Abstract
Human dermal fibroblasts seeded in tense disc-shaped collagen gels organized gradually into a tissue composed of three distinct superimposed layers of cells: a basal layer of aligned myofibroblasts, a middle layer of unoriented fibroblasts, and an upper layer of myofibroblasts oriented orthogonally to the basal myofibroblasts. Confocal observation of α-smooth muscle actin (α-SMA) immunolabelling as a marker of myofibroblasts showed that the upper myofibroblasts disappeared during maturation of the lattices. Observation of morphological modifications of cells, typical chromatin condensation identified by Hoechst 33258 staining, and detection of ssDNA after formamide-induced denaturation suggested the involvement of apoptosis in myofibroblast disappearance. It is suggested that the model of disc-shaped stressed collagen lattice is thus able to mimic conditions in wound repair: on the one hand, wound contraction during which fibroblasts undergo mechanical stress and, on the other, apoptotic disappearance of cells at the end of tissue retraction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The three-dimensional collagen lattice in which fibroblasts are embedded within hydrated type I collagen was developed as an in vitro culture model considered as a dermal equivalent [4]. Collagen lattices have proven to be a valuable model for studying several cellular functions under conditions that resemble the in vivo situation and to test the effect of various drugs [1, 4, 8]. Two different collagen gel systems, contractile and anchored collagen lattices, have been developed and have shown considerable differences in their cell proliferation, gene expression, cell phenotype and mechanical features (for review, see reference 20). In contracting free-floating collagen lattices, the tension is distributed isotropically and the extracellular matrix remains mechanically relaxed during contraction of the gel which results in a decrease in surface and an increase in thickness [15]. There is a marked decline in cellular DNA synthesis and the fibroblasts become quiescent within 1–2 days [26, 33, 38]. During the final stage of contraction, fibroblasts and collagen fibres are oriented perpendicular to the surface of the lattice [28].
In disc-shaped anchored collagen gels, contraction is prevented by attachment of the matrix either to the bottom of the culture dish [3, 41] or to a ring lining the inner edge of the dish made of stainless steel wire [26], glass microfibre filter [28] or nylon filtration mesh [27, 29]. The initial surface is preserved and the dermal equivalent contracts only in thickness. Contraction becomes isometric as the matrix resists the pull of the cells. Fibroblasts in the mechanically loaded matrix resemble proliferative cells of granulation tissue during later stages of remodelling [20] and the isometric tension is equivalent to that found in skin wounds [13, 25]. The cells continue to synthesize DNA and several matrix molecules, including type I, III and VI collagens, fibronectin, elastin and actin, increase [26]. The developed load results in a polarized cell morphology and formation of prominent actin stress fibres [21, 41]. Histological and ultrastructural studies have demonstrated that fibroblasts spread with a bipolar morphology in stratified planes parallel to the surface of the gel [25, 28, 29, 31, 41].
In disc-shaped tense lattices, no clear orientation of the cells is seen, in contrast to rectangular tense lattices anchored at only the two opposite short sides, in which the elongated bipolar fibroblasts become oriented in the direction of the main vectorial force, on the long axis of the gel [11, 14]. Recently, in confocal microscopic studies [5] using α-smooth muscle actin (α-SMA) labelling as a marker of myofibroblasts [9], we have observed that, under our culture conditions, successive morphogenetic phases during disc-shaped tense lattice structure formation result in a tissue composed of three superimposed layers of cells. During the first 5 days of culture, fibroblasts organize gradually into a basal layer where they differentiate into myofibroblasts aligned unidirectionally. Then, spindle-shaped fibroblasts located on the upper surface of the lattice differentiate into myofibroblasts which become oriented orthogonally to the basal myofibroblasts in 11-day-old lattices. In the middle layer of the lattice, the spindle-shaped fibroblasts remain randomly arranged. This process resembles the orthogonal multilayering that occurs in fibroblast hyperconfluent cultures in the presence of accumulated collagen [16, 17].
