Abstract
The free and protein-bound ceramides of dog stratum corneum (SC) were analyzed by thin-layer chromatography after tape stripping of the abdomen of five dogs. The sphingoid bases were identified by gas–liquid chromatography as sphingosine, phytosphingosine, and 6-hydroxysphingosine. Electrospray ionization-ion trap mass spectrometry was used to characterize the protein-bound ceramides containing sphingosine and omega-hydroxy long-chain fatty acids. Although the molecular species were the same ones in all dogs, wide quantitative variations in the patterns of SC ceramides were observed in different breeds of dogs. The free ceramide concentration changed with the depth of SC, with a higher concentration in the deep layers, whereas the concentration of protein-bound ceramides remained constant. These results show that canine SC is close to that of humans with respect to ceramides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lipids of human skin have been extensively studied and analyzed [13]. Among these lipids, ceramides draw considerable interest after some alterations in these sphingolipids were reported in many skin disorders such as psoriasis [10] and atopic dermatitis [2, 6–8]. Ceramides [13] are the most important class of lipids of the stratum corneum (SC) that contain numerous molecular species of ceramides differing in both long-chain bases and fatty acids. In order to study skin disorders, animal models have long been sought as an alternative to carry out investigations that could bring insight into similar pathologies of the human skin. Among those models, dog skin has received little attention, and the biochemical analyses regarding the patterns of ceramides in dog skin are clearly lacking, although a very recent paper [12] brought some interesting data on the free ceramide composition of canine SC. Atopic dermatitis has been shown to present very similar features in humans and dogs [4]. Prior to a more detailed investigation of SC ceramides of atopic dogs, this study was undertaken to analyze the molecular species of ceramides in dog SC and to determine to which extent the ceramide pattern of dog SC is close to human SC in terms of both free and protein-bound ceramides.
Materials and methods
Dog population
Healthy dogs with no diagnosed skin disorders were recruited from patients at the Dermatology Clinic of the National Veterinary School of Lyon. Two Beagles, one Labrador retriever and two Golden Retrievers were selected for the study. The dogs were not washed with shampoo or treated with cream for 2 weeks before tape stripping.
Materials
All solvents were of analytical grade and were purchased from Carlo-Erba (Peypin, France). Solid phase extraction aminopropyl-bonded silica gel columns (Supelclean LC-NH2 columns containing 500 mg bed weight in 3 ml volume) were from Supelco (L’Isle d’Abeau, France). Aluminum-backed HPTLC plates were from Merck (Darmstadt, Germany). Standard ceramides NS and AS were from Matreya (Pleasant Gap, PA, USA).
Tape stripping
Since the Leukoflex tape (Beiersdorf, Hamburg, Germany) which was reported to be suitable in previous studies [15] is no longer available, several brands of adhesive tape were tried in preliminary experiments, and the most satisfactory results were obtained with Magic Tape 3M (Dominique Dutscher, Strasbourg, France). Tape stripping was carried out on the abdomen of five dogs after cutting out the hair with scissors. Precut strips (5 cm × 1.8 cm) were applied on the skin with pressure and removed with tweezers. After 12 consecutive strips were taken, the stripped areas became shiny. In order to have authentic standards for analysis by thin-layer chromatography, SC was also obtained by tape stripping on the forearm of healthy human subjects. The same strips as above were used and 15–18 strips taken on the same area were necessary for the skin to become shiny. The tapes were extracted as pools.
Lipid extraction
Each tape was placed in a glass tube containing 10 ml of n-hexane–isopropanol 4:1 (v/v) and the tubes were sonicated on ice for 5 min in a Bransonic 220 bath sonicator (Branson, Paris, France). In preliminary experiments, this procedure was sufficient to release in the solvent all the cells stuck to the tape, with a minimal contamination by tape-associated compounds and with a negligible solubilization of ceramides. After sonication, the tapes were removed and the tubes were centrifuged 10 mn at 1,500×g to recover the corneocytes. Following centrifugation, the supernatants were discarded and the corneocytes were extracted at room temperature for 1 h in 6 ml of chloroform–methanol 2:1 (v/v) with shaking. After centrifugation 10 mn at 1,500×g, the supernatants were transferred to 25 ml flasks and the pellets were extracted a second time with chloroform–methanol 2:1 (v/v), then a third time with methanol only. The extracts were pooled and evaporated under reduced pressure at 50°C with a rotary evaporator Rotavapor R111 (Büchi, Paris, France). The lipid residues were taken up with 0.5 ml of diethylether.
