Abstract
Psoriasis has been related to metabolic syndrome (MS). Adipocytokines produced by white adipose tissue may be involved in the pathogenesis of psoriasis and its association with MS. Our objectives were to characterize the profile of a number of different inflammatory and atherogenic markers, vitamins, adipokines and cytokines and their potential involvement in MS in patients with moderate-to-severe psoriasis without joint involvement compared to anthropometrically matched controls, and to evaluate correlation with severity of the skin disease and changes after narrow-band UVB (NB-UVB) phototherapy. We designed a prospective cross-sectional study. Baseline waist circumference, body fat composition, lipid, carbohydrate and calcium metabolism profile, inflammation markers, homocysteine and vitamins D, B6, B12 and folic acid, leptin, resistin, omentin, lipocalin-2, adipocyte fatty acid-binding protein, retinol-binding protein-4 (RBP-4), interleukin-6, soluble tumour necrosis factor receptor 1 (sTNFR1) and interleukin-17 of 50 psoriasis patients and 50 gender, age and body mass index-matched controls were recorded, then evaluated after NB-UVB in the patients. The patients had higher baseline serum concentrations of leptin, RBP-4, lipocalin-2 and sTNFR1. Baseline psoriasis area and severity index correlated with serum concentrations of RBP-4 and lipocalin-2 only. Principal components analysis disclosed a component including vitamins B12, B6, folic acid, calcidiol and HDL-cholesterol that was only present in healthy controls and opposed to a cluster of variables which promote MS. This component was absent in the patients. Our results point to lipocalin-2 and RBP-4 as relevant mediators of the trend towards MS in psoriatic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriasis is an inflammatory skin disease that has been related to obesity and metabolic syndrome (MS) [2].White adipose tissue is a known source of adipokines and cytokines that can mediate the development of insulin resistance, endothelial dysfunction and atherosclerosis [5]. Adipocytokines may be involved in the pathogenesis of psoriasis and previously published studies have reported their overexpression in this skin disease [8, 19]. The interaction between adipocytokines and chronic skin and systemic inflammation in psoriasis can be bi-directional. Th1 and Th17 lymphocyte activation in psoriasis is a shared pathway with adipokine activation [19]. Psoriasis has been genetically linked to obesity [18] and may also act as an attracting element in terms of risk factors which eventually lead to the patients developing MS.
Adipokines such as leptin [6, 13, 19], adiponectin [33] and resistin [19] have been extensively studied in psoriatic patients and proposed as a link in the causal relationship between psoriasis and its comorbidities. Published data on lipocalins such as retinol-binding protein-4 (RBP-4) and lipocalin-2 are more sparse [13, 20]. RBP-4 is produced in the liver and the adipose tissue [13]. Lipocalin-2 has been recently identified as adipokine. It is expressed in liver, lung, kidney adipocytes, macrophages and epithelial cells [20]. It has also been known as neutrophil gelatinase-associated lipocalin (NGAL) and it is important in the innate immune response to bacterial infection. It has been reported to be increased in the epidermis of psoriatic lesions [22, 30]. Adipocyte fatty acid-binding protein (A-FABP) has been linked to regulation of insulin sensitivity, lipid metabolism, atherogenesis and inflammation [3], but it has never been specifically studied in psoriasis. Omentin is a newly discovered adipokine [29, 34] that has been studied in obesity [11] and inflammatory arthritis [4], but its role in inflammation and cardiovascular morbidity remains unclear. It has been found decreased in psoriatic patients when compared to healthy controls [17].
Some cytokines that are upregulated as a result of lymphocyte activation in psoriasis have also been described as mediators of atherosclerosis and cardiovascular morbidity [1, 27]. Adipose tissue can also produce some of these cytokines, such as interleukin-6 (IL-6) [24], tumour necrosis factor (TNF) [32] and interleukin-17 (IL-17) [25]. The involvement of Th1 and Th17 lymphocytes in psoriasis and atherosclerosis [1] provides a plausible explanation for their shared pathophysiology. Table 1 shows a summary of known functions and sources of these adipocytokines and their possible role in psoriasis.
