Abstract
Caspase-14 is a seemingly non-apoptotic caspase involved in keratinocyte differentiation and cornification of the skin. Keratin-19 is an epithelial marker and a potential marker of epidermal stem cells that is expressed during human fetal skin development. We examined the immunohistochemical expression of caspase-14 in relation to CK-19 in the human fetal skin during development and perinatally, to assess their role in human skin maturation. Skin samples were received at autopsy. In the fetal epidermis, caspase-14 was predominantly expressed in the more differentiated layers, gradually disappearing from the basal layer toward term. By contrast, keratin-19 expression gradually decreased with epidermal maturation through gestation (rho = −0.949; p = 0.0001) and was a marker of the germinative layers. Keratin-19 was preserved in scarce basal cell nests at term and postnatally. Caspase-14 and keratin-19 were inversely expressed in the differentiating epidermal layers through gestation (p < 0.0001). Concerning the appendages, in hair follicles and sebaceous glands, caspase-14 located preferentially in the more differentiated layers of the inner root sheath, whereas keratin-19 was expressed in the outer sheath. Eccrine sweat glands showed a variable pattern of caspase-14 and keratin-19 expression. In conclusion, caspase-14 emerged as a marker of human skin differentiation during development, while keratin-19 marked the germinative epithelial layers in the fetal epidermis and appendages and possibly the nests of epidermal stem cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caspase-14 (CASP-14), identified in 1998 [30], is a member of the unique family of cysteinyl aspartate-specific proteases, which are mainly involved in the process of inflammation and apoptosis [4, 18, 24]. However, it has been demonstrated on mice that CASP-14 has an active role in the maturation of the epidermis, the prevention of UVB photodamage and water loss, affecting skin osmolarity and moisture [7]. By contrast to the other members of the caspase family, CASP-14 does not participate in any apoptotic pathways [22].
The role of CASP-14 during terminal keratinocyte differentiation was described by Denecker et al. [7] on CASP-14-deficient mice. The lack of CASP-14 had great influence on water homeostasis of the mice as well as the protection against UVB photodamage. Furthermore, CASP-14 participates to the process of cornification, a particular type of programmed cell death, different from apoptosis [8, 21, 27]. In this process, various proteins of the intermediate filaments are known to be involved such as keratins [16].
Keratins belong to heterogeneous family of intermediate filaments mainly present in epithelial cells, comprising over 30 individual keratin polypeptides [12, 19]. Keratin-19 (CK-19) is the smallest known protein of the intermediate filaments found in human skin epithelia and is expressed during human fetal skin development from the embryonic to the late fetal period [6]. CK-19 is localized as a structural protein in the basal layer of human skin, in the Merkel cells, in a minor population (1 %) of mechanosensory cells and also in a distinct region of hair follicles known as the bulge [11, 25, 26].
The development of human skin is mainly classified in four stages [29]. The first is the embryonic period (until 9 weeks of development/11 weeks of gestation) during which the epidermis consists of a double cellular layer. The remaining three stages are within the fetal period: stratification (9–14 weeks of development/11–16 weeks of gestation, with increasing number of layers), follicular keratinization (15–24 weeks of development/17–26 weeks of gestation, when skin appendages begin to form), and interfollicular keratinization (26 weeks onwards, with formation of terminally differentiated cornified cells) [6].
Given the important role of CASP-14 in skin maturation and the involvement of CK-19 in fetal skin development in this study, we examined for the first time the expression of these two molecules in human fetal skin during gestation and postnatally, to assess their role and interrelation in the differentiation of human epidermis and appendages.
Materials and methods
Patients: material
This is a retrospective immunohistochemical study. Fetal skin was included in archival diagnostic material, obtained from 35 fetuses and 5 neonates sent for autopsy and histopathological examination at the first Department of Pathology of the University of Athens. All procedures to sample and retain fetal tissues were approved by the Ethics Committee of Athens University (10651/24-06-08) and informed written consent was always obtained from the parents. Archival samples were selected to represent various gestational ages (GA), ranging from 11 to 40 weeks of gestation (corresponding to 9–38 weeks of development). For first trimester abortuses, the estimated GA by crown-rump length was used for classification. For older fetuses, GA by dates was correlated to the estimated gestational age (EGA), based on somatometric measurements at autopsy (body weight, crown-heel and crown-rump length, right foot length). Fetuses that showed discordance between GA by dates and estimated GA by somatometry were excluded from the study. Fetuses with signs of maceration were also excluded from the study, thus eliminating the postmortem interval between death and delivery to less than 6 h [14]. For liveborn neonates, the corrected age was calculated as the sum of gestational plus postnatal age in weeks. Skin sections including epidermis, chorion, subcutaneous tissue and section of the quadriceps were sampled from the anterolateral surface of the thigh. Sections were fixed in 10 % formalin, embedded in paraffin and routinely stained for hematoxylin–eosin. Two sections of normal adult human skin (obtained from hospital resection specimens) and two sections from benign breast lesions were used as positive controls for CASP-14 and CK-19, respectively.
