Abstract
The aim of this study was to screen the antioxidant, anti-hyaluronidase, and anti-elastase activity of the lyophilized juice of Cucumis sativus fruit (CSLJ). The CSLJ was subjected to DPPH and superoxide radical scavenging assay in reference to butylated hydroxytoluene. The hyaluronidase and elastase inhibitory assay was performed in reference to oleanolic acid. Furthermore, the activities have been rationalized with HPLC analysis of the CSLJ with standard reference compound of ascorbic acid. The CSLJ exhibited DPPH-free radical and superoxide radical scavenging activity, IC50 at a concentration of 14.73 ± 1.42 and 35.29 ± 1.30 μg/mL, respectively. The CSLJ also showed strong anti-hyaluronidase (c P < 0.001) and anti-elastase (c P < 0.001) activity, IC50 at a concentration of 20.98 ± 1.78 and 6.14 ± 1.74 μg/mL, respectively. The HPLC ‘chromatogram’ of standard and CSLJ showed specific peak at retention time 2.905 and 3.066 min, respectively. Content of ascorbic acid was calculated with respect to the standard compound and it was found to be 3.5 ± 0.23% w/w. CSLJ is the rich source of ascorbic acid and this study thereby rationalizes the use of C. sativus as potential anti-wrinkle agent in cosmetic products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Skin produces free radicals or reactive oxygen species due to repeated sun exposure, which leads to oxidative stresses and inflammatory responses in the dermal or epidermal layer of the connective tissues resulting aging and damage to cell membranes, lipids, proteins, and DNA [21]. Degradation of dermal or epidermal network is primarily due to the elevation of dermal enzymatic activities arising from infiltration of inflammatory neutrophils in response to acute UV irradiation to skin [8]. Hyaluronic acid and elastin are responsible for organization, structure, and elasticity of extracellular matrix of connective tissue. It decreases sharply during maturation as well as in premature aging [9, 14]. Polymerized connective tissue fibers lead to loss of skin elasticity and decrease the capacity of the skin to hold water [15]. Therefore, searching new skin-care cosmetic ingredients from natural resources becomes a great interest and could also be used as a cosmetic ingredient to relieve the skin aging [19].
Cucumis sativus L. (Cucurbitaceae) commonly called as cucumber is a seasonal vegetable in India, which has very good economic potential. In Indian traditional medicine it is known as Khira (Hindi), Trapusah (Sanskrit), Sasa (Bengali), and Vellarikkay (Tamil) and cultivated throughout India [12]. The traditional systems of medicine particularly in Ayurveda, the leaves, fruits, and seeds of C. sativus have long been used widely [1] for various skin problems, including swelling under the eyes, sunburn and are believed to promote cooling, healing, soothing, emollient, lenitive, anti itching effect to irritated skin, and extended cosmetic effects [11]. The fruits are sweet, refrigerant, hemostatic, diuretic, and tonic. It is useful in vitiated condition of pitta, hyperdipsia, burning sensation, thermoplegia, fever, insomnia, cephalalgia, bronchitis, jaundice, hemorrhages, strangury, and general debility [20]. A normal fruit contains moisture (96.4%), proteins (0.4%), fats (0.1%), carbohydrates (2.8%), mineral matter (0.3%), calcium (0.01%), phosphorus (0.03%), iron (1.5 mg/100 g), vitamin C (7 mg/100 g), and vitamin B (30 IU/100 g). Seeds contain proteins (42%) and fats (42.5%). Oil extracted from seeds is clear and light yellow with specific gravity of 0.9130; acid value 0.22; saponification value 1930; soluble fatty acid (as butyric acid) and unsaponification matter (0.91%). The fatty acid components are palmitic (0.63%), stearic (16.2%), linoleic (40.11), and oleic acid (38.70) [5]. As folk medicines and folk cosmetics, cucumber pulp and seeds are used in the treatment of skin problems like hyper-pigmentation and cleaning for centuries; and nowadays it is recommended worldwide to prepare cosmetic products [16]. Considering all these aspects the present study was undertaken to evaluate antioxidant, anti-hyaluronidase, and anti-elastase effect of lyophilized juice of C. sativus (CSLJ).
Materials and methods
Plant materials
The fresh fruits of C. sativus were purchased from local market of Jadavpur, Kolkata during the month of July–August 2010; subsequently collected specimen was authenticated and the voucher specimen was deposited as herbarium at School of Natural Product Studies, Jadavpur University, Kolkata, India for future reference (SNPS-JU/2010/1060).
