Abstract
Lysosomes and their components are suspected to be involved in epidermal differentiation. In this study, lysosomal enzyme activities, expression of the lysosome-associated membrane protein 1 (Lamp-1) and expression of the epidermal galectins-1, -3 and -7 were investigated in human keratinocytes cultured at different cell densities (subconfluence, confluence and postconfluence) in order to induce differentiation. Detected by Western blot and immunofluorescence, Lamp-1 expression is transiently upregulated at culture confluence, but reduced at postconfluence. Northern blot analyses performed on subconfluent, confluent and post-confluent cultures of keratinocytes show that Lamp-1 mRNA expression is also upregulated at culture confluence, but downregulated at postconfluence. Measurements of lysosomal enzyme activities indicate a transient upregulation at culture confluence, whereas cathepsins B, C and L are particularly downregulated at postconfluence. Cell density and differentiation of epidermal cells also differentially regulates galectin expression in autocrine cultures. As the expression of galectin-1 mRNA is high in subconfluent cells, it is assumed to be associated with their proliferative state. On the other hand, as the mRNA levels for galectins-3 and -7 are notably upregulated at culture confluence (galectin-7) or at postconfluence (galectin-3), their expression is thought to be related to the differentiated state of keratinocytes. However, we collected evidence by confocal microscopy that galectin-3 and Lamp-1 do not colocalize in vitro in keratinocytes. Altogether, our results suggest that the upregulated Lamp-1 expression at confluence could be involved in keratinocyte differentiation, but apparently not through interaction with galectin-3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Keratinocytes in the basal epidermal layer are proliferative cells, which then leave the cell cycle, migrate into the suprabasal layers and stratify in order to differentiate towards the superficial cornified layer of the epidermis. During this journey, keratinocytes exhibit progressive morphological changes due to programmed terminal differentiation and mature keratinocytes desquamate from the surface of the skin as dead enucleate cells [52]. Epidermal differentiation is a progressive complex phenomenon that depends on the fine regulation of gene expression in keratinocytes. Indeed, many genes are expressed in a coordinated manner during the differentiation of keratinocytes [12, 15, 53]. Beside involucrin, the suprabasal keratins 1 and 10, and other proteins used commonly as differentiation markers (e.g. transglutaminase-1, profilaggrin, loricrin) [13, 15], the involvement of many other genes and their encoded proteins in the development of the mature cornified phenotype of epidermal keratinocytes is increasingly identified.
As a model for in vitro studies of the regulation of differentiation in human epidermal keratinocytes, autocrine cultures of keratinocytes demonstrate initiation of early differentiation when the cell density is high enough to reach confluence of the culture [25, 35, 36], and allow the expression of later markers of differentiation in post-confluent conditions [36, 43]. Thus, autocrine keratinocyte cultures represent a model highly suitable for studying epidermal differentiation.
Lysosomes are intracellular organelles containing acid hydrolases and are responsible for intracellular digestion. Their role in cell differentiation is supported by several studies performed on different cell types including keratinocytes [5, 9, 45, 57]. A particular importance of lysosomes in keratinocytes has been suggested by the high level of biosynthesis and processing of the lysosomal protein ganglioside GM2 activator protein observed in cultured keratinocytes in comparison with fibroblasts [16]. Furthermore, a secretion of lysosomal enzymes from lamellar granules in terminally differentiated keratinocytes has been established [28], and the lysosomal protease cathepsin L was shown to regulate keratinocyte differentiation [46].
The lysosome-associated membrane protein 1 (Lamp-1) is a transmembrane glycoprotein predominantly found in lysosomal membranes. Diverse biological and pathological functions have been proposed for this protein, but its precise role has not been defined yet. Interestingly, it was demonstrated that Lamp-1 is a differentiation marker for mouse mammary epithelial HC11 cells [5]. Hence, the cell surface expression of Lamp-1 on tumour cells [39, 41] and on peripheral blood lymphocytes [24] has suggested that these glycoproteins may play a role as adhesion molecules. Our previous study indicates that Lamp-1 on the surface of tumour cells binds galectin-3, thus possibly promoting tumour invasion and/or metastasis [41]. Galectins are involved in cell proliferation and death, but also in cell adhesion [33], as well as in inflammation, immunity [37] and cancer [30]. Galectin-1 is detected in normal human skin and its gene expression is localized in keratinocytes of the basal and spinous epidermal layers [1]. During the first trimester of human embryogenesis, epidermal galectin-1 expression becomes detectable in basal keratinocytes at 14 weeks, suggesting a possible developmental regulation pattern of the gene [49]. Galectin-1, galectin-3 and galectin-7 expressions have been observed in normal human epidermis [1, 55].