Immunohistochemistry
Sections were treated with 3 % H2O2 to block endogenous peroxidase and antigen retrieval followed the requirements of CASP-14 and CK-19 antigens: heating in microwave oven to 95 °C in citric acid buffer (pH 9.0) for 15 min and slowly cooling to room temperature. Immunohistochemical staining was performed by the use of a single step kit (EnVision™, DAKO, Glostrup, Denmark). The monoclonal antibodies used were mouse anti-human CASP-14 (D-10, sc48336, Santa Cruz Biotechnology, Santa Cruz CA, USA) and CK-19 (RCK108, DAKO, Glostrup, Denmark), at 1:50 dilution. None of the specific antibodies can cross-react with other antigens.
Evaluation of CASP-14 and CK-19 staining/image analysis
Images of immunohistochemically stained sections were captured with a Nikon DS-2Mv color CCD digital camera mounted on a Nikon Eclipse 80i microscope (Nikon Co., Tokyo, Japan) using a 20× objective and stored as tagged image file format (TIFF) files. Three to six images per section were captured under 200× and 400×. Images were then analyzed with Image-Pro Plus 5.1 software (Media Cybernetics, Silver Spring, MD, USA). In each image, the parameters measured by the image analysis program were (1) the percentage of tissue-stained area in the epidermis or appendages and (2) the staining intensity of CASP-14 or CK-19 in the epidermis or appendages. The staining intensity levels of CASP-14 were measured using arbitrary units (AU) on a linear scale ranging from 0 (non-detectable) to 255 (highest intensity) as described previously [32]. Averaging the quantitative computerized image analysis data from 3 to 6 images of each tissue section yielded an average staining intensity and an average percentage of stained area. Through the interactive message screen, cells that should not be included in the analysis, such as stromal or vascular endothelial cells, were eliminated, concentrating the analyses on skin cells. Skin appendages (hair follicles, sebaceous glands and eccrine glands) were collectively assessed by image analysis.
Statistical analysis
Numerical values in this study were evaluated as continuous parameters and included: weeks of gestational or corrected age, percentage of CASP-14 and CK-19 stained area in epidermis and appendages, arbitrary units (AU) of CASP-14 and CK-19 staining intensity.
CASP-14 and CK-19 stained area percentages were also examined separately in the epidermal layers (basal, intermediate and cornifying) according to graded positivity and used as categorical variables (positive (+): >50 %; positive/negative (±): 10–50 %; negative/positive (∓): <10 %; and negative (−): scarce or no expression).
The normality of distributions was tested with the Kolmogorov–Smirnov test. Spearman rank correlation coefficient (rho) was used to determine the strength of association between all continuous variables. Associations between categorical variables were determined either by the χ 2 test or Fisher’s exact test. Wilcoxon signed-rank test was used to examine any possible paired difference between CASP-14 and CK-19. Statistical calculations were performed using the SPSS for Windows software (SPSS, Chicago, IL, USA) on an IBM compatible PC. Statistical significance was attributed to p values lower than 0.05.
Results
Patients
We collected 40 skin samples: 5 from first trimester abortuses (11–13 weeks), 17 from second trimester fetuses (15–26 weeks), 13 from third trimester fetuses (27–38 weeks) and 5 from liveborn neonates (range of corrected age 34–52 weeks). Fetal skin samples were classified into three groups, according to the stage of skin differentiation (Table 1).
Immunohistology
CASP-14: epidermis
CASP-14 had a nuclear localization during the first trimester of gestation, while in the 2nd and 3rd trimester it was seen in the cytoplasm (Fig. 1a–c).
