Abstract
Oral lichen planus (OLP) is a chronic muco-cutaneous inflammatory disease defined as a precancerous condition. We determined the expression patterns of proliferation markers topoisomerase IIα (topo IIα) and Ki-67 and an intermediated filament protein cytokeratin-19 (CK-19) in atrophic OLP. These markers were selected because our recent microarray analysis indicated they might aid in identification of potentially malignant lesions. The expression patterns were correlated with the DNA content of these lesions shown to be useful in detection lesions at risk for malignant transformation of OLP. A series of 81 formalin-fixed, paraffin-embedded biopsies from 70 patients suffering from atrophic OLP were immunostained with monoclonal antibodies against topo IIα, Ki-67 and CK-19 using standard methods. Of the 70 patients, there were eight patients who had dysplastic changes in OLP lesions. During the follow-up, altogether five patients got cancer in the OLP area even though no dysplastic changes were present in the preceding lesion. On light microscopy, 500 cells were examined in the basal and parabasal epithelial layers of biopsy samples at 400× magnification. All biopsy samples were topo IIα positive and approximately 70% of the counted cells were positive. Strong staining of topo IIα was significantly correlated with dysplasia (P = 0.019), basal cell hyperplasia (P = 0.005) and ulceration (P = 0.008) in the samples. Ki-67 was expressed in all samples but only 36% of the cells were positive. CK-19 positivity was found in 29% of the specimens. Histological parameters were not related to either Ki-67 or CK-19. The comparison of the staining patterns with the DNA content of lesions showed that strongly stained cells with topo IIα were significantly more frequent in the samples with 2.5cER higher than 15% than in those below 15% (P = 0.013; Mann–Whitney). The percentage of the measured cells is 2.5cER exceeding the 2.5c value on the DNA scale. We earlier showed that this cut-off value of 2.5cER discriminated DNA aneuploidy. To conclude, topo IIα is a proliferation and also an apoptotic marker in atrophic OLP lesions and it might have a predictive value in oral lichen planus lesions prone to develop malignancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral lichen planus (OLP) is a chronic muco-cutaneous inflammatory disease, which WHO has defined as a potentially precancerous condition, representing a generalized state, associated with a significantly increased risk of cancer [32]. Because of its unknown etiology, the management of OLP is still not satisfactory and the optimal treatment remains to be defined [27]. Currently, there are no prognostic markers to identify the lesions at increased risk for malignant transformation among the chronic OLP lesions.
Topoisomerase II alpha (topo IIα) is an enzyme that can modify (isomerise) the tertiary structure of DNA without changing its primary structure, determined by the nucleotide sequence. It exerts an important role in DNA topology, repair and replication by breaking and rejoining the DNA double helix [2]. Topo IIα is a cell cycle-related protein and is expressed in normal as well as neoplastic cells in the S, G2 and M phases. The lowest level of expression is found in the G0 and G1 phases. Consequently, topo IIα is related to cell proliferation [13, 14] but also related to cell viability and apoptosis. Previous studies have shown that aberrant expression of topo IIα is associated with the induction of apoptosis [19, 30].
A variety of other markers of cell proliferation are available as well. Ki-67 antigen is a nuclear matrix protein that is expressed in proliferating cells during the G1, S, G2 and M phases of the cell cycle but not in resting cells (G0 phase) [4, 26]. Ki-67 is the most frequently used proliferation marker in routine diagnostics [31]. There are studies suggesting Ki-67 as a useful prognostic factor in oral squamous cell carcinoma [8], although some other studies did not find any association between Ki-67 and oral cancer [11, 24].
Cytokeratin 19 (CK-19) is an intermediated filament protein, which is found in most of the simple and non-keratinizing stratified epithelia [21]. It was shown as a useful marker of cellular atypia, associated with pre-malignant lesions in oral epithelium [16]. In addition a sparse basal expression of CK-19 is usually detected in OLP lesions [30]. CK-19 expression in suprabasal cell layers of oral mucosa might also be a useful marker for diagnosing oral precancerous lesions. CK-19 expression has been regarded important in the initial events during oral carcinogenesis [22].
We have recently described changes in the DNA content in atrophic oral lichen planus lesions measured by static cytometry and found that during the follow-up DNA content might be a useful predictor of OPL lesions prone to malignant transformation [18]. Based on our microarray studies we also found that topo IIα, Ki-67 and CK-19 were among the up-regulated genes in oral cancer cell lines derived from oral lichen planus [25]. Thus these markers were tested in atrophic OLP lesions to estimate their usability in predicting the lesions at risk for malignancy.