We have recently shown that Lamp-1, and also Lamp-2, are expressed on the surface of keratinocytes treated with ionomycin in order to increase the intracellular calcium concentration [23]. Here, we studied the regulation of Lamp-1 and lysosomes during epidermal differentiation induced in vitro by cell culture density. An upregulation of Lamp-1 at culture confluence, together with differential regulation of the expression of galectins around confluence strongly suggest that lysosomes and galectins may play a role during the differentiation of epidermal keratinocytes.
Materials and methods
Skin samples and culture of normal human keratinocytes
Human adult abdominal skin samples were obtained at plastic surgery (Dr. B. Bienfait, Clinique St. Luc, Namur-Bouge). Samples for microscopy were frozen immediately in Tissue-Tek O.C.T. compound (Sakura) and stored at −80°C until further processing. Keratinocytes were isolated by the trypsin float technique as described [54], and primary cultures were initiated in KGM-2 medium (BioWhittaker, Europe). Keratinocytes harvested from trypsinized primary proliferating subconfluent cultures were then plated into secondary cultures at 5×103 cells/cm2 in the same medium. All experiments were performed on cells cultured in autocrine conditions [35], which means that, when approximately 40% of the culture substratum was covered by cells, secondary cultures were washed repeatedly with KBM-2 medium prepared by excluding bovine pituitary extract, insulin, transferrin, epinephrine and EGF from the culture medium. Cultures were re-fed every other day with KBM-2 medium until use at culture subconfluence (4 days before the expected confluence), at confluence, or 4 days after confluence, at postconfluence.
Confluence of the cultures induces growth arrest and terminal differentiation of the keratinocytes, characterized by the induction of early epidermal differentiation markers such as the suprabasal keratins 1 and 10 [35]. In post-confluent autocrine keratinocyte cultures, the later marker of differentiation involucrin is upregulated [36].
Immunofluorescent labelling
Normal skin samples coated in O.C.T. were cut at 5 μm in a Microm HM 500 OM cryostat at −20°C, air-dried and fixed on ice with acetone for 4 min. Sections were then washed three times in PBS solution (pH 7.2) and blocked in PBS containing 0.1% bovine serum albumin. Incubation with the primary H4A3 antibody, specific to human Lamp-1 and diluted in the blocking solution, was performed for 1 h at room temperature in a humidified chamber. The mouse H4A3 antibody developed by Drs. J.T. August and J.E.K. Hildreth was obtained from the Developmental Studies Hybridoma Bank maintained at The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, under contract N01-HD-7-3263 from the NICHD. Incubation with the secondary antibody (fluorescein isothiocyanate (FITC)-conjugated anti-mouse) (Amersham, USA) was for 30 min as above in the dark. Sections incubated either without primary antibodies or with irrelevant mouse antibodies were used as controls. Tissue sections were observed by fluorescent microscopy in a Provis microscope (Olympus, USA).
Immunofluorescent labelling for Lamp-1 and galectin-3 was performed on cultured keratinocytes plated and grown on coverslips. Cells were then fixed and processed as described for frozen sections, or double labelled with the mouse antibody H4A3 as above for Lamp-1, followed by a goat anti-mouse antibody linked to Alexa 568 (red, Molecular Probes, Eugene, OR, USA). Coverslips were also incubated with a rabbit antibody to galectin-3 (Abcam Ltd, Cambridge, UK) followed by a goat anti-rabbit antibody linked to Alexa 488 (green, Molecular Probes, Eugene, OR, USA). The double labelling was then observed on a Leica confocal microscope with both red and green fluorescence combined in order to detect an eventual co-localization.
Western blot analysis
Cell lysates were prepared by scraping cultures into Triton X-100-containing PBS buffer. The protein concentration was determined in each sample and 13 μg of protein from each sample were then resolved by 10% sodium dodecylsulfate–polyacrylamide gel electrophoresis. The proteins were electro-transferred to Immobilon-P membranes (Millipore, Bedford, MA, USA) and the membranes blocked in 3% skimmed milk/0.5% BSA. Lamp-1 protein is detected by incubation for 2 h with H4A3 antibody, followed by three 10 min washes and 1 h incubation with a peroxidase-conjugated anti-mouse antibody.
The final detection was performed with 1:1000 dilutions of horse radish peroxidase-conjugated secondary anti-rabbit or anti-mouse antibodies (DAKO, Hamburg, Germany) and visualized using the BM Chemiluminescence Blotting Substrate (Roche, Penzberg, Germany).
RNA isolation and NORTHERN blot analysis
Poly(A)RNA was isolated and analyzed by Northern blotting as described previously [35]. The probes used to analyze epidermal differentiation are cDNAs specific to the human basal keratin 14 (K14) or specific to the suprabasal keratins 1 (K1) or 10 (K10) [38], and cDNA specific to the human involucrin [11]. The 36B4 cDNA specific to the human acidic ribosomal phosphoprotein PO [27] was used to assure equivalent loading and transfer of RNA.