Extraction
The fruits were washed with water, ground, and filtered through a nylon mesh to obtain crude juice (yield 80.56% v/w), which was centrifuged (Remi centrifuge 10,000 rpm, 5 min) and the supernatant clear solution was collected. This solution was concentrated by using rotary evaporator (EYELA, Tokyo, Japan) and lyophilized. The yield of CSLJ was found to be 36.85% w/v.
Sources of materials
1,1-Diphenyl 2-picrylhydrazyl, xanthine (X), xanthine oxidase (XO), nitroblue tetrazolium dye (NBT), hyaluronidase (from bovine testes), human leukocyte elastase (HLE), hyaluronic acid potassium salt (from human umbilical cord), N-Succinyl-(Ala) 3-p-nitroanilide, and standard ascorbic acid were purchased from Sigma-Aldrich, Inc (St. Louis, MO, USA). Solvents used for chromatography were methanol, water, and glacial acetic acid (all are HPLC grade) procured from Merck (Darmstadt, Germany). A 0.45 μm pore size membrane filter was used for mobile phase filtration and Whatman NYL 0.45 μm syringe filter was used for the filtration of samples. All other reagents and chemicals used in this study were of analytical grade.
HPLC analysis
Chromatographic analysis of CSLJ was performed on Waters liquid chromatographic system equipped with Waters (Milford, MA, USA) 600 quaternary solvent delivery system (pump), a Rheodyne-7725i injector (USA) with a 20 μl loop volume, waters 2489 UV–Vis dual wavelength detector, and Waters Spherisorb column (C18; 250 × 4.6 mm, 5 μ particle size) as stationary phase. Mobile phase consisting of a mixture of methanol:water (10:90) acidified with 1% acetic acid, pH (7.2), was delivered at a flow rate of 1 mL/min. Standard calibration curve was prepared using standard ascorbic acid in a concentration range of 50–1,000 μg/mL (injection volume 20 μl). CSLJ sample solution was prepared in the same way and in the same concentration range. Each sample was injected three times in three independent experiments and content of ascorbic acid was determined.
Experimental procedure
Preliminary phytochemical screening
The CSLJ was subjected to qualitative test [10] for the identification of various phyto constituents including carbohydrates, amino acids, vitamin-C, saponins, flavonoids, etc.
DPPH radical scavenging assay
The 1,1-diphenyl 2-picrylhydrazyl (DPPH) free-radical scavenging assay was performed as previously described [13]. Reaction mixture containing 75 μl DPPH solution (1.3 mg/mL), 100 μl of test solution, and 2,825 μl of methanol was used for the assay. Butylated hydroxytoluene as a standard [22] and different concentrations of test sample ranging from 1.56 to 50 μg/mL were used in the experiment. Reaction mixtures were incubated at 25°C for 15 min. After incubation the absorbance was determined at 517 nm by using UV spectrophotometer (Aquaris-CE 7200, CECIL) and the mean of six readings was obtained. Scavenging activity was determined by the following equation:
The IC50 value was obtained through extrapolation from linear regression analysis and denoted the concentration of sample required to scavenge 50% of DPPH radicals.
Superoxide radical-scavenging activity
A superoxide radical was generated by the xanthine oxidase (XO) system and was monitored with the product of nitroblue tetrazolium dye (NBT). The reaction mixtures contain different concentrations of test sample and standard ranging from 1.56 to 50 μg/mL, X, NBT, and phosphate buffer (pH 7.4). The reaction mixtures were incubated at ambient temperature for few minutes. Further reaction was initiated with the addition of XO. Butylated hydroxytoluene was used as a standard [22]. The absorbances of standard and test sample were obtained at 560 nm by using UV spectrophotometer (Aquaris-CE 7200, CECIL) [18]. The mean of six readings was considered and activity was determined by same equation as described earlier in DPPH radical scavenging assay.