Materials and methods
The material of this study consists of 81 biopsy specimens taken from 70 consecutive patients with atrophic OLP defined as criteria by WHO [32], identified between years 1991 and 2002, and retrieved from the files of the Department of Oral Pathology and Radiology, University of Turku. All patients, born before 1928, those with any medication or suffering from any systemic disease, were excluded from this study. Of the 70 patients, 53 (75.7%) were women with the mean age of 53.3 years (range 29–73 years) and 17 (24.3%) were men with the mean age of 52.7 years (range 36–72 years). Thus the patients represented a typical age and sex distribution of OLP patients. The mean follow up time of the patients was 63.5 months (range from 7.2 to 117 months). Five patients with atrophic oral lichen planus developed oral squamous cell carcinoma during the follow-up. At the first visit all lesions were clinically typical atrophic OLP fulfilling the WHO criteria, such as presence of bilateral lesions on oral mucosa with atrophic or ulcerative outlook. All the specimens stained here were the same as were used earlier for DNA content measurements allowing the comparison between immunohistochemistry and DNA data [18].
Histological examination
The biopsies were fixed in formalin and processed to paraffin-embedded tissue blocks according to the routine practice as described earlier. Haematoxylin and eosin stained slides (6 μm) were cut for routine diagnostics. Re-examination of the slides confirmed the diagnosis of OLP in all cases. The WHO criteria [32] for OLP were used when re-examining the histopathology of the sections. In addition, the following morphological parameters were recorded: keratosis, atrophy, ulceration, hyperplasia and dysplasia. Also the location of OLP lesion in the oral mucosa was recorded.
The presence of dysplasia was graded as mild, moderate or severe. Dysplasia was mild when dysplastic changes were present in lower third of the epithelia, moderate when two thirds of the epithelia were affected and severe when the whole thickness of epithelia was involved. Basal cell hyperplasia was defined as increased density of basal cells within a unit area.
Immunohistochemistry
For immunohistochemical staining of topo IIα, Ki-67 and CK-19, 4 μm thick sections from formalin-fixed, paraffin-embedded tissue blocks were cut. Briefly, the sections were de-paraffinized and boiled in microwave for 2 × 5 min in 0.01 M citrate buffer pH 6, then after cooling in room temperature for 20 min. The monoclonal antibodies for topo IIα, Ki-76 and CK-19 were used (DAKO, Glostrup, Denmark) at a 1:100 dilution, ready-to-use, and 1:100, respectively. The staining was made by DAKO TechMate™ 500 Plus staining system, following the manufacturer’s instructions. Sections from breast carcinoma were used as a positive control for topo IIα and CK-19, while a biopsy of oral verruca vulgaris was used as a positive control for Ki-67. Negative controls were processed in parallel to test samples, by replacing the primary antibody by buffer.
Evaluation of immunostaining
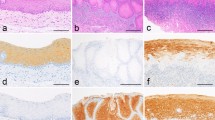
On light microscopy, 500 cells in each section were examined for the staining of topo IIα and Ki-67 in the basal and parabasal epithelial layer at 400× magnifications. The staining pattern was graded as negative, diffuse staining (<50%), and strong staining (≥50%) comparing to the intensity and frequency of stain among the cells (Figs. 1, 2). The cell was graded as diffuse when the staining of the nucleus was punctuated and as strong when the whole nucleus was in dark staining. Staining pattern of CK-19 was graded into four groups: (1) no staining, (2) only few cells staining, (3) less than 50% of the cells stained, and (4) 50% or more of the basal cells stained (Fig. 3). For immunohistochemical stainings consecutive sections were made from the specimens. Thus, it was possible to correlate the location of expression of topo IIα, Ki-67 and CK-19 with each other.
Immunohistochemical staining of CK-19 of the same sample as presented in Fig. 2 (original magnification ×200)
DNA analysis
For analysis of the DNA content, we used static cytometry, as previously described, analyzing a wide variety of parameters [18]. DNA ploidy is 1 (one) in a normal diploid cell and 2 (two) in a dividing or tetraploid cell. In an aneuploid cell the ploidy could be approximately between 1.2 and 1.8. In our study the approximately value for ploidy discriminating DNA content in cells was 1.3. Its importance proved to be 2.5c exceeding rate (2.5cER) and the proliferation indexes (PI). The rate of deviation from the 2.5c value (sign of diploidy with cell in G0 phase) is represented by 5cER, is the percentage of nuclei that are over 5c (polyploidy or aneuploidy). Proliferation index is the sum of S and G2/M phase fractions. Determined on the basis of the previous analysis, the value of 15.3% for 2.5cER was selected here as the cut-off value. The statistically significant value for 2.5cER explaining the presence of DNA aneuploidy was 15.3% in our previous study. All these values recorded in static cytometry were compared with the immunohistological staining for topo IIα, Ki-67 and CK-19.