The study reported here dealt with cellular changes during maturation of disc-shaped lattices maintained in culture for a longer period than 11 days, up to 26 days. Confocal microscopic observation of α-SMA labelling suggested that some cells disappeared. The question arose as to the process that was responsible for myofibroblast disappearance. As mechanically stressed collagen gels resemble wound granulation tissue during later stages of remodelling [20], we wanted to determine if apoptosis, that has been implicated in regression of myofibroblasts in granulation tissue in vivo [12], might also account for the disappearance of myofibroblasts in disc-shaped tense collagen lattices. We tested this hypothesis by means of morphological indications of apoptosis at the light microscopic level. Apoptosis is a controlled cell death process [42] involving specific distinct morphological, biochemical and molecular alterations including DNA fragmentation
Material and methods
Cells
Fibroblast cultures were obtained from cell outgrowth from explants of abdominal dermis collected during plastic surgery on five women (30–51 years of age). Fibroblast monolayer cultures were maintained using a routine technique in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Cergy-Pontoise, France) supplemented with 10% fetal calf serum (FCS, Gibco) and gentamicin (10 mg/ml). For seeding into collagen lattices, cells from subcultures 14–19 were used. As fibroblasts from different donors behaved in the same way, we report the morphological events in only one series of lattices populated with fibroblasts from a 30-year-old woman.
Preparation of disc-shaped tense collagen lattices
Just before reaching confluency, fibroblasts were detached from the culture flasks by trypsin-EDTA, collected by centrifugation for 10 min at 800 g and suspended in DMEM. Each lattice was prepared by mixing fibroblasts and a rat tail collagen type I solution (Bioetica Institut J. Boy, Reims, France) in a sterile Erlenmeyer flask at 4°C, so that the final collagen concentration was 0.6 mg/ml and the cell concentration was 8×104/ml. Each lattice was prepared by mixing, in the following order: 3 ml of 1.28× concentrated DMEM containing 0.5% NaHCO3, 0.083 N NaOH, 10 mg/ml gentamicin and 15% FCS, with 1.5 ml of a 2 mg/ml rat tail collagen type I solution in 0.1% acetic acid, and then with 0.5 ml of fibroblast suspension. The viscous mixture was then quickly poured into 50-mm bacteriological dishes (Falcon) containing a 7-mm O-ring of sterilized nylon filtration mesh (Scrynel, NY 150 HC; Polylabo, Strasbourg, France) with an inner diameter of 35 mm. After polymerization of the gel in an incubator at 37°C under an atmosphere comprising 95% air/5% CO2, 3 ml of culture medium were added to the culture dish and changed every 48 h. The disc-shaped lattice became attached to the ring of nylon mesh and the whole system was floating in the culture medium.
Confocal laser microscopic observations
The depth of the lattices varied from about 20 to 40 μm. They could therefore be examined easily as a series of horizontal optical sections to localize the cells containing α-SMA. As primary antibody we used a mouse monoclonal antibody against α-SMA (A 2547, clone A4; Sigma) diluted 1/100. Antigen-antibody complexes were visualized by a goat anti-mouse IgG antibody conjugated to fluorescein (F 2012, Sigma) diluted 1/40. The antibodies were diluted in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and 0.1% Triton X100. Two lattices per day of culture were fixed under tension in the culture dish in 3% paraformaldehyde (PFA) in PBS (pH 7.4) for 1 h. After rinsing with PBS, square pieces about 3×3 mm were punched out of the lattice in the area around the centre. The fibroblasts in the lattice were permeabilized by treatment with acetone for 5 min at −20°C followed by three changes of PBS. Nonspecific binding sites were blocked at room temperature by a 10-min incubation in 1% glycine in PBS followed by a 1-h incubation in 3% BSA solution in PBS containing 10% normal goat serum and 0.1% Triton X100.