The probe for the human Lamp-1 was obtained by RT-PCR. Briefly, RNA from human keratinocytes was reverse transcribed to generate first strand cDNA using 5 μM random hexamers, Superscript reverse transcriptase (Life Technologies, Mississauga, ON, Canada) and reaction mix. This cDNA was used as a template for PCR in 20 μl containing 0.25 mM dNTP, 0.5 μM of each primer and two units of Taq DNA polymerase (Life Technologies, Mississauga, ON, Canada) in a buffer containing 20 mM Tris–HCl pH 8.4, 1.5 mM MgCl2 and 50 mM KCl. The reaction conditions involved initial denaturation for 4 min at 94°C, followed by 25 cycles of 0.5 min at 94°C, 0.5 min at 59°C and 1 min at 72°C. The primers used for PCR were TGACACCATCCGTCTGTCTTGG and CATTGTACACAGCGCAGAACAGG (sense and antisense, respectively) designed to amplify a 1,050 bp fragment localized in exon 9 of Lamp-1 [42].
Galectin RNA expression was detected by using human cDNAs specific for galectin-1, galectin-3 and galectin-7 [48]. Relative gene expression was determined by comparison of the specific gene expression with 36B4 expression, based on densitometric measurements obtained with the NIH Image Analysis 1.60 software.
Measurement of lysosomal enzymatic activities
Enzymatic activities were assayed according to the following references: β-glucuronidase [7], α-mannosidase, β-galactosidase and β-glucosidase [34], hexosaminidase [47], cathepsin C [22], cathepsin B and cathepsin L [2], and acid phosphatase [51]. α-glucosidase and α-fucosidase were measured as described for α-mannosidase but using 4-methylumbelliferyl-α-D-glucoside and 4-methylumbelliferyl-α-L-fucoside, respectively, as substrates.
Results
Lamp-1 expression in normal human keratinocytes
Immunofluorescent staining of Lamp-1 on skin sections reveals expression of the protein in cells present in the dermis and in the epidermis (Fig. 1a). A few cells in the basal layer of the epidermis contain some particular staining for Lamp-1 in their cytoplasm localized in a supranuclear position. By the use of silver affinity staining to detect melanin on a tissue section and immunofluorescent staining to localize Lamp-1 in a successive section, we found that Lamp-1 in basal cells co-localizes mainly with melanosomes (data not shown). This observation is in accordance with the lysosomal identity of these organelles [32]. In suprabasal layers, Lamp-1 is present at higher concentration in keratinocytes of the granular layer versus keratinocytes of the spinous layer (Fig. 1a). The epidermal layers are identified by differential interference contrast microscopy (Fig. 1b). The same immunofluorescent staining of Lamp-1 in monolayer cultures of keratinocytes clearly illustrates the elevated number of lysosomes in this cell type (Fig. 1c).
Lamp-1 protein expression in normal human skin and in cultured human keratinocytes. Frozen section of the skin was labeled by indirect immunofluorescence (FITC) using the H4A3 antibody to human Lamp-1 and observed by fluorescence microscopy (a; magnification as in b) and by differential interference contrast microscopy (b; bar 100 μm). The expression of Lamp-1 is particularly demonstrated in keratinocytes as illustrated in the basal layer (insert of a; arrow). Lamp-1 protein is visualized by immunofluorescence in subconfluent keratinocyte culture (c; bar 40 μm)
Lysosomal enzymatic activities are upregulated in correlation with the induction of keratinocyte differentiation at culture confluence
Keratinocyte cultures grown in autocrine conditions were collected when still highly proliferating at subconfluence, when growth-arrested at confluence, or 4 days later at postconfluence in order to measure specific lysosomal activities of several acid hydrolases. The specific activities measured at confluence or postconfluence were compared with the respective specific activities obtained in subconfluent keratinocytes in order to determine ratios of activities in differentiating keratinocytes versus activities in non-differentiating cells (Table 1). The ratios for culture confluence indicate an increase in all lysosomal specific enzymatic activities in differentiating cells, except for cathepsin L, which is decreased. Interestingly, at postconfluence, no further increase of hydrolases activities is detected. Conversely, several enzymes rather show decreased specific activities. In particular, the cathepsins B, C and L exhibit relatively low specific activities in post-confluent keratinocytes (Table 1).