In vitro hyaluronidase inhibition assay
Hyaluronidase inhibitory assay was performed by the method described previously [6]. A test sample of 5 μl (concentration range 1.56–50 μg/mL) of CSLJ in DMSO (dimethyl sulphoxide) was pre-incubated with hyaluronidase (1.50 U in 100 μl), sodium phosphate buffer 20 mM (pH 7.0) with sodium chloride 77 mM, and bovine serum albumin (BSA) 0.01% for 10 min at 37°C. Subsequently the assay was initiated by adding hyaluronic acid 100 μl (0.03% in 300 mM sodium phosphate, pH 5.35) to the incubation mixture and incubated further for 45 min at 37°C. Hyaluronic acid (undigested) was precipitated with acid albumin solution 1 mL, made up of bovine serum albumin 0.1% in sodium acetate 24 mM and acetic acid 79 mM, pH 3.75. It was allowed to stand at room temperature for 10 min and then absorbance was measured at 600 nm. The assay was performed with oleanolic acid as a positive control under exactly the same experimental condition. An absorbance value of intact undigested hyaluronic acid at 600 nm was considered as 100%. For calculation of percentage of enzyme activity, the following formula was used:
Elastase inhibitory assay
The elastase inhibition assay was performed spectrophotometrically according to the method of Kraunsoe et al. [7] using N-Succ-(Ala) 3-p-nitroanilid (SANA) as substrate, and monitoring the release of p-nitroaniline by measuring the absorbance at 410 nm. For experiment, 1.015 mM Succinyl-(Ala) 3-p-nitroanilide was prepared in a 0.1232 M Tris–HCl buffer (pH 8.0) and this solution (1,300 mL) was added to the stock sample solution (100 mL). The sample solution was diluted to final concentrations of 1.56–50 μg/mL. The solutions were vortexed and preincubated for 10 min at 25°C and than an elastase (0.0375 unit/mL) solution (100 mL) was added. After vortexing, each solution was placed in a water bath for 10 min at 25°C and the absorbance was measured at 410 nm. The performance of the assay was verified using oleanolic acid as a positive control under exactly the same experimental conditions.
The percentage of inhibition was calculated as: Inhibition (%) = (1 − B/A) × 100, where A is the enzyme activity without sample and B is the activity in presence of the sample.
Statistical analysis
The IC50 values were expressed as mean ± standard error mean by plotting the curve with percentage of inhibition versus concentrations of the individual experiments measured (n = 6). Statistical analysis was performed by two way analysis of variance (Two way ANOVA) followed by Bonferroni post test using Graph Pad prism version 4.01. P < 0.05 was considered as significance difference as compared to reference standard.
Results and discussion
Apart from genetic factors, the human skin is being directly affected with external factors such as nutrition, smoking, alcohol, contaminated environment like air, solar radiations, etc. and thereby severs damage to the skin resulting in enhancement of the aging process [3, 4]. The flesh of C. sativus is primarily composed of water, ascorbic acid (vitamin C), and caffeic acid; and is being used extensively in various skin-care products for the improvement of soothe skin irritations and reduce swelling with cooling enhancement [17]. Cho et al. [2] have also been reported the anti-wrinkling effects of the antioxidant mixture of vitamin C (ascorbic acid), vitamin E, pycnogenol and evening primrose oil along with its possible molecular mechanisms on hairless mouse skin caused by chronic ultraviolet B irradiation.
In the present study, preliminary phytochemical screening of the CSLJ showed positive results, for the presence of carbohydrates, amino acids, vitamin-C, saponins, flavonoids, sterols, and triterpenes. Further the CSLJ was screened by in vitro antioxidant, anti-hyaluronidase, and anti-elastase enzyme assay which have been correlated with HPLC analysis in reference to ascorbic acid. Absorption values were taken six times for each concentration (n = 6). Inhibition curves were obtained by plotting the percent inhibition versus the inhibitory concentration in the assay solutions. The linear regression parameters were determined for each curve and the IC50 values were extrapolated.
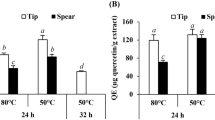
The CSLJ dose-dependently exhibited significant (c P < 0.001) DPPH and superoxide radical scavenging activity (Figs. 1, 2) with 50% inhibition (IC50), at a concentration of 14.73 ± 1.42 and 35.29 ± 1.30 μg/mL compared to butylated hydroxytoluene IC50 at 31.38 ± 1.43 and 51.79 ± 1.05 μg/mL, respectively. The CSLJ (Figs. 3, 4) dose-dependently exhibited significant (c P < 0.001, b P < 0.01) anti-hyaluronidase and anti-elastase activity with 50% inhibition (IC50), at a concentration of 20.98 ± 1.78 and 6.14 ± 1.74 μg/mL compared to oleanolic acid IC50 at 41.54 ± 1.57 and 18.47 ± 1.4 μg/mL, respectively. These activities have been supported by HPLC analysis with reference to ascorbic acid as a standard phyto-marker. The calibration curve for ascorbic acid was constructed by plotting mean peak area against concentrations. Linearity of the calibration curve was tested by linear regression analysis and found to be linear in the concentration range 50–1,000 μg/mL with correlation coefficient (R 2) of 0.995. The HPLC ‘chromatograms’ of the standard ascorbic acid (Fig. 5) and CSLJ (Fig. 6) showed specific peak at retention time 2.905 and 3.066 min, respectively on scanned wavelength 254 nm. The content of ascorbic acid was found to be 3.5 ± 0.23% w/w in CSLJ.