The protein-bound lipids were extracted from the cell pellet residues left in the glass tubes by mild saponification with 3 ml of KOH 0.1 N in methanol–water 10:1 (v/v) for 2 h at 50°C [14]. After neutralization with HCl 1 N, the protein residue was removed by centrifugation 10 mn at 3,000 rpm and assayed by the Coomassie blue method [16]. The supernatants were partitioned to remove the salts after addition of 2 ml of distilled water, then 3 ml of chloroform to the neutralized solution. After thorough shaking, the tubes were centrifuged 5 mn at 3,000 rpm. The upper (aqueous) phases containing the salts were discarded and the lower (organic) phases containing the lipids recovered by saponification were dried off under nitrogen. The lipid residues were taken up in 0.5 ml of diethylether.
Lipid analysis
The lipids were applied in diethylether onto a LC-NH2 silica gel column and the different classes were eluted as previously described [11]. The neutral lipids were eluted with 3 ml of diethylether. Then, the ceramides were eluted in the second fraction with 3 ml of chloroform–methanol 23:1 (v/v) and evaporated to dryness under nitrogen. The free and protein-bound ceramides were analyzed by thin-layer chromatography using a Linomat IV (Camag, Muttenz, Switzerland) to apply the lipids on aluminum-backed HPTLC silica gel plates under a flow of nitrogen.
The total ceramide fraction purified from two consecutive strips was applied. The plates were developed with chloroform–methanol 50:3 (v/v) along with appropriate standards; then the lipids were visualized with a spray reagent made of cupric acetate 3% in 8% phosphoric acid by charring 5 mn at 150°C. Ceramides were tentatively identified by co-migration with authentic standards.
The distribution of components was determined by scanning densitometry at 450 nm with a CS-930 Chromatoscan (Shimadzu, Kyoto, Japan). For quantitative densitometry, calibration curves were established with standard ceramide NS. Increasing amounts (1–10 μg) of ceramide were applied to HPTLC silica gel plates that were run and chemically visualized simultaneously with the plates containing, along with the samples to be analyzed, a known amount of ceramides NS and/or AS. For each plate, scanning densitometry was performed three times and the variations were always below 5%.
The nomenclature system of Motta et al. (1993) was used to identify the ceramide species. The long-chain base composition was established by gas–liquid chromatography (GLC) of the bases released by alkaline methanolysis (KOH 4 M in 95% methanol at 80°C for 6 h). The bases were extracted with diisopropylether and analyzed as TMS derivatives on a gas chromatograph HP 9680 (Hewlett-Packard, Paris, France) as described [1]. The whole ceramide fraction was purified from human forearm SC after tape stripping to be used as a standard. Each strip was sonicated in n-hexane–isopropanol 4:1 (v/v) and after centrifugation and the cell pellets were extracted as described above. The extracts from all strips were pooled and processed together. The ceramide fraction was isolated by chromatography on LC-NH2 columns.
Mass spectrometry of CerOS
The ceramide fraction eluted from LC-NH2 columns, after applying the covalently bound lipids released by mild saponification, was developed with chloroform–methanol 50:3 (v/v) along with known standards on a HPTLC plate prewashed by three consecutive migrations with chloroform–methanol 1:1 (v/v). The ceramides were visualized with iodine vapors and the spot migrating like CerOS was scrapped and eluted with chloroform–methanol 2:1 (v/v).
The eluted product was analyzed using an ESI ion-trap instrument Esquire 3000 (Bruker, Bremen, Germany) in negative mode with a scan rate of 13,000 Th/s using a m/z range of 3,000 Th (ion ejection to βz 2:3). Sequential mass spectrometry experiments were performed under resonant excitation conditions. The automated ion charge control was set at 10,000 to avoid a space charge effect. The low mass cut-off used during the collision-induced dissociation experiments (related to the excitation at particular βz values) was set automatically by the instrument software.
Results
The experimental procedure detailed in “Materials and methods” allowed an excellent recovery of the corneocytes taken from the skin by tape stripping, with a minimal contamination by solvent-soluble compounds from the tapes that are a major problem in this kind of lipid analysis. In order to carry out the extraction of covalently bound lipids, the SC material collected by tape stripping must be released from the tapes because the saponification procedure cannot be carried out on material stuck to the adhesive tapes. Otherwise, this would result in a heavy contamination from the glue precluding all possibilities of analysis. These problems led us to develop the method used to release the SC from the adhesive tapes before lipid extraction.