Immunostaining for CASP-14 (left) and CK-19 (right) during fetal skin development. a Nuclear immunoreactivity in all epidermal layers (GA 12 weeks), b CASP-14 expression is more prominent in the differentiating epidermal layers and in the inner sheath of hair follicles (GA 17 weeks). c The basal layer no more expresses CASP-14. Inset eccrine sweat glands negative for CASP-14 (GA 38 weeks). d Perimembranous localization of CK-19 in the periderm, basal and intermediate layer (GA 11 weeks). e CK-19 is positive only in the basal layer. Inset there is no obvious CK-19 expression in hair follicles and sebaceous glands (GA 17 weeks). f CK-19 positive basal cell nests (arrows). Inset eccrine sweat glands display inner luminal positivity (GA 37 weeks)
First trimester samples, belonging to stage I of fetal skin differentiation, showed immunoreactivity mainly in the nucleus of all three layers of the epidermis (basal, intermediate and periderm wherever present) (Fig. 1a). At the 2nd trimester (mostly differentiation stage II), immunoexpression was prominent in the intermediate layer, being less pronounced in the basal layer and the cornifying layer (Fig. 1b). At the 3rd trimester, represented by samples of differentiation stage III, CASP-14 expression was intense in the differentiated epidermis, including mostly the granular and cornified layers, having disappeared from the basal layer (Fig. 1c). This pattern remained unchanged postnatally (data not shown).
CASP-14: appendages
The skin appendages showed variability in their staining pattern. Hair follicles and sebaceous glands were seen positive to CASP-14 since their appearance by the beginning of differentiation stage II, and remained positive until the postnatal period (Table 2). CASP-14 expression started from the basal layer surrounding the outer root sheath of hair follicles but soon moved and remained in the inner sheath (Fig. 2a). Eccrine sweat glands were faintly immunoreactive (both layers of the bilayered epithelium) between the 23rd and 28th week (Fig. 2b); thereafter, CASP-14 expression was gradually eliminated, until it disappeared by the 35th week and remained absent until postnatal life (Table 2; Fig. 1c-inset). CASP-14 staining in appendages had initially a nuclear and later a cytoplasmic localization (Fig. 2).
CK-19: epidermis
CK-19 immunoreactivity had a perimembranous localization throughout the fetal and postnatal period (Fig. 1d–f). CK-19 in the first trimester (differentiation stage I) was detected in all epidermal layers (Fig. 1d). For the remaining of gestation, CK-19 remained positive only in the basal layer, diffusely during the 2nd trimester (differentiation stage II) and focally in cell nests during the 3rd trimester (differentiation stage III). CK-19 was constantly absent from the more mature epidermal layers throughout the 2nd and 3rd trimester (Fig. 1e, f) until the postnatal age (data not shown).
CK-19: appendages
CK-19 was positive in the epidermal ridges of appearing hair follicles (17–19 weeks); thereafter it was seen focally positive in cell groups of the outer sheath throughout the 2nd and 3rd trimesters (Fig. 3a), being significantly restricted toward term and postnatally (data not shown). CK-19 was constantly negative in well formed sebaceous glands (Fig. 1e-inset). By contrast, in eccrine sweat glands, CK-19 appeared gradually positive from the 22nd week on and peaked until week 31 (Fig. 3); by term, positivity was restricted in about half of the glands in the coil and remained so until the postnatal period (Table 3; Fig. 1f-inset). CK-19 was always expressed in the luminal layer of the bilayered epithelium (Figs. 1f-inset, 3b).
Statistical relations
CASP-14
Quantitative assessment of CASP-14 expression is shown in Table 1. CASP-14 expression in the epidermis tended to increase with gestational age, with a marginal statistical significance though (Spearman’s rho = 0.377; p = 0.057). In appendages (collectively evaluated), a positive linear correlation was found between CASP-14 staining intensity and gestational age (Spearman’s rho = 0.452; p = 0.023) (Table 1). No significant correlation was found between CASP-14 staining area and gestational age in appendages.
CK-19
In the epidermis, CK-19 staining area showed a negative linear correlation with gestational age (Spearman’s rho = −0.949; p = 0.0001) (Table 4; Fig. 4). There was no correlation between CK-19 staining intensity and gestational age.