Statistical analysis
SPSS for Windows, version 11.5 (Chicago, IL, USA) was used for statistical analysis. Frequency tables were analyzed using the Chi-square test, with Pearson’s R or likelihood ratio (LR) being used to assess the significance of the correlation between the categorical variables. Differences in the means of continuous variables between the groups were analyzed using ANOVA (when appropriate) or non-parametric tests (Mann–Whitney, Kruskal–Wallis) tests. Binary logistic regression models were used to analyze the power of different variables as predictors of dysplasia, using the stepwise backward approach. In all analyses, the probability values less than 0.05 were regarded as significant.
Results
During the follow-up the oral lichen lesions progressed to squamous cell carcinoma in five patients. Table 1 shows the results of immunohistological stainings in the original OLP lesions of these patients before cancer development. Topo IIα was expressed in 75% of the counted cells in four of the five biopsy samples. One atrophic OLP progressed to carcinoma in only 7 months. In this lesion 20% and 45% of the cells were strongly stained with topo IIα and Ki-67, respectively.
In eight of the 81-biopsy specimen there were dysplastic changes present in the original biopsy samples. During the follow-up (mean 63.5 months) there was no progression of dysplasia in seven of those lesions. Only one of the eight lesions progressed to cancer in 20 months from the original diagnose of atrophic OLP. The rest of the OLP lesions had no changes during the follow-up time.
We compared the expression of topo IIα in patients who developed oral SCC and in those who did not. There was a borderline significant difference of topo IIα expression between these two groups (Fisher’s exact test; P = 0.054). There was no such difference in expression of Ki-67 and CK-19.
Table 2 summarizes the staining pattern of topo IIα and Ki-67 in OLP. The assessment was based on 500 cells counted in each biopsy sample. In every sample there were cells without any staining, with diffuse staining and strong staining. For topo IIα, diffuse staining pattern was detected in 59% of the cells, while 11% of the cells were strongly stained. Diffuse staining for Ki-67 was detected in 12% of the cells, while strong staining was detected in 24% of the cells.
The staining pattern of CK-19 is shown in Table 3. CK-19 expression was detected in 24 of the 81-biopsy samples (29%). Of the positive samples, 11/24 was classified in category <50% of the cells stained while only in four samples 50% or more of the cells were stained.
Histological variables related to immunohistochemical staining patterns
In dysplastic lesions, there were statistically significantly more strongly (P = 0.019) (mean 58.7 ± 16.6 SD) and diffusely stained (P = 0.030) (mean 11.5 ± 6.3 SD) topo IIα positive cells than in non-dysplastic lesions. Strong and diffuse staining of topo IIα was also related to basal cell hyperplasia (P = 0.005) (mean 14.0 ± 5.4 SD) and ulceration (P = 0.008) (mean 48.4 ± 18.9 SD), respectively. There was no significant relation with topo IIα and location of the lesions.
The staining pattern of Ki-67 was not statistically significantly associated with any specific histological parameters or location of the lesions.
The staining of CK-19 was significantly more frequent in the tongue than in other sites of oral mucosa (P = 0.047). However, CK-19 had no statistically significant relation to any of those histological parameters mentioned above. Moderate dysplastic changes occurred in one of the strongly stained lesions and one lesion with only few stained cells.
We have earlier determined the DNA content in these biopsy samples. Table 4 summarized the relationship of topo IIα expression with the DNA content. There were 34 samples with 2.5cER higher than 15% shown earlier to be useful cut-off value to detect premalignant lesions. The proportion of topo IIα-stained cells in these lesions was 65.9 (±31.8 SD). Strongly stained cells with topo IIα were significantly more frequent in samples with 2.5cER higher than 15% than in those below 15% (P = 0.013; Mann–Whitney). Based in our previous study we have found 1.3 as a useful cut-off value for ploidy. Strongly stained cells with topo IIα were significantly more frequent in samples with ploidy higher than 1.3 than those below 1.3 (P = 0.034; Mann–Whitney) (Table 4). However, there were only two samples with ploidy higher than 1.3 of the percentage of stained cells was 20.4 (±2.3 SD). Finally, topo IIα was correlated to G2/M parameter. In strong staining topo IIα correlated significantly to G2/M fraction (P = 0.036).
