobiol Sci 5(2):208–214
Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R (1999) Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis 20(10):1939–1944
Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, Coldiron BM (2010) Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol 146(3):283–287
Rundhaug JE, Fischer SM (2008) Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem Photobiol 84(2):322–329
Said-Sadier N, Ojcius DM (2012) Alarmins, inflammasomes and immunity. Biomed J 35(6):437–449
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418(6894):191–195
Schafer M, Werner S (2008) Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol 9(8):628–638
Sharma A, Ray R, Rajeswari MR (2008) Overexpression of high mobility group (HMG) B1 and B2 proteins directly correlates with the progression of squamous cell carcinoma in skin. Cancer Invest 26(8):843–851
Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA (2001) Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem 276(1):125–132
Straino S, Di Carlo A, Mangoni A, De Mori R, Guerra L, Maurelli R, Panacchia L, Di Giacomo F, Palumbo R, Di Campli C, Uccioli L, Biglioli P, Bianchi ME, Capogrossi MC, Germani A (2008) High-mobility group box 1 protein in human and murine skin: involvement in wound healing. J Invest Dermatol 128(6):1545–1553
Thomas-Ahner JM, Wulff BC, Tober KL, Kusewitt DF, Riggenbach JA, Oberyszyn TM (2007) Gender differences in UVB-induced skin carcinogenesis, inflammation, and DNA damage. Cancer Res 67(7):3468–3474
Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, Egger M, Juni P (2011) Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 342:c7086
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285(5425):248–251
Wang W, Jiang H, Zhu H, Zhang H, Gong J, Zhang L, Ding Q (2013) Overexpression of high mobility group box 1 and 2 is associated with the progression and angiogenesis of human bladder carcinoma. Oncol Lett 5(3):884–888
Wilgus TA, Koki AT, Zweifel BS, Kusewitt DF, Rubal PA, Oberyszyn TM (2003) Inhibition of cutaneous ultraviolet light B-mediated inflammation and tumor formation with topical celecoxib treatment. Mol Carcinog 38(2):49–58
Wilgus TA, Ross MS, Parrett ML, Oberyszyn TM (2000) Topical application of a selective cyclooxygenase inhibitor suppresses UVB mediated cutaneous inflammation. Prostaglandins Other Lipid Mediat 62(4):367–384
Wulff BC, Kusewitt DF, VanBuskirk AM, Thomas-Ahner JM, Duncan FJ, Oberyszyn TM (2008) Sirolimus reduces the incidence and progression of UVB-induced skin cancer in SKH mice even with co-administration of cyclosporine A. J Invest Dermatol 128(10):2467–2473
Zampell JC, Yan A, Avraham T, Andrade V, Malliaris S, Aschen S, Rockson SG, Mehrara BJ (2011) Temporal and spatial patterns of endogenous danger signal expression after wound healing and in response to lymphedema. Am J Physiol Cell Physiol 300(5):C1107–C1121
Acknowledgments
The authors are supported in part by the following grants from the National Institutes of Health: R01CA109204 (TMO), R01CA127109 (TAW) and R21ES020462 (TAW).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnson, K.E., Wulff, B.C., Oberyszyn, T.M. et al. Ultraviolet light exposure stimulates HMGB1 release by keratinocytes. Arch Dermatol Res 305, 805–815 (2013). https://doi.org/10.1007/s00403-013-1401-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-013-1401-2