Culture confluence induces transient upregulation of Lamp-1 in epidermal keratinocytes
It has been demonstrated that cell confluence in autocrine cultures of keratinocytes controls the initiation of epidermal differentiation, including the expression of suprabasal K1 and K10 [35, 36, 43]. We tested both at protein and mRNA levels whether Lamp-1 expression varied when epidermal keratinocytes were grown to confluence and committed to differentiation. Indeed, Western blot (Fig. 2) reveals a transient upregulation of Lamp-1 when the keratinocyte cultures reach cell confluence. Four days later, post-confluent cells show a decreased expression of Lamp-1 when compared to confluent and subconfluent cells (Fig. 2). The apparent molecular weight of Lamp-1 on SDS-PAGE is reduced in the post-confluent keratinocytes, suggesting partial degradation, probably deglycosylation of the protein.
Confluence of cultured keratinocytes transiently upregulates Lamp-1 protein expression. Proteins from subconfluent (SC), confluent (C) and post-confluent (PC) cultures of keratinocytes were extracted and identical amounts of proteins (13 μg) were analyzed by Western blotting with an antibody to Lamp-1. Positions of molecular weight markers (expressed in kDa) are indicated on the left
At the mRNA level, culture confluence induces an elevated Lamp-1 mRNA expression, concomitant with the induction of the suprabasal K1 and K10, and with the increase in involucrin mRNA expression (Fig. 3). In post-confluent cultures, the amount of Lamp-1 mRNA is decreased (Fig. 3) in accordance with the detection of the protein (Fig. 2). This decrease in Lamp-1 expression at high cell density correlates with the suppressed expression of K1 and K10, whereas involucrin expression remains relatively high. This suggests that Lamp-1 expression can be modulated during the differentiation of epidermal keratinocytes and particularly upregulated in cultured keratinocytes at confluence.
Lamp-1 mRNA expression is transiently upregulated in confluent differentiating keratinocytes. Poly(A)RNA from subconfluent (SC), confluent (C) and post-confluent (PC) cultures of keratinocytes were extracted and analyzed by Northern blot hybridization. Two micrograms of each sample were used in each lane. Hybridization was performed with probes specific for Lamp-1, K14, K1, K10, involucrin and 36B4 transcripts. Ratios to 36B4 were determined by quantitative densitometry using the NIH image software program and normalized to levels in subconfluent (SC) cultures
This transient upregulation of Lamp-1 in the culture model seemed intriguing and we wondered whether there could be a particular reason for this regulation. Because of the possible interaction between Lamp-1 and galectins [41], we studied the expression of members of this family of animal lectins known to be expressed in the epidermis.
Cell density differentially regulates galectin expression in autocrine keratinocyte cultures
Northern blot analysis reveals that galectin-1 transcripts are only detected in subconfluent proliferating keratinocytes (Fig. 4). Indeed, following the onset of terminal differentiation at culture confluence, the gene expression of galectin-1 is apparently abrogated. Conversely, the gene expression of galectin-3 and galectin-7 is already detectable in subconfluent cultures of proliferating keratinocytes, but the expression of galectin-7 is notably upregulated as soon as keratinocytes are committed to differentiate at culture confluence (Fig. 4). Simultaneously, the galectin-3 expression is also regulated with epidermal differentiation and reaches its highest level in post-confluent cultures (Fig. 4), concomitantly with the expression of later markers of epidermal differentiation. These results show that galectins-1, -3 and -7 are expressed by epidermal keratinocytes and that the expression of their transcripts is regulated with the typical phenotypic variations demonstrated when the density of this cell type increases in culture.
Galectin-1, -3 and -7 mRNA expressions are differentially regulated in autocrine keratinocyte cultures. a Expression of galectins mRNA in keratinocytes cultured at different cell density. Keratinocytes were grown in autocrine conditions and poly(A)RNA were extracted from subconfluent, confluent and post-confluent cultures. Then poly(A)RNA were analyzed using Northern blotting and hybridization with probes specific to galectin-1, -3 or -7. Exposures of each hybridized membrane were performed with screens fitting the PhosphorImager apparatus (Perkin-Elmer). The duration of the exposure was identical for each culture condition. Hybridization with the 36B4 probe was done in order to estimate gel loading. b Densitometric analysis of galectins expression. The results are presented as relative expression levels of galectins-1, -3 and -7 calculated as ratios to the expression of the 36B4 house-keeping gene
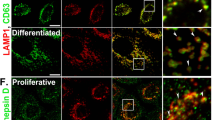
The localization of galectin-3 detected by immunolabelling and confocal microscopy suggests that galectin-3 is already present in subconfluent keratinocytes and remains well expressed in post-confluent cells in both basal or suprabasal position in the culture (Fig. 5). Galectin-3 is partly found in keratinocytes in a position, which may correspond to the nucleus, an observation compatible with the splicing activity of this protein [6] and with its normal pattern in cells able to differentiate [50]. Lamp-1 does not particularly co-localize with galectin-3 in any culture condition (Fig. 5).