Most studies aiming to clarify the role of systemic inflammation in psoriasis and its involvement in the evolution towards metabolic abnormalities have included a significant number of patients with psoriatic arthritis and obesity [13, 21]. Those patients have a higher systemic inflammatory burden than patients whose disease is limited to the skin. However, both skin and joint inflammation might contribute to the final outcome, so evaluation of patients exclusively with skin involvement would be very valuable.
Although statistical analysis has often pointed to psoriasis as an independent factor of MS, not all studies match their patients to a control group with comparable anthropometric and inflammatory profiles.
In a previous study [28], we identified a distinctive body fat composition and atherogenic pattern in moderate-to-severe psoriatic patients treated with narrow-band UVB (NB-UVB) phototherapy. Using the same cohort, our aim was to characterize the profile of a number of different adipokines and cytokines in patients and controls and to evaluate correlation with severity of the skin disease and the changes after NB-UVB.
Materials and methods
We studied the profile of a number of different adipokines and cytokines in patients with moderate-to-severe psoriasis compared to controls matched for gender, age and body mass index (BMI). Subjects with psoriatic arthritis, along with a list of other inflammatory diseases (inflammatory bowel disease, rheumatoid arthritis, active metastatic neoplasia or infection, granulomatous diseases, thyroid disease and advanced renal or liver failure), were excluded from the study.
We further studied the changes in this profile after NB-UVB and the relationship with different anthropometric and laboratory variables.
Inclusion and exclusion criteria, treatment, clinical evaluation and laboratory studies have been described previously [28]. In short, psoriatic patients with psoriasis area and severity index (PASI) ≥10 aged 18 years or over were recruited in the Phototherapy Unit of Hospital Parc Taulí from January 2010 to May 2011, not including the months of strongest sunlight (April–September) to avoid interference from UV sources other than phototherapy. The patients should have not received any systemic treatment at least 1 month prior to inclusion. During the same period, we recruited matched controls without psoriasis. We recruited patients and controls excluding a list of inflammatory and neoplastic diseases [28], including psoriatic arthritis, which might interfere with the evaluation of psoriasis as a single factor of chronic systemic inflammation. Anthropometric measurements (including BMI, waist circumference and body fat composition as determined by electrical bioimpedance) and fasting venous blood samples were taken for assessment and repeated on patients after completion of the phototherapy course. The standard serum determinations included a lipid, carbohydrate and calcium metabolism profile, inflammation markers (standard and high-sensitivity C-reactive protein and ferritin), homocysteine, uric acid, vitamins D, B6, B12 and folic acid. Samples were frozen and stored at −80 °C until the end of the study protocol, and then shipped to the laboratory for analyses for leptin, resistin, omentin, A-FABP, RBP-4, IL-6, soluble tumour necrosis factor-alpha receptor-1 (sTNFR1) and IL-17. The following commercially available ELISA kits were used: for omentin, IL-17, A-FABP, lipocalin-2 and resistin, Biovendor Laboratory Medicine, Inc., Palackeho, Czech Republic; for IL-6 and RBP-4, R&D systems, Minneapolis, MN, USA; for Leptin, Assaypro, St Charles, MO, USA; and for sTNFR1, Linco (Millipore), Billerica, MA, USA. The ELISA tests were performed in duplicate, following the manufacturer’s instructions, in the patients and controls at baseline and in the patients after completion of the phototherapy course.
Statistical analysis
Data sets were tested for normality with the appropriate tests, and distribution was normal. Differences were tested using Student’s paired t test for quantitative variables and the McNemar test for qualitative variables. For quantitative variables divided in categories, one-way ANOVA (analysis of variance) was applied. Linear regression analysis further determined multivariate relationships. For the variables that were statistically different in patients and controls, a multivariate logistic regression model was applied to detect a significant risk for certain values being predictive of having psoriasis.