The fractionation of the total lipids by chromatography on aminopropyl silica gel extracted from corneocytes into several lipid classes gives a fraction of ceramides readily purified from other lipid contaminants. Thin-layer chromatography analysis of the ceramides isolated from pools of 12 strips taken from two Beagle dogs is shown in Fig. 1. At least eight of the ceramides reported in humans [15] were also found in the SC of these dogs. The analysis of long-chain bases by GLC (not shown) demonstrated the presence of sphingosine, phytosphingosine, and 6-hydroxysphingosine as in humans.
As for the ceramides bound to proteins of the SC, Fig. 2 shows the profile of the ceramides released by mild saponification of the delipidated protein residue. The three ceramides that can be clearly seen migrate like the molecular species reported in the human SC which contain omega hydroxylated long-chain fatty acids coupled, respectively, to sphingosine (CerOS), phytosphingosine (CerOP), and 6-hydroxysphingosine (CerOH) [5, 14]. The major compound migrating like CerOS was purified by preparative thin-layer chromatography, and its structure was analyzed using electron spray ionization–mass spectrometry (ESI–MS).
TLC of protein-bound ceramides of SC taken from two dogs. Solvent system and visualization as in Fig. 1
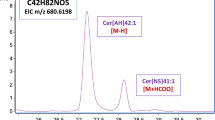
Figure 3 shows the mass spectrometry profile in negative mode of CerOS purified from canine SC. Since sphingosine was found by GLC to be mostly as d18:1, the molecular ions displayed were likely to correspond to the molecular species varying in the fatty acid chain length. The fragmentation pattern by ESI–MS/MS of the major parent ion at m/z 748.9 is given in Fig. 4. As already reported for human CerOS [3], the ion at 263.2 is known to reflect the presence of d18:1 sphingosine, and the product ion at m/z 492.6 is a fragment related to a C30:0 omega-hydroxy fatty acid (ω-OH C30:0). The ion at m/z 467.6 could also be a breakdown product due to the loss of a water molecule from the ion of ω-OH C30:0 after fragmentation of the parent ion m/z 748.9. The possible presence of sphingosine with different chain length is not supported by any of the fragments, and it can be assumed that the ions seen in Fig. 3 are indeed corresponding to d18:1 sphingosine with fatty acids of different chain length. In this respect, the major omega-hydroxy fatty acid species of CerOS would be in decreasing order ω-OH C30:0 (m/z 748.9), ω-OH C29:0 (m/z 734.9), ω-OH C28:0 (m/z 720.9), and ω-OH C32:0 (m/z 776.9). The two ions at m/z 666.8 and 638.8 could be, respectively, produced by dihydrosphingosine d18:0 coupled to C24:0 and C22:0. The presence of dihydrosphingosine in the ceramides of canine SC, which has been already documented in human SC [9], would be possible in this fraction since these species cannot be resolved from CerOS by the solvent system used for purification by thin-layer chromatography. However, MS–MS fragmentation of these minor ions did not allow a clear identification of the long-chain base.
The SC of three other dogs gave different patterns of ceramides. Figures 5 and 6 show, respectively, the free and protein-bound ceramides of the Labrador. In this dog, ceramides containing 6-hydroxysphingosine were almost absent. In the other dogs, sphingosine was the major long-chain base of ceramides, along with a low amount of phytosphingosine and trace amounts of 6-hydroxysphingosine. The distribution of ceramides in all dogs included in this study is detailed in Table 1 (free ceramides) and Table 2 (covalently bound ceramides).
TLC of free ceramides of SC taken from dog 3. Legends as in Fig. 1
TLC of protein-bound ceramides of SC taken from dog 3. Legends as in Fig. 1
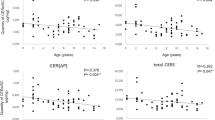
The 12 tapes taken from the abdomen of the Labrador were pooled by two and analyzed. The concentration of free ceramides that are seen in Fig. 7 was lower in the upper layers and increased along with the depth to reach a maximal concentration in layers 9 and 10. There were no significant variations between the protein content of each tape that could account for the differences in ceramide content. However, the protein-bound ceramides did not show the same depth-related change and the concentration was equivalent in each lane as can be seen in Fig. 8. Similar observations were made with the two other dogs, i.e., variations in free ceramides with depth, but no variation with protein-bound ceramides (not shown). The results suggest that wide breed-specific differences in the patterns of free and protein-bound ceramides exist in dogs, but these differences remain only quantitative and the molecular species of ceramides are similar in all dogs.