The samples were then incubated overnight at room temperature in a moist chamber with primary antibody. As a control, a piece removed from the lattice was incubated in PBS instead of the primary antibody. Excess antibody was removed in three changes, each of 10 min, of PBS + 1% BSA + 0.1% Triton X100. The fluorescein-conjugated secondary antibody was applied for 1 h at room temperature in a moist chamber. After washing in three changes of PBS for a minimum of 1 h, the tissue was gently carried onto a histological slide and coverslipped without pressure with Vectashield mounting medium (Biosys, Compiegne, France). The coverglasses were sealed with nail polish for viewing with a laser scanning confocal Leica microscope (Centre de Microscopie, University of Besançon, France). The images were obtained in red artificial colour.
Morphological and histological assessment of fibroblast apoptosis
To identify the cellular changes characteristic of apoptosis, the collagen lattices were examined using an Olympus IX 5D inverted microscope. Cells showing morphological changes consistent with apoptosis were identified using criteria including cellular rounding, cytoplasmic condensation and budding of the plasma membrane [42]. Condensed and fragmented nuclei typical of apoptotic cells were identified by staining with bisbenzimide. Fragments removed from the central area of the fixed collagen lattices were incubated with 2.5 μg/ml Hoechst 33258 (Sigma) in PBS for 10 min in the dark at room temperature. After extensive washing in PBS, the lattice fragments were mounted on histological slides with Vectashield mounting medium and visualized microscopically under UV illumination. The blue fluorescent Hoechst 33258 stained the condensed chromatin of apoptotic cells more brightly than the less-dense chromatin of normal cells. Condensed and fragmented nuclei were counted per microscopic field (×40 objective) to calculate the mean number of apoptotic cells per 0.228 mm2 field. At least, ten contiguous fields per lattice were examined in two lattices per stage. Statistical analysis was performed using Student-Newman-Keul’s multiple comparisons test. Each bar represents the mean (±SD) apoptotic density per microscopic field in one lattice.
To further confirm the presence of apoptosis, we used an assay to determine ssDNA, which has been shown to be a specific method for detection of apoptotic cells [19]. The procedure was adapted from the protocol accompanying the antibody to ssDNA (Alexis Biochemicals). Briefly, fragments removed from PFA-fixed lattices were incubated in a methanol/PBS (6/1) solution for 30 min. They were then mounted on Superfrost/plus slides in a drop of distilled water and heated in an oven at 56–60°C for 1–2 h. They were stored dry at room temperature. Slides were incubated in 0.2 mg/ml saponin in PBS for 20 min, rinsed with PBS, incubated in 20 μg/ml proteinase K in PBS for 20 min, and rinsed with three changes of distilled water. The slides were then incubated for 20 min in 50% formamide (v/v, distilled water) preheated in a water bath to 56°C. The slides were transferred into ice-cold PBS for 5 min. Then 40 μl 3% nonfat dried milk in PBS was added to the lattice fragments for 30 min to block nonspecific antibody binding. After elimination of milk solution, anti-ssDNA monoclonal antibody (mouse IgM) diluted in 1% nonfat dried milk in PBS was applied for 30 min at room temperature and then rinsed with three changes of PBS. FITC-conjugated goat anti-mouse IgM diluted 1/100 was applied for 30 min. After rinsing with PBS, the coverslips were mounted with Vectashield.