Given the number of variables gathered in this study, we applied the principal components analysis (PCA) [26] to better ascertain multivariate relationships and with the aim of clustering groups of variables into components that explained a common effect. Principal components analysis can reduce the dimensionality of a data set, retaining the characteristics that contribute to its variance.
Statistical calculations were performed with SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). All p values were two-sided and a p value <0.05 was considered statistically significant. Results are presented as mean ± standard deviation.
Results
The study included 50 psoriatic patients (49 of them presenting chronic plaque psoriasis and only one patient with guttate psoriasis that subsequently evolved into plaque psoriasis) with a mean age of 46.38 ± 17.29 and 50 matched controls with a mean age of 46.06 ± 17.53. Mean duration of the disease was 18.09 ± 15.28 years (range 6 months–59 years). Mean basal PASI was 15.59 ± 5.42. Mean BMI was 27.5 kg/m2. The main parameters of the study with their values at baseline in patients and controls and after therapy in patients are presented in Table 2.
Adipocyte fatty acid-binding protein (A-FABP), omentin, resistin and IL-6 did not differ at baseline between patients and controls. A-FABP positively correlated with percentage body fat (p = 0.004, R 2 = 0.367).
Soluble TNFR1 was higher in patients than in controls (p = 0.007) and there was no change after treatment.
Leptin concentrations were higher in women than in men as it has previously been described. Leptin was higher in patients than in controls (p = 0.032) due to the marked differences in women, and patients that met IFD (International Diabetes Federation) criteria for MS had a higher, although not statistically significant, leptin concentration (one-way ANOVA, p = 0.051). There was no change after treatment although in the analysis of the patients divided into two groups according to the presence of MS there was a significant decrease in leptin after treatment in those patients with MS (p = 0.024). After therapy leptin values concerning patients did not differ from those of controls as it happened before therapy.
In multivariate analysis, leptin positively correlated with percentage body fat as calculated by electrical bioimpedance, LDL cholesterol, apo-B and A-FABP.
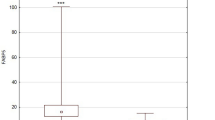
Lipocalin-2 was higher in patients than in controls (p = 0.001) and it did not change significantly after treatment. Multivariate linear regression detected a positive correlation between baseline lipocalin-2 and PASI (p = 0.023, R 2 = 0.078), RBP-4 (p = 0.038, R 2 = 0.069) and percentage body fat (p = 0.012, R 2 = 0.706). Multivariate logistic regression showed an odds ratio of 1.682 for psoriasis for every 10-unit change in lipocalin-2 (confidence interval 1.62–1.74).
RBP-4 concentration was higher in patients than in controls (p < 0.001), but it did not decrease after therapy. However, after treatment, the difference in its levels between controls and patients lost statistical significance. Multivariate analysis showed a correlation between RBP-4 and baseline PASI (p = 0.024, R 2 = 0.009), sTNFR1 (p = 0.031, R 2 = 0.028), IL-6 (p = 0.026, R 2 = 0.005) and resistin (p = 0.04, R 2 = 0.027). Multivariate logistic regression was applied, with the odds ratio for psoriasis found to be 1.03966 for RBP-4 concentration (confidence interval 1.03947–1.03985), for every 100-unit change in the variable.
Interleukin-6 (IL-6) decreased significantly after phototherapy (p = 0.027). In a multivariate regression model, the decrease in IL-6 was only significantly correlated with the decrease in ferritin (p = 0.005, R 2 = 0.157).
Interleukin-17 (IL-17) was undetectable in both patients and controls, with and without the 1:2 dilution recommended by the kit’s manufacturer, with all values below the last standard.