Localization of galectin-3 and Lamp-1 proteins in autocrine cultures of keratinocytes. Keratinocytes of autocrine cultures were fixed when subconfluent, confluent, or post-confluent and analyzed after immunofluorescent labelling for galectin-3 (green) and Lamp-1 (red) using a Leica confocal microscope. In each culture condition, galectin-3 is distributed in the cytoplasm, and also in the nucleus, although Lamp-1 is found mainly in the cytoplasm, mostly with perinuclear localization. Galectin-3 and Lamp-1 are not predominantly co-localized in any culture condition
Discussion
In the present study, we show expression of Lamp-1 in the normal human epidermis and in normal cultured keratinocytes. In the epidermis, lysosomes of keratinocytes revealed by staining of Lamp-1 are mainly granules observed in the granular layer, maybe the lamellar granules involved in the keratinization and desquamation processes [14, 18]. Lysosomes in cultured keratinocytes seem abundant as soon as in subconfluent conditions, especially in comparison with cultured fibroblasts [16].
Looking at Lamp-1 during the differentiation of keratinocytes, we identify upregulation of Lamp-1 and upregulation of the specific activities of several lysosomal enzymes, particularly at the onset of differentiation concomitant with confluence of autocrine-cultured keratinocytes [35, 36]. Other studies of several cell types in culture have already suggested that lysosomes play a role in cells undergoing differentiation. For instance, in the intestinal cell lines HT-29 or Caco-2, the activity levels of lysosomal proteinases [9] and glycosidases [57] have been shown elevated in differentiating cells, compared with proliferating cells. Increased mRNA levels and enzymatic activities of certain lysosomal acid hydrolases were also observed in differentiated myocytes [44]. Only a few studies examined the activity of lysosomal hydrolases in human keratinocytes. Cathepsin B and L activities were found increased in differentiated rat-cultured keratinocytes [45]. Cathepsin L was shown to be involved in keratinocyte and melanocyte differentiation during mice hair follicle morphogenesis [46]. Our results reveal increased specific activities of lysosomal hydrolases upon differentiation. However, the decreased activities in post-confluent cultures could suggest that, for instance, some release of lysosomal proteins may occur after the triggering of differentiation. Such a release is proposed to contribute to desquamation at the surface of the epidermis [21]. Interestingly, we have recently demonstrated that such a lysosomal exocytosis can be induced by an intracellular elevation of calcium [23], a phenomenon accompanying the differentiation of epidermal cells and concomitant with a localization of Lamp-1 and -2 at the cell surface [23].
In the present study, the expression of Lamp-1 is transiently elevated at the onset of epidermal differentiation, when their clonogenic potential of keratinocytes is drastically suppressed [35, 54], and K1/K10 expression is upregulated [35]. However, the Lamp-1 expression is decreased 96 h later in post-confluent cultures containing keratinocytes expressing later markers of differentiation [36]. A similar concomitant elevation of Lamp-1 with cellular differentiation has been noticed in HC11 mammary epithelial cells [5]. Interestingly, only minor effects on Lamp-1 mRNA expression were observed in that study, suggesting a post-transcriptional control of Lamp-1 expression in HC11 cells [5]. A possible explanation for the discrepancy we noticed when comparing levels of Lamp-1 protein and transcript in our cultures of keratinocytes could be that Lamp-1 is partly degraded and deglycosylated in post-confluent keratinocytes. Indeed, Lamp-1 shows a reduced apparent molecular weight on Western blots in the post-confluent state (Fig. 3), a change also observed in differentiating Caco-2 intestinal cells [56]. Additionally, in both Caco-2 cells and keratinocytes, there is a clear increase in lysosomal enzyme activities with differentiation [57]. The specific activities of most lysosomal enzymes did not increase in post-confluent keratinocytes and those of cathepsins remained very low or even decreased in comparison with subconfluent cultures. Perhaps, it may be relevant to notice that endogenous inhibitors of cathepsins were reported as induced by differentiation in keratinocytes [45]. Moreover, the regulation of expression of a highly glycosylated Lamp-1 with epidermal differentiation is reminiscent of much older studies in which, by the use of labelled lectins, alterations in the carbohydrate content of differentiating keratinocytes had been reported [3, 4].