In appendages, collectively assessed, CK-19 staining area presented a positive linear correlation with gestational age (Spearman’s rho = 0.727; p = 0.001) (Table 4). This correlation reflected the staining pattern of eccrine glands rather than the remaining appendages, as there was a statistically significant difference in CK-19 expression (when analyzed as a categorical variable) between subgroups of appendages: with increasing gestational age, eccrine glands were CK-19 positive as opposed to the subgroups of hair follicles and sebaceous glands (Fisher’s Exact Test, p = 0.01) (Table 3).
CASP-14/CK-19 interrelation
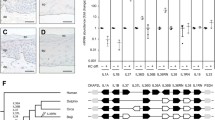
There was no significant correlation between CASP-14 and CK-19 collective quantitative expression in the epidermis. However, when the two proteins were compared separately in the basal layer and in the differentiating layers (intermediate + cornifying) and analyzed as categorical variables (positive versus negative graded expression), there was a significant difference between CASP-14 and CK-19 in the differentiating layers (Wilcoxon signed-rank test, p < 0.0001). In the whole sample, 92 % of cases were CASP14-positive/CK19-negative, with confidence limits (CL) 95 % (80–97 %). This difference applied for every differentiation stage, particularly for stages II and III, as follows: Stage I: 71, 95 % CL (35–91 %); Stage II: 93 %, 95 CL (68–98 %); Stage III 100 %. Figure 5 shows the distribution of our samples through gestation, according to their graded positivity to CASP-14 and CK-19 in the differentiated epidermal layers.
Comparison of CASP-14 and CK-19 expression in the differentiating epidermal layers (intermediate + corneum). The expression is evaluated as graded positivity throughout gestational and postnatal age (positivity scores 0, negative; 1, rare scattered positive cells; 2, intermittent positivity; 3, over 70 % positive cells). The distribution of the samples shows an inverse relation between the two proteins (Wilcoxon signed-rank test, p < 0.0001)
In appendages, in the subgroup of hair follicles and sebaceous glands, a significant difference was shown between CASP-14 and CK-19 expression when they were analyzed as categorical variables (positive versus negative expression) (Fisher’s Exact Test, p = 0.05).
Discussion
In contrast to the ubiquitously expressed other members of the caspase family, caspase-14 is expressed and activated mainly in the epidermis and is absent from most other adult tissues [17, 20, 23]. Our study investigated for the first time the expression of caspase-14 in the human skin during fetal development, in relation to keratin-19, a structural molecule known to be expressed in the human fetal skin [6].
Previous studies on keratinocyte cell cultures and on caspase-14 deficient mice have demonstrated that caspase-14 has a critical role in terminal keratinocyte differentiation of the adult skin, as well as in the development of fetal mouse epidermis [7, 9, 10, 22]. Despite the limitation that the monoclonal antibody used in our study does not detect the activated molecule, our results suggest that caspase-14 plays a role in the differentiation of the human fetal epidermis as well. Caspase-14 expression was expressed in the nucleus of immature keratinocytes of the first trimester of pregnancy and moved to the cytoplasm by the second trimester, while showing a gradual predilection for the more differentiated epidermal layers, including the cornified layer in the third trimester till postnatal life. The tendency of caspase-14 not only to increase with age the percentage of positive-staining epidermal cells but also to move from the basal toward the upper differentiating layers of the fetal epidermis appears to be an indication of epidermal maturation. Given the protective properties of this protein, by inducing cornification and preventing water loss [7], the evolution in the pattern of caspase-14 expression in the fetal epidermis suggests the possibility that it represents a preparation of the skin to its protective role.
The expression pattern of caspase-14 in human fetal skin bore similarities with that of the mouse fetal and adult epidermis in that it was predominantly located on the differentiating keratinocytes. The early human fetal epidermis in the youngest of our samples (aged 11.5 and 12 weeks of gestation) expressed caspase-14 in the nuclei of all layers, including the periderm and basal layer, while the mouse embryo of embryonic day E15.5 [22] and E16.5 [10] (equivalent to the 8th week of human development, i.e. the 10th week of human gestation) shows a suprabasal expression pattern that corresponds to that of the more matured second trimester human fetus. This discordance is a difference between the two species and may reflect the very advanced maturation rate of the murine skin as compared to the human.
Epithelial skin appendages of adult human skin also express caspase-14 [1]. In the fetus, hair follicles expressed caspase-14 since their appearance by the 17th week till the end of gestation and postnatally. The pattern of expression paralleled that of the fetal epidermis: the initial positivity of the basal layer of the outer sheath during the 2nd trimester gradually moved inwards to the more differentiated hair forming layers by the 3rd trimester. In studies made on skin samples of various mammals excluding humans, caspase-14 was diffusely present in cornifying cells of the outer root sheath of hair follicles, and most abundantly in the inner root sheath of all mammalian species studied [2].