Results
Confocal observations of α-SM actin staining in 11-day to 26-day cultures
In 11-day cultures, the structure of the lattice had attained a stable vertical stratification comprising:
-
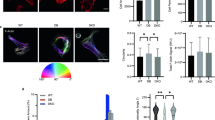
A basal cell layer composed of elongated unidirectionally oriented myofibroblasts accommodated in large winding parallel arrays (Fig. 1c1)
Fig. 1a–c Comparison of confocal observation of α-SMA staining in 11-day (a 1, b 1, c 1) and 26-day (a 2, b 2, c 2) cultured lattices. a 1, b 1, c 1 Three of 12 consecutive optical sections within a 29-μm Z-series starting at the upper surface from an 11-day cultured lattice. The distance between each image is 4.8 μm. At the base of the gel (c 1), closely packed fibroblasts are oriented in one direction whereas the cells located in the middle layer are unoriented (b 1). Elongated fibroblasts at the surface (a 1) of the gel are clearly oriented orthogonally to the basal layer. α-SMA staining is faint in the middle layer (b 1) as compared to cells in the basal (c 1) and upper (a 1) layers of the lattice. a 2, b 2, c 2 Three of 12 consecutive optical sections within a 30-μm Z-series starting at the upper surface from a 26-day cultured collagen lattice. The distance between each image is 7.5 μm. Orientation of elongated fibroblasts persists in the basal layer (c 2) of the lattice. Compared to a 1, b 1, c 1, the oriented upper α-SMA-stained fibroblasts have disappeared. Unoriented fibroblasts (a 2, b 2) remain in the middle layer where α-SMA staining appears less intense than in the basal fibroblasts (c 2)
-
A middle thick layer (about 12 μm) populated with filiform cells having few cell contacts and oriented in all directions (Fig. 1b1)
-
An upper cell layer whose morphology and myofibroblast differentiation were similar to those observed in the basal layer except that the orientation of the parallel arrays of cells was perpendicular to the orientation of cells in the basal layer (Fig. 1a1).
In 14-day-old lattices, the upper cells had a tendency to rounding and the α-SMA labelling intensity decreased (not shown). In 19-day and 26-day cultured lattices, staining of oriented upper cells was no longer observed (Fig. 1a2), whereas oriented myofibroblasts persisted in the basal layer (Fig. 1c2).
Morphological and histological assessment of apoptosis
Using an inverted microscope, we observed obvious morphological modifications consistent with apoptosis at the upper surface of lattices from the 14th day of culture. The upper oriented myofibroblasts showed loss of their elongated shape and cell-cell contacts. Cytoplasm contraction resulted in cell rounding. At 19 days of culture, numerous refringent globular bodies appeared at the upper surface of the lattices (Fig. 2). Then they floated in the culture medium. Their impermeability to trypan blue indicated that their plasma membrane remained intact as in apoptotic bodies. Globular bodies were not visible with confocal microscopy because they were not immunostained for α-SMA. Visualization of Hoechst 33258 fluorescence demonstrated typical chromatin condensation and margination in some cells (Fig. 3). ssDNA-immunopositive cells were found (Fig. 4) after formamide-induced DNA denaturation [19] confirming that fibroblasts underwent apoptosis in maturing mechanically stressed lattices. Quantitation of apoptosis (Fig. 5) showed that in cultures up to 10 days there were a very few positively stained apoptotic nuclei which coexisted with dividing nuclei. There was then a gradual increase in apoptotic cells until the highest density was evident in cultures at 26 days, at the end of the experiment. No significant difference in apoptotic density was observed until the 14th day in spite of a 5-fold increase in mean density (mean of two lattices per stage of culture) in 11-day and 14-day lattices compared with 8-day lattices and a 3.5-fold increase compared with 10-day lattices. In 19-day cultured lattices, there were significant increases in mean apoptotic density compared with 8-day and 10-day lattices of, respectively, 8.4-fold and 5.9-fold, but there were no significant differences compared with 11-day and 14-day lattices. In 26-day cultured lattices, the mean apoptotic density was significantly higher than in all younger lattices: 8 days (20-fold), 10 days (13-fold), 11 and 14 days (4-fold), 19 days (2.3-fold).