This regulation of Lamp-1 in keratinocytes focused our interest towards testing epidermal galectins in keratinocytes, knowing for instance that galectin-7 is found especially in the epidermis [29]. Our results indicate that galectin-7 and -3 are upregulated with keratinocyte differentiation in culture, simultaneously with Lamp-1. It has been suggested that changes in galectin-3 level might be associated with differentiation of monocytes into macrophages, and with the cellular response to altered environmental conditions [10]. Our previous studies indicate that Lamps on the surface of some tumour cells serve as ligands for galectin-3 [41]. Based on that finding, we propose that the concomitant increase of Lamp-1 and galectins-3 and -7 with keratinocyte confluence may reflect a possible involvement in cell–cell interactions and perhaps in the epidermal stratification. Although galectin-3 is considered as a multifunctional protein, it still retains its unique role as a mediator of cell-to-matrix adhesion [26]. Epithelial cells lacking galectin-3 interact poorly with their extracellular matrices [31]. On the other hand, galectin-3 binds to cytokeratins, which might serve as cytoplasmic anchors regulating the transport and function of galectins in MCF7 and other cells [17]. It is not excluded that in differentiating keratinocytes, galectin-3, which is involved in protein sorting [8], may interplay with Lamp-1, perhaps on the cell surface, in order to direct the secretion of lamellar bodies [40]. Recently, it was reported that cultured keratinocytes at confluence do not require complementary signals for the onset of terminal differentiation except those given by cell–cell contact [25], but the localization of galectin-3 in cellular structures different than the ones where Lamp-1 is found denies any special interaction between the two proteins in those culture conditions.
Our results also show that the expression of galectin-1 is sharply regulated by cell density in autocrine cultures of keratinocytes. Galectin-1 transcripts were indeed detected in subconfluent highly proliferating keratinocytes only. Galectin-1 might thus play a role during keratinocyte proliferation.
Finally, one may wonder why keratinocytes exhibit so many lysosomes in the proliferating state. One possibility is that cells committed to differentiate in the epidermis must degrade adhesion molecules of the integrin family found exclusively in the basal layer [19]. This degradation is performed by integrin internalization and processing in lysosomes [20].
In summary, our results reveal that lysosomes are regulated by differentiation in human keratinocytes, resulting in increased activity of lysosomal hydrolases and Lamp-1 expression in confluent cultures. Expressions of galectins-1, -3 and -7 are differentially regulated in the same conditions. However, although Lamp-1 might be implied in the normal epidermal differentiation in vitro, it does not co-localize with galectin-3.
References
Akimoto Y, Hirabayashi J, Kasai K, Hirano H (1995) Expression of the endogenous 14-kDa beta-galactoside-binding lectin galectin in normal human skin. Cell Tissue Res 280:1–10
Barret AJ, Kirschke H (1981) Cathepsin B, cathepsin H and cathepsin L. Methods Enzymol 80:535–561
Brown R, Wu WW, Bernstein IA (1987) Changes in lectin binding by differentiating cutaneous keratinocytes from the newborn rat. J Invest Dermatol 88:719–726
Brysk MM, Snider JM (1982) The effect of the state of differentiation on labeling of epidermal cell surface glycoproteins. J Invest Dermatol 78:366–370
Cella N, Cornejo-Uribe RR, Montes GS, Hynes NE, Chammas R (1996) The lysosomal-associated membrane protein Lamp-1 is a novel differentiation marker for HC11 mouse mammary epithelial cells. Differentiation 61:113–120
Dagher SF, Wang JL, Patterson RJ (1995) Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci USA 92:1213–1217
de Duve C, Pressman BC, Gianetto R, Wattiaux R, Appelmans F (1955) Tissue fractionation studies. 6. Intracellular distribution pattern of enzymes in rat liver tissue. Biochem J 60:604–617
Delacour D, Cramm-Behrens CI, Drobecq H, Le Bivic A, Naim HY, Jacob R (2006) Requirement for galectin-3 in apical protein sorting. Curr Biol 16:408–414
de Stefanis D, Demoz M, Dragonetti A, Houri JJ, Ogier-Denis E, Codogno P, Baccino FM, Isidoro C (1997) Differentiation-induced changes in the content, secretion, and subcellular distribution of lysosomal cathepsins in the human colon cancer HT-29 cell line. Cell Tissue Res 289:109–117
Dumic J, Lauc G, Hadzija M, Flogel M (2000) Transfer to in vitro conditions influences expression and intracellular distribution of galectin-3 in murine peritoneal macrophages. Z Naturforsch 55:261–266