G2/M fraction was the only DNA parameter, which was significantly associated either with diffuse staining pattern of Ki-67 (P = 0.046) or without any staining (P = 0.024). CK-19 had no significant associations with any of the DNA parameters tested.
Mutual correlations showed that topo IIα was statistically significantly correlated to strong staining of Ki-67 (P = 0.000; Pearson correlation), (P = 0.002; Spearman’s rho).
Discussion
The aim this study was to investigate the expression of topoisomerase IIα, Ki-67 and cytokeratin 19 in atrophic oral lichen planus as a prognostic and proliferation marker. The results were correlated with histological parameters and our recent results with static cytometry [18].
Previous immunohistological studies have shown topo IIα to be a reliable indicator of cell proliferation in carcinomas of vulva [6], breast [17], uterine [9] and head and neck [12, 28]. In prostate carcinoma expression of topo IIα seems to relate to the grade of the carcinoma being lowest in well-differentiated neoplasms and highest in poorly differentiated tumours [33]. In the present study, the topo IIα was expressed in 70.2% of the counted cells. Our results indicate that atrophic lichen planus is a highly proliferative lesion in oral mucosa. Besides proliferation expression of topo IIα could be due to DNA repairing role of topo IIα. Similar results of high cell proliferation in oral lichen planus lesions have been reported earlier, even though in those studies the used proliferation marker is Ki-67 together with p53 [10, 29].
We also found that the other cell proliferation marker, Ki-67 was much lesser expressed in OLP lesions than topo IIα (35.9 vs. 70.2%). However, strong staining of topo IIα correlated statistically significantly with Ki-67 (P = 0.002). Our results support the earlier view that topo IIα might be a better proliferation marker than Ki-67 [12, 17]. Topo IIα expression in the S, G2, and M phases gives a better estimate of the number of the actively cycling cells than Ki-67 does, as shown with static cytometry. Difference can also be explained by the repairing or apoptotic role of topo IIα, which Ki-67 does not have. Static cytometry alone is unable to prove cell repairing, thus further studies are needed.
Besides proliferation marker topo IIα repairs DNA damages during cell proliferation [2]. Apoptosis seems to be increased in oral lichen planus [3]. In addition, it is shown that topo IIα plays a role in apoptosis by inducing apoptotic cell death [1, 19, 30]. Topo IIα could be a marker of unsuccessful DNA repair, which finally will result in apoptosis. This would explain the more frequent expression of topo IIα than Ki-67 in atrophic OLPs. Our finding that topo IIα correlated with the changes in DNA content further supports the need of DNA repairing in these lesions. In atrophic oral lichen apoptosis is increased but the reason for this is totally unknown. It might be that one key event is DNA failure leading to increased expression of repair markers as topo IIα, but the repair might failure by depletion of topo IIα leading to apoptosis of basal or parabasal cells [1]. It is also shown, that protein kinase C delta (PKCδ), which has an essential role in the genotoxic stress response, activates topo IIα alpha to induce apoptotic cell death in response to DNA damage [34]. Previous studies have shown that intense expression of topo IIα was also significantly related to response to chemotherapy [28].
Our results of static cytometry showed that strong staining of topo IIα correlated with G2/M fraction (P = 0.036), while the association with Ki-67 was slightly different. Not only the diffuse staining of Ki-67 correlated statistically significantly with G2/M (P = 0.046) but there was also the no colour pattern (P = 0.024). It is known that Ki-67 accumulates during S phase and it rapidly disappears from post-mitotic cells [5]. The rapid disappearing and short half-life of Ki-67 during G2/M phase could be one explanation for the negative staining pattern of Ki-67 found in this study.
Ki-67 is widely used as a molecular marker of cellular proliferation that predicts prognosis of many different tumours. Some studies however cannot prove Ki-67 as a useful indicator for cancer development or recurrence [7, 15]. When we compare the expression of Ki-67 with those of static cytometry the results are not fully concordant. In atrophic lichen planus expression of Ki-67 is of limited value as a proliferation marker.
CK-19 expression was detected in 29% of the OLPs whereas only 4/81 samples were strongly stained. Suprabasal CK-19 staining has been regarded as a marker of premalignant change in oral epithelium [16]. Our results further support the view that CK-19 can be used as marker of cellular atypia in pre-malignant oral lesions [16, 22]. However, it has to be noted that biopsy sample might not always cover the whole histological picture present in a clinically extensive lesion. Sometimes it might be difficult to target the correct biopsy site.