In eccrine sweat glands, the period of caspase-14 positivity covers roughly the development of the first eccrine unit when secretory portions are formed. The loss of positivity in the more mature fetal eccrine glands indicates that caspase-14 is not involved in the differentiation of non-keratinizing epithelium into tall columnar secretory or to outer myoepithelial cells.
While caspase-14 can be considered as a marker of fetal epidermal differentiation, keratin-19, which is a cytoplasmatic intermediate filament protein of epithelial cells, was confirmed as a marker of the germinative epidermal layers, i.e. the periderm and the basal layer. The periderm is the proliferating layer of the first trimester fetus, while by the second trimester the mitotic activity of the basal layer predominates over that of the periderm and soon the basal becomes the germinative layer, from which rows of cells are added between the basal and the periderm. By the beginning of the third trimester, keratinization has taken place in the upper stratum and the cells of the periderm have already been shed [29]. According to this scheme, keratin-19 marked the germinative skin layers of the early fetus. These findings confirm the observations made in previous studies [30], which also described a positive epidermal expression of keratin-19, beginning in the post-implantation embryonic and early fetal life and getting gradually restricted to the basal layer, while sparing the more differentiated layers and stratum corneum of the more mature fetal epidermis. In our samples, only limited nests of basal cells remained positive for keratin-19 in the epidermis of the mature fetus and the neonate, as described in the adult human skin [26]. Similarly, keratin-19 was restricted to outer root sheath reservoirs of the hair follicles, as previously depicted in adult human skin [5, 15]. The localization of keratin-19 on the germinative epidermal layers of the early fetus and its persistence in few cell nests of the basal layer of the mature skin and the outer sheath of hair follicles is intriguing, as this molecule is presumed to be expressed by undifferentiated epidermal stem cells [3, 28]. We failed to detect any keratin-19 expression in the developing sebaceous glands, despite the fact that they are also thought to contain stem cells [13].
Evidence has been provided that epidermal stem cells are the source of sweat glands [31]. This could be compatible with the positive keratin-19 expression that we observed in the fetal sweat glands. However, the appearance and peaking of immunoexpression at the third trimester of gestation, its fading toward term and the restricted immunolocalization in the inner epithelial layer along with its gradual transportation toward an inner apical cytoplasmic localization could indicate the localization of keratin-19 intermediate filaments in the developing eccrine secretory epithelium.
In conclusion, analysis of caspase-14 and keratin-19 expression in the fetal skin demonstrates the former as a marker of fetal epidermal differentiation and the latter as a marker of the germinative epidermal layers and possibly of the stem cell nests that remain in the basal layer and the outer sheath of hair follicles.
References
Alibardi L, Dockal M, Reinisch C, Tschachler E, Eckhart L (2004) Ultrastructural localization of caspase-14 in human epidermis. J Histochem Cytochem 52:1561–1574
Alibardi L, Tschachler E, Eckhart L (2005) Distribution of caspase-14 in epidermis and hair follicles is evolutionarily conserved among mammals. Anat Rec A Discov Mol Cell Evol Biol 286:962–973
Barthel R, Aberdam D (2005) Epidermal stem cells. J Eur Acad Dermatol Venereol 19:405–413
Callus BA, Vaux DL (2007) Caspase inhibitors: viral, cellular and chemical. Cell Death Differ 14:73–78
Commo S, Gaillard O, Bernard BA (2000) The human hair follicle contains two distinct K19 positive compartments in the outer root sheath: a unifying hypothesis for stem cell reservoir? Differentiation 66(4–5):157–164
Dale BA, Holbrook KA, Kimball JR, Hoff M, Sun TT (1985) Expression of epidermal keratins and filaggrin during human fetal skin development. J Cell Biol 101:1257–1269