Eckert RL, Green H (1986) Structure and evolution of the human involucrin gene. Cell 46:583–589
Eckert RL, Crish JF, Banks EB, Welter JF (1997) The epidermis: genes on—genes off. J Invest Dermatol 109:501–509
Esposito C, Caputo I (2005) Mammalian transglutaminases. Identification of substrates as a key to physiological function and physiopathological relevance. FEBS J 272:615–631
Freinkel RK, Traczyk TN (1985) Lipid composition and acid hydrolase content of lamellar granules of fetal rat epidermis. J Invest Dermatol 85:295–298
Fuchs E (1993) Epidermal differentiation and keratin gene expression. J Cell Sci Suppl 17:197–208
Glombitza GJ, Becker E, Kaiser HW, Sandhoff K (1997) Biosynthesis, processing, and intracellular transport of GM2 activator protein in human epidermal keratinocytes. J Biol Chem 272:5199–5207
Goletz S, Hanisch F-G, Karsten U (1997) Novel αGalNAc containing glycans on cytokeratins are recognized in vitro by galectins with type II carbohydrate recognition domains. J Cell Sci 110:1585–1596
Grayson S, Johnson-Winegar AG, Wintroub BU, Isseroff RR, Epstein EH Jr, Elias PM (1985) Lamellar body-enriched fractions from neonatal mice: preparative techniques and partial characterization. J Invest Dermatol 85:289–294
Hertle MD, Kubler MD, Leigh IM, Watt FM (1992) Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest 89:1892–1901
Hotchin NA, Gandarillas A, Watt FM (1995) Regulation of cell surface β1 integrin levels during keratinocyte terminal differentiation. J Cell Biol 128:1209–1219
Horikoshi T, Arany I, Rajaraman S, Chen SH, Brysk H, Lei G, Tyring SK, Brysk MM (1998) Isoforms of cathepsin D and human epidermal differentiation. Biochimie 80:605–612
Jadot M, Wattiaux-De Coninck S, Wattiaux R (1985) Effect on lysosomes of invertase endocytosed by rat liver. Eur J Biochem 151:485–488
Jans R, Sartor M, Jadot M, Poumay Y (2004) Calcium entry into keratinocytes induces exocytosis of lysosomes. Arch Dermatol Res 296:30–41
Kanan K, Stewart RM, Bounds W, Carlsson SR, Fukuda M, Betzing KW, Holcombe RF (1996) Lysosome-associated membrane proteins h-Lamp-1 (CD107a) and h-Lamp-2 (CD107b) are activation-dependent cell surface glycoproteins in human peripheral blood mononuclear cells, which mediate cell adhesion to vascular endothelium. Cell Immunol 171:10–19
Kolly C, Suter MM, Müller EJ (2005) Proliferation, cell cycle exit, and onset of terminal differentiation in cultured keratinocytes: pre-programmed pathways in control of c-Myc and Notch1 prevail over extracellular calcium signals. J Invest Dermatol 124:1014–1025
Krzeslak A, Lipinska A (2004) Galectin-3 as a multifunctional protein. Cell Mol Biol Lett 9:305–328
Laborda J (1991) 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res 19:3998
Madison KC, Sando GN, Howard EJ, True CA, Gilbert D, Swartzendruber DC, Wertz PW (1998) Lamellar granule biogenesis: a role for ceramide glucosyltransferase, lysosomal enzyme transport, and the Golgi. J Investig Dermatol Symp Proc 3:80–86
Magnaldo T, Bernerd F, Darmon M (1995) Galectin-7, a human 14-kD S-lectin, specifically expressed in keratinocytes and sensitive to retinoic acid. Dev Biol 168:259–271
Nangia-Makker P, Conklin J, Hogan V, Raz A (2002) Carbohydrate-binding proteins in cancer, and their ligands as therapeutic agents. Trends Mol Med 8:187–192
Ochieng J, Leite-Browning ML, Warfield P (1998) Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem Biophys Res Commun 246:788–791
Orlow SJ (1995) Melanosomes are specialized members of the lysosomal lineage of organelles. J Invest Dermatol 105:3–7
Perillo NL, Marcus ME, Baum LG (1998) Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med 76:402–412
Peters TJ, Müller M, de Duve C (1972) Lysosomes of the arterial wall. J Exp Med 136:1117–1139
Poumay Y, Pittelkow MR (1995) Cell density and culture factors regulate keratinocyte commitment to differentition and expression of suprabasal K1/K10 keratins. J Invest Dermatol 104:271–276
Poumay Y, Herphelin F, Smits P, De Potter I, Pittelkow MR (1999) High-cell-density phorbol ester and retinoic acid upregulate involucrin and downregulate suprabasal keratin 10 in autocrine cultures of human epidermal keratinocytes. Mol Cell Biol Res Commun 2:138–144
Rabinovich GA, Baum LG, Tinari N, Paganelli R, Natoli C, Liu FT, Iacobelli S (2002) Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol 23:313–320
Roop DR, Krieg TM, Mehrel T, Cheng CK, Yuspa SH (1988) Transcriptional control of high molecular weight keratin gene expression in multistage mouse skin carcinogenesis. Cancer Res 48:3245–3252