Conclusion
From the present study, it was found that the lyophilized juice of C. sativus potentially inhibited the hyaluronidase and elastase enzyme activity. It also showed potent-free radical scavenging activity. Thus, it can be concluded that C. sativus may have potential role on skin care and can be explored further for its use in cosmetics for its enzyme inhibition activity.
References
Anonymous (2001) Ayurvedic pharmacopoeia of India. The Controller of Publication (NISCOM), New Delhi
Cho HS, Lee MH, Lee JW, No KO, Park SK, Lee HS, Kang S, Wan-Goo Cho, Park HJ, Oh KW, Hong JT (2007) Anti-wrinkling effects of the mixture of vitamin C, vitamin E, pycnogenol and evening primrose oil, and molecular mechanisms on hairless mouse skin caused by chronic ultraviolet B irradiation. Photodermatol Photoimmunol Photomed 23:155–162
Chung JH (2003) Photoaging in Asians. Photodermatol Photoimmunol Photomed 19:109–121
Getoff N (2007) Anti-aging and aging factors in life. The role of free radicals. Radiat Phys chem 76:1577–1586
Kapoor LD (1990) CRC handbook of Ayurvedic medicinal plants. CRC Press, Florida
Kim JH, Byun JC, Bandi AKR, Hyun CG, Lee NH (2009) Compounds with elastase inhibition and free radical scavenging activities from Callistemon lanceolatus. J Med Plant Res 3:914–920
Kraunsoe JA, Claridge TDW, Lowe G (1996) Inhibition of human leukocyte and porcine pancreatic elastase by homologues of bovine pancreatic trypsin inhibitor. Biochemistry 35:9090–9096
Labat-Robert J, Fourtanier A, Boyer-Lafargue B, Robert L (2000) Age dependent increase of elastase type protease activity in mouse skin: effect of UV-irradiation. J Photochem Photobiol B: Biol 57:113–118
Manuskiatti W, Maibach H (1996) Hyaluronic acid and skin wound healing and aging. Int J Dermatol 35:539–544
Mukherjee PK (2002) Quality control of herbal drugs. Business Horizons, New Delhi
Patri G, Silano V, Anton R (2002) Plants in cosmetics. Council of Europe Publishing, Strasbourg
Peter KV, Abraham Z (2007) Biodiversity in horticultural crops. Daya Publishing House, New Delhi
Raja S, Nazeer Ahamed KFH, Mukherjee K, Bandyopadhyay A, Mukherjee PK (2005) Antioxidant potential of Aerial part of Asclepias curassavica Linn (Family—Asclepiadaceae). Orient Pharm Exp Med 5:92–99
Reed RK, Saito M, Qiu G (1988) Hyaluronan in the rat with special reference to the skin. Acta Physiol Scand 134:405–411
Ryu A, Naru E, Arakane K (1997) Cross-linking of collagen by singlet oxygen generated with UV-A. Chem Pharm Bull 45:1243–1247
Sotiroudis G, Melliou E, Sotiroudis TG, Chinou I (2010) Chemical analysis, antioxidant and antimicrobial activity of three Greek cucumber (Cucumis sativus) cultivars. J Food Biochem 34:61–78
Sumathi T, Ponnuswami V, Senthamizh BS (2008) Anatomical changes of cucumber (Cucumis sativus L.) leaves and roots as influenced by shade and fertigation. Res J Agric Biol Sci 4:630–638
Valentao P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, Bastos ML (2001) Studies on the antioxidant activity of Lippia citriodora infusion: scavenging effect on superoxide radical, hydroxyl radical and hypochlorous acid. J Agric Food Chem 49:3476–3479
Wang KH, Lin RD, Hsu FL, Huang YH, Chang HC, Huang CY, Lee MH (2006) Cosmetic applications of selected traditional Chinese herbal medicines. J Ethnopharmacol 106:353–359
Warrier PK (1994) Indian medicinal plants: a compendium of 500 species. Orient Longman, Chennai
Yamamoto YJ (2001) Role of active oxygen species and antioxidants in photoaging. J Dermatol Sci 27:S1–S4
Yang J, Guo J, Yuan J (2008) In vitro antioxidant properties of rutin. LWT Food Sci Technol 41:1060–1066
Acknowledgments
The study was supported by Department of Science and Technology, through Drug and Pharmaceutical Research Programme [DST-DPRP, File No. VI-D&P/287/08-09/TDT], Government of India, New Delhi and Parker Robinson Pvt. Ltd., Kolkata, India.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nema, N.K., Maity, N., Sarkar, B. et al. Cucumis sativus fruit-potential antioxidant, anti-hyaluronidase, and anti-elastase agent. Arch Dermatol Res 303, 247–252 (2011). https://doi.org/10.1007/s00403-010-1103-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-010-1103-y