Principal components analysis (PCA)
Factor maps from the PCA including the most relevant study variables along with our previous results [24] are shown in Fig. 1. A clustering of variables can be seen (red arrows) that exert an atherogenic and inflammatory action commonly linked to the evolution towards MS. They include, among others, LDL cholesterol, body fat percentage, waist circumference, BMI, ferritin, resistin, A-FABP, leptin and sTNFR1. The component we have named as “promoter” of MS is present both in patients and controls. However, controls show a second clustering of variables (in green) opposed to the “promoter” component and comprising vitamin D (calcidiol), vitamins B6 and B12, folic acid and HDL-cholesterol, all of which could be associated with protective actions against MS. We named this component as “protector”. We see that it is expressed in the controls but not in the patients, even after improvement of psoriasis with treatment.
Variables factor map resulting from the principal components analysis (PCA) in controls (left), patients before treatment (centre) and patients after completion of the phototherapy course (right). Red arrows show metabolic syndrome (MS)-promoter variables. Green arrows show variables protective against MS. Lipocalin-2 is represented with blue arrows. Omentin is represented with black arrows. Retinol-binding protein-4 (RBP-4) is shown by orange arrows. IL-6 interleukin-6, hs-CRP high-sensitivity C-reactive protein, bmi body mass index, wc waist circumference, pbf percentage body fat (calculated by electrical bioimpedance), a-fabp adipocyte fatty acid-binding protein, sTNFR soluble tumour necrosis factor receptor
Some variables changed position in the factor map. Lipocalin-2 (blue arrow) is within the promoter component in controls, but opposed to it in the patients. Omentin (black arrow) was opposed to the promoter component in the controls, but within it in the patients. RBP-4 (orange arrow) was near the protector component in controls, but within the promoter component in patients.
Discussion
In our study, which included psoriatic patients without arthritis, we have shown differences in the adipokine profile between psoriatic patients and controls that seem to be independent of joint involvement. Lipocalin-2 and RBP-4 in particular appeared to be related to severity of the skin disease.
Our results provide new insight into the relationship between psoriasis and adipokines, particularly the lipocalins, which have been shown to be involved in the initial stages of insulin resistance development and the response to bacterial infection [10, 12, 16, 36, 37]. In order to describe the metabolic and inflammatory profiles in our patients, we studied a set of adipokines, some of them already explored in psoriasis. We searched for a possible correlation between these metabolic pathways and cytokines that are overexpressed by the different lymphocytic subpopulations involved in the pathogenesis of psoriasis (TNF, IL-6 and IL-17) and parameters of atherogenesis, insulin resistance and inflammation. These had been explored in our previous publication [28] where we found significant differences in body fat content as determined by electric bioimpedance, apo-B, LDL-cholesterol and uric acid, between patients and controls with a matched BMI.
In the present study, we found a positive correlation between leptin, lipocalin-2 and A-FABP, and body fat content. This result points to the close relationship between psoriasis pathogenesis and abdominal fat which is shared with MS [5].
Leptin, lipocalin-2 and RBP-4 were higher in patients than in controls, whereas the rest of the study variables were not different between the two groups. The increase in the production of lipocalins and leptin should be attributed to the differences in body fat content in psoriatic patients, although we cannot rule out that psoriatic disease acts as an attractant for unknown risk factors that would determine a distinctive fat distribution and adipokine secretion pattern.
Resistin, IL-6 and IL-17 have been linked to inflammatory activity and PASI in previous publications, but displaying heterogeneity in their results [8, 9, 19, 31]. IL-6 has been associated with inflammatory activity and cardiovascular risk [27], but our results were comparable in patients and controls. However, IL-6 decreased significantly after treatment, correlating with a decrease in ferritin. The design of the study, together with the weak correlation between the improvement in these parameters and PASI improvement, does not enable us to conclude whether the decrease in inflammation is the result of clinical improvement of psoriasis or an intrinsic effect of UVB phototherapy.