Saitoh O, Wang WC, Lottan R, Fukuda M (1992) Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem 267:5700–5711
Sando GN, Zhu H, Weis JM, Richman JT, Wertz PW, Madison KC (2003) Caveolin expression and localization in human keratinocytes suggest a role in lamellar granule biogenesis. J Invest Dermatol 120:531–541
Sarafian V, Jadot M, Foidart JM, Letesson JJ, van den Brule F, Castronovo V, Wattiaux R, Wattiaux-De Coninck S (1998) Expression of Lamp-1 and Lamp-2 and their interactions with galectin-3 in human tumor cells. Int J Cancer 75:105–111
Sawada R, Jardine KA, Fukuda M (1993) The genes of major lysosomal membrane glycoproteins, lamp-1 and lamp-2. Flanking sequence of lamp-2 gene and comparison of exon organization in two genes. J Biol Chem 268:9014–9022
Smits P, Poumay Y, Karperien M, Tylzanowski P, Wauters J, Huylebroeck D, Ponec M, Merregaert J (2000) Differentiation-dependent alternative splicing and expression of the extracellular matrix protein 1 gene in human keratinocytes. J Invest Dermatol 114:718–724
Szebenyi G, Rotwein P (1991) Differential regulation of mannose 6-phosphate receptors and their ligands during the myogenic development of C2 cells. J Biol Chem 266:5534–5539
Tanabe H, Kumagai N, Tsukahara T, Ishiura S, Kominami E, Nishina H, Sugita H (1991) Changes of lysosomal proteinase activities and their expression in rat cultured keratinocytes during differentiation. Biochim Biophys Acta 1094:281–287
Tobin DJ, Foitzik K, Reinheckel T, Mecklenburg L, Botchkarev VA, Peters C, Paus R (2002) The lysosomal protease cathepsin L is an important regulator of keratinocyte and melanocyte differentiation during hair follicle morphogenesis and cycling. Am J Pathol 160:1807–1821
Vaes G (1966) Subcellular localization of glycosidases in lysosomes. Methods Enzymol 8:509–514
van den Brûle FA, Buicu C, Baldet M, Sobel ME, Cooper DN, Marschal P, Castronovo V (1995) Galectin-1 modulates human melanoma cell adhesion to laminin. Biochem Biophys Res Commun 209:760–767
van den Brûle FA, Fernandez PL, Buicu C, Liu FT, Jackers P, Lambotte R, Castronovo V (1997) Differential expression of galectin-1 and galectin-3 during first trimester human embryogenesis. Dev Dyn 209:399–405
van den Brûle F, Waltregny DW, Liu FT, Castronovo V (2000) Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int J Cancer 89:361–367
van der Zee J, Dubbelman TMAR, Raap TK, Van Steveninck J (1987) Toxic effects of ozone on murine L929 fibroblasts. Biochem J 242:707–712
Watt FM (1989) Terminal differentiation of epidermal keratinocytes. Curr Opin Cell Biol 1:1107–1115
Welss T, Sun J, Irving JA, Blum R, Smith AI, Whisstock JC, Pike RN, von Mikecz A, Ruzicka T, Bird PI, Abts HF (2003) Hurpin is a selective inhibitor of lysosomal cathepsin L and protects keratinocytes from ultraviolet-induced apoptosis. Biochemistry 42:7381–7389
Wille JJ Jr, Pittelkow MR, Shipley GD, Scott RE (1984) Integrated control of growth and differentiation of normal human prokeratinocytes cultured in serum-free medium: clonal analyses, growth kinetics, and cell cycle studies. J Cell Physiol 121:31–44
Wollenberg A, de la Salle H, Hanau D, Liu FT, Bieber T (1993) Human keratinocytes release the endogenous beta-galactoside-binding soluble lectin immunoglobulin E (IgE-binding protein) which binds to Langerhans cells where it modulates their binding capacity for IgE glycoforms. J Exp Med 178:777–785
Youakim A, Romero PA, Yee K, Carlsson SR, Fukuda M, Herscovics A (1989) Decrease in polylactosaminoglycans associated with lysosomal membrane glycoproteins during differentiation of CaCo-2 human colonic adenocarcinoma cells. Cancer Res 49:6889–6895
Zhang Y, Wick DA, Haas AL, Seetharam B, Dahms NM (1995) Regulation of lysosomal and ubiquitin degradative pathways in differentiating human intestinal Caco-2 cells. Biochim Biophys Acta 1267:15–24
Acknowledgments
The authors are grateful to Prof. M. Jadot for his helpful ideas and critical remarks. The technical assistance provided by R. Deom, F. Dubois and F. Herphelin is gratefully acknowledged. We thank Dr B. Bienfait (Namur-Bouge) for providing skin specimens. Special thanks are addressed to Drs D.R. Roop (Houston), R.L. Eckert (Cleveland) and F. Van Den Brûle (Liège) for their gift of keratins, involucrin and galectins cDNA probes, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarafian, V., Jans, R. & Poumay, Y. Expression of lysosome-associated membrane protein 1 (Lamp-1) and galectins in human keratinocytes is regulated by differentiation. Arch Dermatol Res 298, 73–81 (2006). https://doi.org/10.1007/s00403-006-0662-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-006-0662-4