Graphical representation of apoptotic cell density during maturation of disc-shaped tense lattices. Hoechst-stained apoptotic nuclei were counted per microscopic field in two lattices per stage of culture. Student-Newman-Keul’s test indicated that the density of apoptotic cells increased significantly from the 19th day of culture. Each bar represented the mean (±SD) density in each lattice
Discussion
The observations demonstrate dynamic changes in cells that are contained within tethered fibroblast-populated collagen lattices. Human dermal fibroblasts in disc-shaped tense collagen lattices developed three distinct cell layers. Myofibroblasts were found in the top and bottom layer of the lattice, whereas fibroblasts in the middle layer were consistent with the non-aligned in vivo dermal fibroblasts [34]. As lattices matured the myofibroblasts disappeared and underwent apoptosis. It is proposed that fibroblasts under mechanical stress produce a highly oriented myofibroblast population which undergoes apoptosis.
Mechanical tension is crucial for differentiation and maintenance of the myofibroblast phenotype characterized by development of contractile features such as stress fibre formation and α-SMA expression in vivo [22] and in vitro in high or low lattice contraction [3, 20, 21, 41]. In disc-shaped collagen gels under high contraction, it is reasonable to suggest that the contractile activity of precocious differentiated myofibroblasts in the basal layer may participate in generating an isometric tension responsible for stretching the extracellular matrix. As it is known that an altered mechanical load on the matrix stimulates fibroblasts to adjust their contractile activity to maintain tensional homeostasis by reacting in the opposite direction to the perceived changes in mechanical loading [7], it can be suggested that the tension induced in the basal layer may be responsible for phenotypic modulation of fibroblasts into orthogonally oriented myofibroblasts in the upper layer of lattices. This could explain why the fibroblasts in the upper layer of disc-shaped lattices aligned orthogonally to the direction of cellular orientation in the lower layer. The reason why a layer of myofibroblasts appeared first at the basal surface and not at the upper one is not known.
The process of orthogonal layering of myofibroblasts is consistent with previous findings in other fibroblasts grown beyond confluence [2, 16], where the basal layer persists after destruction of upper cell layers [17]. Heterogeneity of the cellular phenotype has also been found by confocal microscopy in tense lattices obtained on the bottom of the culture dish by self-production of extracellular matrix [23]. In these lattices, elongated large polygonal fibroblasts in the basal layer are aligned unidirectionally whereas cells in the upper layer exhibit a spindle shape and are oriented in all directions.
Our observation in disc-shaped tense lattices of myofibroblast differentiation and disappearance suggest some analogies with in vivo evolution of granulation tissue. It is known that fibroblasts in the mechanically loaded matrix resemble proliferating cells of granulation tissue [20], where fibroblasts differentiated in oriented contractile myofibroblasts promote closure of the wound [39] by a coordinated cellular contraction [37] and disappear at the end of wound healing [9].
The disappearance of upper myofibroblasts in maturing disc-shaped lattices by apoptosis may be a process similar to that occurring in the in vivo decrease in tissue granulation cellularity including disappearance of typical myofibroblasts as the wound closes [9, 12]. Evidence that apoptosis is the mechanism responsible for downregulation of granulation tissue fibroblasts in rodents has been reported [9, 10, 12, 30]. In humans, the data are contradictory regarding the kind of scar. Apoptosis of myofibroblasts has been reported to occur from granulation tissue through hypertrophic scarring [24] whereas apoptosis does not appear to be a significant mechanism for fibroblasts downregulation in normally healing granulation tissue [35]. In disc-shaped tense collagen lattices, morphological assessment of apoptosis detected myofibroblast apoptosis after 10 days of culture. The gradual increase in apoptotic cells appeared to start after the disc-shaped collagen lattices had been submitted to mechanical forces by establishment of orthogonal layers of contractile myofibroblasts. This is in line with the gradual resorption of granulation tissue after wound closure at the end of wound contraction [12].