Soluble TNF receptor was higher in patients than in controls but did not decrease after treatment. These results are in contrast with those from Coimbra et al. [9], who found no differences in TNF levels between their patients and controls, but a significant decrease after phototherapy. The disparity among results published in the dermatology literature [8, 9, 13, 14, 21, 31] can be attributed to heterogeneity in the study designs, non-standardised laboratory techniques and the inclusion of psoriatic patients with variable severity and inflammatory profiles. Our results indicate that there is an inflammatory state in psoriatic patients without arthritis which is not completely corrected with clinical improvement of psoriasis after treatment. Lipocalin-2 and sTNFR levels persisted higher than those of controls after phototherapy. On the other hand, leptin and RBP-4, which had been significantly different between patients and controls prior to treatment, did not differ after phototherapy, meaning that the treatment had at least some effects on the metabolic and inflammatory balance.
Principal components analysis is useful for finding patterns in high-dimensional data [26]. In the whole data set from our study, which contained a large number of variables, we applied PCA to identify clusters of variables with a common effect and the way they differed between patients and controls and in patients before and after treatment.
Our results showed a protector component that seemed to be present in the control group but not in psoriatic patients. This component included a cluster of variables including HDL-cholesterol, calcidiol, folate and vitamin B12, which can counteract components of MS. Patients with psoriasis had a distinctive factor map, where those protective variables were intermingled with the cluster of MS-promoter variables rather than forming an opposed structured component as in the controls. These findings suggest a peculiar metabolic environment in psoriasis, probably associated with a pro-inflammatory state, which makes psoriatic patients more prone to MS than controls. Moreover, this state is not modified with clinical improvement after phototherapy.
Lipocalin-2 and RBP-4 correlated with baseline PASI, although they did not decrease after treatment. High values of these variables meant a risk of psoriasis in our study sample. Lipocalin-2 forms part of the promoter component in the controls, but is opposed to it in the patients, remaining unchanged by treatment. It also shows a good correlation with body fat content. We might speculate that the increment in lipocalin-2 in our psoriatic patients could be a protective mechanism against inflammation or fat content increase, as has been suggested in other diseases [10, 38]. Improvement of psoriasis with phototherapy did not change either its position in the factor map or its higher levels when compared with controls. This finding could signify the presence of a metabolic disturbance in psoriatic patients that is not corrected even after clinical control of the disease with phototherapy.
RBP-4 correlates with inflammatory markers such as IL-6, sTNFR, and also with basal disease activity (PASI), although it does not show correlation with body fat content. It does not decrease in parallel with PASI, as it could be expected. These results reflect the complexity of analysing the interaction of inflammatory and adiposity markers in psoriatic patients, given that some adipokines can exert diverse actions, being pro-inflammatory or anti-inflammatory depending on the patient’s anthropometric or inflammatory profile, or on lifestyle and genetic factors.
We found higher levels of lipocalin-2, RBP-4, leptin, sTNFR, LDL, uric acid and apo-B in our study patients compared to the controls. Some of these parameters are markers for atherogenesis and inflammation. The increase in lipocalins can be interpreted as a marker for insulin resistance and development of MS, but they can also increase in response to an initial metabolic disturbance that prompts protective, counteracting mechanisms. In fact, it has been speculated that lipocalins may be activated in initial stages of insulin resistance, in moderately obese subjects or in patients with a genetic predisposition to insulin resistance [12, 16]. The theory of psoriatic pathogenic cascade being triggered by an unknown antigen fits well with the activation of lipocalins, which have been shown to exert dual modulatory actions both in front of infections and lipid metabolism. Adipose tissue can be actually considered an immunoreactive organ [23], and some triggers including infection, stress or diet overload could stimulate the secretion of adipokines and cytokines contributing to an inflammatory state and a deposition of fat.
Phototherapy with NB-UVB achieved an improvement in PASI associated with a decrease in some indicators of systemic inflammation. However, PASI improvement did not correlate with variables that mark an evolution towards MS. Control of classical cardiovascular risk factors is thus essential in a population such as psoriasis patients, which is prone to developing MS.