In the present study, 41% of the OPL lesions were aneuploid [18]. We have reported earlier that 2.5cER and PI values were significantly higher in ulcerated OLP lesions than in nonulcerated ones (P < 0.001 and P = 0.012, respectively). Furthermore, OLPs located in the tongue had significantly higher values of 2.5cER and PI than lesions at other sites (P < 0.001 and P = 0.013). We also concluded that atrophic OLP lesions with both ulceration and dysplastic changes were at risk of developing an oral cancer. Based on the present results we can add that an atrophic OLP biopsy sample with strongly topo IIα staining and high 2.5cER and DNA ploidy values could be a risk lesion to progress to malignancy.
According to the best of our knowledge, there are no previous studies that compare the results of static cytometry to topo IIα or CK-19 staining. Ki-67 has earlier been regarded as a useful prognostic marker for prostate and head and neck carcinomas, although conflicting results exists [20, 23].
Abbreviations
- OLP:
-
Oral lichen planus
- topo IIα:
-
Topoisomerase IIα
- CK-19:
-
Cytokeratin-19
- ER:
-
Exceeding rate
- PI:
-
Proliferating index
References
Akimitsu N, Kamura K, Tone S, Sakaguchi A, Kikuchi A, Hamamoto H, Sekimizu K (2003) Induction of apoptosis by depletion of DNA topoisomerase IIalpha in mammalian cells. Biochem Biophys Res Commun 307:301–307
Berger JM, Gamblin SJ, Harrison SC, Wang JC (1996) Structure and mechanism of DNA topoisomerase II. Nature 379:225–232
Bloor BK, Malik FK, Odell EW, Morgan PR (1999) Quantitative assessment of apoptosis in oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88:187–195
Brown DC, Gatter KC (1990) Monoclonal antibody Ki-67: its use in histopathology. Histopathology 17:489–503
Bruno S, Darzynkiewicz Z (1992) Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif 25:31–40
Brustmann H, Naude S (2002) Expression of topoisomerase IIalpha, Ki-67, proliferating cell nuclear antigen, p53, and argyrophilic nucleolar organizer regions in vulvar squamous lesions. Gynecol Oncol 86:192–199
Davies L, Hardin NJ, Beatty BG (2006) Can Ki-67 predict recurrence of NO squamous cell carcinoma of the tongue? Ann Otol Rhinol Laryngol 115:12–17
Dissanayake U, Johnson NW, Warnakulasuriya KA (2003) Comparison of cell proliferation in the centre and advancing fronts of oral squamous cell carcinomas using Ki-67 index. Cell Prolif 36:255–264
Gibbons D, Fogt F, Kasznica J, Holden J, Nikulasson S (1997) Comparison of topoisomerase II alpha and MIB-1 expression in uterine cervical squamous lesions. Mod Pathol 10:409–413
Girod SC, Pfeiffer P, Ries J, Pape HD (1998) Proliferative activity and loss of function of tumour suppressor genes as ‘biomarkers’ in diagnosis and prognosis of benign and preneoplastic oral lesions and oral squamous cell carcinoma. Br J Oral Maxillofac Surg 36:252–260
Gonzalez-Moles MA, Caballero R, Rodriguez-Archilla A, Ruiz-Avila I, Bravo I (1996) Prognosis value of the expression of Ki-67 for squamous cell carcinoma of the oral cavity. Acta Stomatol Belg 93:159–165
Hafian H, Venteo L, Sukhanova A, Nabiev I, Lefevre B, Pluot M (2004) Immunohistochemical study of DNA topoisomerase I, DNA topoisomerase II alpha, p53, and Ki-67 in oral preneoplastic lesions and oral squamous cell carcinomas. Hum Pathol 35:745–751
Heck MM, Earnshaw WC (1986) Topoisomerase II: a specific marker for cell proliferation. J Cell Biol 103:2569–2581
Heck MM, Hittelman WN, Earnshaw WC (1988) Differential expression of DNA topoisomerases I and II during the eukaryotic cell cycle. Proc Natl Acad Sci USA 85:1086–1090
Jalava P, Kuopio T, Juntti-Patinen L, Kotkansalo T, Kronqvist P, Collan Y (2006) Ki67 immunohistochemistry: a valuable marker in prognostication but with a risk of misclassification: proliferation subgroups formed based on Ki67 immunoreactivity and standardized mitotic index. Histopathology 48:674–682