Denecker G, Hoste E, Gilbert B (2007) Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol 9:666–674
Denecker G, Ovaere P, Vandenabeele P, Declercq W (2008) Caspase-14 reveals its secrets. J Cell Biol 180:451–458
Eckhart L, Declercq W, Ban J, Rendl M et al (2000) Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J Invest Dermatol 115:1148–1151
Fischer H, Rossiter H, Ghannadan M, Jaeger K et al (2005) Caspase-14 but not caspase-3 is processed during the development of fetal mouse epidermis. Differentiation 73(8):406–413
Fradette J, Godbout MJ, Michel M, Germain L (1995) Localization of Merkel cells at hairless and hairy human skin sites using keratin 18. Biochem Cell Biol 73:635–639
Fuchs E (1990) Epidermal differentiation: the bare essentials. J Cell Biol 111(6 Pt 2):2807–2814
Gambardella L, Barrandon Y (2003) The multifaceted adult epidermal stem cell. Curr Opin Cell Biol 15:771–777
Genest DR, Singer DB (1992) Estimating the time of death in stillborn fetuses: III. external fetal examination; a study of 86 stillborns. Obstet Gynecol 80:593–600
Gho CG, Braun JE, Tilli CM, Neumann HA, Ramaekers FC (2004) Human follicular stem cells: their presence in plucked hair and follicular cell culture. Br J Dermatol 150(5):860–868
Heid HW, Moll I, Franke WW (1988) Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. II. Concomitant and mutually exclusive synthesis of trichocytic and epithelial cytokeratins in diverse human and bovine tissues (hair follicle, nail bed and matrix, lingual papilla, thymic reticulum). Differentiation 37:215–230
Kam DW, Charles AK, Dharmarajan AM (2005) Caspase-14 expression in the human placenta. Reprod Biomed Online 11:236–243
Kumar S (2007) Caspase function in programmed cell death. Cell Death Differ 14:32–43
Langbein L, Schweizer J (2005) Keratins of the human hair follicle. Int Rev Cytol 243:1–78
Li HH, Zhou G, Fu XB, Zhang L (2009) Antigen expression of human eccrine sweat glands. J Cutan Pathol 36:318–324
Lippens S, Denecker G, Ovaere P, Vandenabeele P, Declercq W (2005) Death penalty for keratinocytes: apoptosis versus cornification. Cell Death Differ 12(Suppl 2):1497–1508
Lippens S, Kockx M, Knaapen M (2000) Epidermal differentiation does not involve the pro-apoptotic executioner caspases, but is associated with caspase-14 induction and processing. Cell Death Differ 7:1218–1224
Lippens S, Vandenbroecke C, Van Damme E, Tschachler E, Vandenabeele P, Declercq W (2003) Caspase-14 is expressed in the epidermis, the choroid plexus, the retinal pigment epithelium and thymic Hassall’s bodies. Cell Death Differ 10:257–259
Martinon F, Tschopp J (2007) Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ 14:10–22
Michel M, Torok N, Godbout MJ, Lussier M, Gaudreau P, Royal A, Germain L (1996) Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci 109(Pt 5):1017–1028
Moll I (1994) Merkel cell distribution in human hair follicles of the fetal and adult scalp. Cell Tissue Res 277:131–138
Nicotera P, Melino G (2007) Caspase-14 and epidermis maturation. Nat Cell Biol 9:621–622
O’Shaughnessy RF, Christiano AM (2001) Stem cells in the epidermis. Skin Pharmacol Appl Skin Physiol 14:350–357
Urmacher CD (2006) Normal skin. In: Histology for pathologists, 3rd edn, Lippincott Williams & Wilkins, Philadelphia, USA, pp 25–40
Van De Craen M, Van Loo G, Pype S et al (1998) Identification of a new caspase homologue: caspase-14. Cell Death Differ 5:838–846
Xiaobing F, Jiannfu L, Xiaoqing S, Tongzhou S, Zhiyong S (2005) Epidermal stem cells are the source of sweat glands in human fetal skin: evidence of synergetic development of stem cells, sweat glands, growth factors, and matrix metalloproteinases. Wound Repair Regen 13:102–108
Zafirellis K, Agrogiannis G, Zachaki A, Gravani K, Karameris A, Kombouras C (2008) Prognostic significance of VEGF expression evaluated by quantitative immunohistochemical analysis in colorectal cancer. J Surg Res 147:99–107
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gkegkes, I.D., Aroni, K., Agrogiannis, G. et al. Expression of caspase-14 and keratin-19 in the human epidermis and appendages during fetal skin development. Arch Dermatol Res 305, 379–387 (2013). https://doi.org/10.1007/s00403-013-1319-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-013-1319-8