References
Armstrong AW, Voyles SV, Armstrong EJ et al (2011) A tale of two plaques: convergent mechanisms of T-cell mediated inflammation in psoriasis and atherosclerosis. Exp Dermatol 20:544–549
Boehncke WH, Boehncke S, Tobin AM, Kirby B (2011) The “psoriatic march”: a concept of how severe psoriasis may drive cardiovascular morbidity. Exp Dermatol 20:303–307
Cabré A, Babio N, Lázaro I et al (2012) FABP4 predicts atherogenic dyslipidemia development. The PREDIMED study. Atherosclerosis 222:229–234
Cantarini L, Simonini G, Fioravanti A et al (2011) Circulating levels of the adipokines vaspin and omentin in patients with juvenile idiopathic arthritis, and relation to disease activity. Clin Exp Rheumatol 29:1044–1048
Carr DB, Utzschneider KM, Hull RL et al (2004) Intra-abdominal fat is a major determinant of the national cholesterol education program adult treatment panel III criteria for the metabolic syndrome. Diabetes 53:2087–2094
Chen YJ, Wu CY, Shen JL et al (2008) Psoriasis independently associated with hyperleptinemia contributing to metabolic syndrome. Arch Dermatol 144:1571–1575
Chodorowska G (1998) Plasma concentrations of IFN-gamma and TNF-alpha in psoriatic patients before and after local treatment with dithranol ointment. J Eur Acad Dermatol Venereol 10(2):147–151
Coimbra S, Oliveira H, Reis et al (2010) Circulating adipokine levels in Portuguese patients with psoriasis vulgaris according to body mass index, severity and therapy. J Eur Acad Dermatol Venereol 24:1386–1394
Coimbra S, Oliveira H, Reis F et al (2010) Interleukin (IL) 22, IL-17, IL-23, IL-8, vascular endothelial growth factor and tumor necrosis factor-alpha levels in patients with psoriasis before, during and after psoralen-ultraviolet A and narrowband ultraviolet B therapy. Br J Dermatol 163:1282–1290
Corripio R, Gónzalez-Clemente JM, Pérez-Sánchez J et al (2010) Weight loss in prepubertal obese children is associated with a decrease in adipocyte fatty-acid-binding protein without changes in lipocalin-2: a 2 year longitudinal study. Eur J Endocrinol 163:887–893
De Souza Batista CM, Yang RZ, Lee MJ et al (2007) Omentin plasma levels and gene expression are decreased in obesity. Diabetes 56:1655–1661
El-Mesallamy HO, Hamdy NM, Sallam AA (2012) Effect of obesity and glycemic control on serum lipocalins and insulin-like growth factor axis in type 2 diabetic patients. Acta Diabetol. doi: 10.1007/s00592-012-0373-6
Gerdes S, Osadtschy S, Rostami-Yazdi M et al (2012) Leptin, adiponectin, visfatin and retinol-binding protein-4—mediators of comorbidities in patients with psoriasis? Exp Dermatol 21:43–47
Gerdes S, Rostami-Yazdi M, Mrowietz U (2011) Adipokines and psoriasis. Exp Dermatol 20(2):81–87
Gisondi P, Lora V, Bonauguri C et al (2012) Serum chemerin is increased in patients with chronic plaque psoriasis and normalizes following treatment with infliximab. Br J Dermatol. doi: 10.1111/bjd.12118
Graham TE, Yang Q, Bluher M et al (2006) Retinol binding protein-4 and insulin resistance in lean, obese and diabetic subjects. New Eng J Med 354(24):2552–2563
Ismail SA, Mohamed SA (2012) Serum levels of visfatin and omentin-1 in patients with psoriasis and their relation to disease severity. Br J Dermatol 167:436–439
Jin Y, Zhang F, Yang S et al (2008) Combined effects of HLA-Cw6, body mass index and waist–hip ratio on psoriasis vulgaris in Chinese han population. J Dermatol Sci 52:123–129
Johnston A, Arnadottir S, Gudjonsson JE (2008) Obesity in psoriasis: leptin and resistin as mediators of cutaneous inflammation. Br J Dermatol 159:342–350