Lindberg K, Rheinwald JG (1989) Suprabasal 40 kd keratin (K19) expression as an immunohistologic marker of premalignancy in oral epithelium. Am J Pathol 134:89–98
Lynch BJ, Guinee DG, Jr. Holden JA (1997) Human DNA topoisomerase II-alpha: a new marker of cell proliferation in invasive breast cancer. Hum Pathol 28:1180–1188
Mattila R, Alanen K, Syrjanen S (2004) DNA content as a prognostic marker of oral lichen planus with a risk of cancer development. Anal Quant Cytol Histol 26:278–284
McPherson JP, Goldenberg GJ (1998) Induction of apoptosis by deregulated expression of DNA topoisomerase IIalpha. Cancer Res 58:4519–4524
Mirtti T, Kallajoki M, Aaltonen M, Alanen K (2001) Cyclin A and Ki-67 with DNA content in benign and malignant prostatic epithelial lesions. Anal Quant Cytol Histol 23:229–237
Moll R (1998) Cytokeratins as markers of differentiation in the diagnosis of epithelial tumors. Subcell Biochem 31:205–262
Nie M, Zhong L, Zeng G, Li B (2002) The changes of cytokeratin 19 during oral carcinogenesis. Zhonghua Kou Qiang Yi Xue Za Zhi 37:187–190
Raybaud H, Fortin A, Bairati I, Morency R, Monteil RA, Tetu B (2000) Nuclear DNA content, an adjunct to p53 and Ki-67 as a marker of resistance to radiation therapy in oral cavity and pharyngeal squamous cell carcinoma. Int J Oral Maxillofac Surg 29:36–41
Roland NJ, Caslin AW, Bowie GL, Jones AS (1994) Has the cellular proliferation marker Ki67 any clinical relevance in squamous cell carcinoma of the head and neck? Clin Otolaryngol 19:13–18
Ruutu M, Johansson B, Grenman R, Syrjanen S (2005) Two different global gene expression profiles in cancer cell lines established from etiologically different oral carcinomas. Oncol Rep 14:1511–1517
Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182:311–322
Scully C, Beyli M, Ferreiro MC, Ficarra G, Gill Y, Griffiths M, Holmstrup P, Mutlu S, Porter S, Wray D (1998) Update on oral lichen planus: etiopathogenesis and management. Crit Rev Oral Biol Med 9:86–122
Stathopoulos GP, Kapranos N, Manolopoulos L, Papadimitriou C, Adamopoulos G (2000) Topoisomerase II alpha expression in squamous cell carcinomas of the head and neck. Anticancer Res 20:177–182
Taniguchi Y, Nagao T, Maeda H, Kameyama Y, Warnakulasuriya KA (2002) Epithelial cell proliferation in oral lichen planus. Cell Prolif 35 Suppl 1:103–109
van der Velden LA, Manni JJ, Ramaekers FC, Kuijpers W (1999) Expression of intermediate filament proteins in benign lesions of the oral mucosa. Eur Arch Otorhinolaryngol 256:514–519
Warnakulasuriya KA, Johnson NW (1996) Importance of proliferation markers in oral pathology. Curr Top Pathol 90:147–177
WHO World Health Organization Collaborating Centre for Oral Precancerous Lesions (1978) Definition of leukoplakia and related lesions; an aid to study precancer. Oral Surg Oral Med Oral Pathol 46:518–539
Willman JH, Holden JA (2000) Immunohistochemical staining for DNA topoisomerase II-alpha in benign, premalignant, and malignant lesions of the prostate. Prostate 42:280–286
Yoshida K, Yamaguchi T, Shinagawa H, Taira N, Nakayama KI, Miki Y (2006) Protein kinase C delta activates topoisomerase IIalpha to induce apoptotic cell death in response to DNA damage. Mol Cell Biol 26:3414–3431
Acknowledgments
The authors thank Mrs. Marja Uola for her assistance in histological sections. We acknowledge Dr. Kari Syrjänen for the statistical analysis. This study was supported by a grant from the Hilkka Brusiin Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mattila, R., Alanen, K. & Syrjänen, S. Immunohistochemical study on topoisomerase IIα, Ki-67 and cytokeratin-19 in oral lichen planus lesions. Arch Dermatol Res 298, 381–388 (2007). https://doi.org/10.1007/s00403-006-0711-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-006-0711-z