TLC of free ceramides of SC taken from dog 4 by tape stripping. The tapes were pooled by two before extraction. Solvent system and visualization as in Fig. 1
TLC of protein-bound ceramides of SC taken from dog 4 by tape stripping. Legends as in Fig. 7
Discussion
In the present study, the structures of ceramides in dog SC were established by means of biochemical analysis and mass spectrometry. Most molecular species of free and protein-bound ceramides reported in human SC were also found in dog SC. In a recent paper [12], five ceramides were identified in healthy dog SC, namely CerEOS, CerNS, CerNP, CerAS, and CerEOP, but ceramides containing 6-hydroxysphingosine were absent although we found the latter ones to be clearly present in some breed of dogs. Differences in the methods used to take the SC could possibly account for the observed discordances. Reiter et al. used cyanoacrylate for stripping, whereas adhesive tape was selected as the most satisfactory material in our study. Cyanoacrylate stripping is not suitable for investigations of the lipid profile with relation to depth and the method makes very difficult the recovery of protein-bound lipids. However, although covalently bound ceramides are thought to have a major role in the function of the skin barrier [14], there is very little information available about their depth-related profile in SC. Our study identified in dog SC the presence of the three ceramides containing omega-hydroxylated long-chain fatty acids, namely CerOS, CerOP, and CerOH found in human SC. The fatty acid composition, as revealed by mass spectrometry of CerOS, was almost similar to that of human CerOS [3].
In conclusion, we have shown that dog SC has a ceramide profile close to the one described in human SC. Our data suggest that dog SC could be considered as a suitable model to study human skin diseases. A detailed study is currently in progress in our laboratory to assess the variations of the ceramide profile with relation to depth in the SC of lesional and non-lesional areas of dogs suffering of atopic dermatitis.
References
Bouchon B, Portoukalian J, Orgiazzi J, Bornet H (1987) Selective enrichment of phytosphingosine in glycosphingolipids of isolated human thyrocytes as compared to the whole thyroid. Biochem Biophys Res Commun 143:827–831
Di Nardo A, Wertz P, Giannetti A, Seidenari S (1998) Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol 78:27–30
Farwanah H, Pierstorff B, Schmelzer CE, Raith K, Neubert RH, Kolter T, Sandhoff K (2007) Separation and mass spectrometric characterization of covalently bound skin ceramides using LC/APCI-MS and Nano-ESI-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 852:562–570
Griffin CE, De Boer DJ (2001) The ACVD task force on canine atopic dermatitis (XIV): clinical manifestations of canine atopic dermatitis. Vet Immunol Immunopathol 81:255–269
Hill J, Paslin D, Wertz PW (2006) A new covalently bound ceramide from human stratum corneum, omega-hydroxyacylphytosphingosine. Int J Cosmet Sci 28:225–230
Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A (1991) Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Investig Dermatol 96:523–526
Macheleidt O, Kaiser HW, Sandhoff K (2002) Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Investig Dermatol 119:166–173
Melnik B, Hollmann J, Plewig G (1988) Decreased stratum corneum ceramides in atopic individuals–a pathobiochemical factor in xerosis? Br J Dermatol 119:547–549
Mizutani Y, Mitsutake S, Tsuji K, Kihara A, Igarashi Y (2009) Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie 91:784–790
Motta S, Monti M, Sesana S, Caputo R, Carelli S, Ghidoni R (1993) Ceramide composition of the psoriatic scale. Biochim Biophys Acta 1182:147–151
Popa I, Bennaceur K, Abdul-Malak N, Perrier E, Schmitt D, Portoukalian J (2006) Studies of compounds that enhance sphingolipid metabolism in human keratinocytes. Int J Cosmet Sci 28:53–59
Reiter LV, Torres SM, Wertz PW (2009) Characterization and quantification of ceramides in the nonlesional skin of canine patients with atopic dermatitis compared with controls. Vet Dermatol 20:260–266
Wertz PW (2000) Lipids and barrier function of the skin. Acta Derm Venereol Suppl 208:7–11
Wertz PW, Madison KC, Downing DT (1989) Covalently bound lipids of human stratum corneum. J Investig Dermatol 92:109–111
Weerheim A, Ponec M (2001) Determination of stratum corneum lipid profile by tape stripping in combination with high-performance thin-layer chromatography. Arch Dermatol Res 293:191–199
Zor T, Selinger Z (1996) Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem 236:302–308
Acknowledgments
The study was partially supported through funding agreement with Virbac.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Popa, I., Thuy, L.H., Colsch, B. et al. Analysis of free and protein-bound ceramides by tape stripping of stratum corneum from dogs. Arch Dermatol Res 302, 639–644 (2010). https://doi.org/10.1007/s00403-010-1049-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-010-1049-0