Kamata M, Tada Y, Tatsuta A et al (2012) Serum lipocalin-2 levels are increased in patients with psoriasis. Clin Exp Dermatol 37:296–299
Kaur S, Zilmer K, Leping V, Zilmer M (2011) The levels of adiponectin and leptin and their relation to other markers of cardiovascular risk in patients with psoriasis. J Eur Acad Dermatol Venereol 25:1328–1333
Mallbris L, O’Brien KP, Hulthén A et al (2002) Neutrophil gelatinase-associated lipocalin is a marker for dysregulated keratinocyte differentiation in human skin. Exp Dermatol 11:584–591
Meijer K, De Vries M, Al-Lahham S et al (2011) Human primary adipocytes exhibit immune cell function: adipocytes prime inflammation independent of macrophages. Plos One 6:1–13
Mohamed-Ali V, Goodrick S, Rawesh A et al (1997) Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 12:4196–4200
Nakajima H, Nakajima K, Tarutani M et al (2011) Kinetics of circulating Th17 cytokines and adipokines in psoriasis patients. Arch Dermatol Res 303:451–455
Pearson K (1902) “On lines and planes of closest fit to systems of points in space” (PDF). Philos Mag 2:559–572
Ridker PM, Rifai N, Stampfer MJ, Hennekens CH (2000) Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101:1767
Romaní J, Caixàs A, Carrascosa JM, Ribera M, Rigla M, Luelmo J (2012) Effect of narrow-band UVB therapy on inflammatory markers and body fat composition in moderate to severe psoriasis. Br J Dermatol 166:1237–1244
Schäffler A, Neumeier M, Herfarth H et al (2005) Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim Biophys Acta 1732(1–3):96–102
Seo SJ, Ahn JY, Hong CK et al (2006) Expression of neutrophil gelatinase-associated lipocalin in skin epidermis. J Invest Dermatol 126:510–512
Serwin AB, Sokolowska M, Chodynicka B (2005) Soluble tumor necrosis factor alpha receptor type 1 in psoriasis patients treated with narrowband ultraviolet B. Photodermatol Photoimmunol Photomed 21:210–211
Sethi JK, Hotamisligil GS (1999) The role of TNF alpha in adipocyte metabolism. Semin Cell Dev Biol 10:19–29
Shibata S, Saeki H, Tada Y (2009) Serum high molecular weight adiponectin levels are decreased in psoriasis patients. J Dermatol Sci 55:62–63
Tan BK, Adya R, Randeva HS (2010) Omentin: a novel link between inflammation, obesity, and cardiovascular disease. Trends Cardiovasc Med 20:143–148
Takahashi H, Tsuji H, Takahashi I et al (2008) Plasma adiponectin and leptin levels in Japanese patients with psoriasis. Br J Dermatol 159(5):1207–1208
Vaisbuch E, Mazaki-Tovi S, Kusanovic JP et al (2010) Retinol binding protein 4: an adipokine associated with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 23:111–119
Wang Y, Chen J, Zhao Y et al (2008) Psoriasis is associated with increased levels of serum leptin. Br J Dermatol 158:1134–1173
Zhang J, Wu Y, Zhang Y et al (2008) The role of lipocalin-2 in the regulation of inflammation in adipocytes and macrophages. Mol Endocrinol 22:1416–1426
Acknowledgements
This study was financially supported by a research grant from the Comite Institucional de Recerca of the Fundació Parc Taulí. The sponsors had no role in the design and conduct of the study; in the collection, analysis and interpretation of data; or in the preparation, review or approval of the manuscript.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romaní, J., Caixàs, A., Ceperuelo-Mallafré, V. et al. Circulating levels of lipocalin-2 and retinol-binding protein-4 are increased in psoriatic patients and correlated with baseline PASI. Arch Dermatol Res 305, 105–112 (2013). https://doi.org/10.1007/s00403-012-1306-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-012-1306-5