Up to 10 days of culture, the absence of apoptosis is in agreement with the findings of others in dermal equivalents anchored on the bottom of culture dish, under low lattice contraction conditions, in contrast to free contractile collagen gel in which fibroblast apoptosis has clearly been demonstrated [18]. In these mechanically relaxed matrices, disturbance by cytochalasin D of cytoskeletal integrity and thereby the cells’ ability to build up contraction forces abrogates apoptosis, suggesting a dependence of apoptosis on mechanical forces and/or cell shape. It has been suggested that α2-integrin is responsible for transducing an apoptosis-promoting signal [32]. In anchored lattices under low contraction condition, release of mechanical tension by removing matrices from the underlying culture dish triggers an apoptotic response which reaches a plateau by 24 h [21]. This appears to suggest that mechanical tension prevents fibroblast apoptosis.
Studies on the mechanism regulating apoptosis caused by release of mechanical tension have established that the apoptosis does not require differentiation of cells into myofibroblasts but is governed by a combination of mechanical tension and growth factors in the matrix [21]. In contrast to our observations, we note that apoptotic events were not investigated beyond 10 days of culture, either in contractile gels or in anchored gels under low contraction. Furthermore, contractile mechanically relaxed collagen gels and anchored lattices under low contraction are known as in vitro models of the very early stage of wound healing [3, 20, 32], whereas the high mechanically stressed disc-shaped collagen lattices tensed on a nylon ring resemble granulation tissue in the later stages of wound healing [20].
Our results show discrepancies with the observations reported by others using the same model of tense lattice for 14 days of culture [27]. The authors demonstrated a significant decrease in cell number, an increase in annexin V labelling which could imply apoptosis and alterations in the cytoplasm without the classical morphology of apoptosis such as chromatin condensation, nuclear fragmentation and apoptotic bodies [6]. They suggested that a new form of programmed cell death, paraptosis [40], is involved in disappearance of mechanically stressed fibroblasts. We have also observed such cytoplasmic alterations in ultrastructural studies of MRC5 fibroblast-populated lattices [29]. The absence of typical chromatin condensation might correlate with discrepancies compared with our conditions such as the width of the nylon ring which could affect the degree of stress, the heterogeneity of the fibroblast type and age, and the quality of the collagen.
Our observations in disc-shaped tense collagen lattices appear to be in line with the suggestion that the process of myofibroblast differentiation in granulation tissue ends with the death of these cells which could be considered terminally differentiated fibroblasts [12].
This study indicates that tense collagen lattices which retain the same diameter, and which are known to closely resemble the dermal wound in which fibroblasts organize repair against the mechanical resistance of the wound margins [36], appear to offer a valuable in vitro model to investigate factors involved in myofibroblast differentiation and disappearance that occur in wound healing.
References
Adams LW, Priestley GC (1988) Contraction of collagen lattices by skin fibroblasts: drug-induced changes. Arch Dermatol Res 280:114–118
Bard J, Hay E (1975) The behavior of fibroblasts from the developing avian cornea: morphology and movement in situ and in vitro. J Cell Biol 67:400–418
Baschong W, Sütterling R, Aebi U (1997) Punch wounded, fibroblast populated collagen matrices: a novel approach for studying cytoskeletal changes in three dimensions by confocal laser scanning microscopy. Eur J Cell Biol 72:189–201
Bell E, Ivarson B, Merill C (1979) Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A 76:1274–1278
Bride J, Lucarz-Bietry A, Viennet C, Humbert Ph (2002) Organisation spatiale des lattices de collagène tendus observés au microscope confocal. In: Actualités en ingénierie cutanée, vol 2. Eska, Paris, pp 229–240
Chamson A, Sudre F, Le Guen C, Lee J, Rattner A, Frey J (1997) Morphological alteration of fibroblasts mechanically stressed in a collagen lattice. Arch Dermatol Res 289:596–599
Brown RA, Prajapati R, MacGrouther DA, Yannas IV, Eastwood M (1998) Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J Cell Physiol 175:323–332
Coulomb B, Dubertret L, Bell E, Touraine R (1984) The contractility of fibroblasts in a collagen lattice is reduced by corticosteroids. J Invest Dermatol 82:341–344
Darby I, Skalli O, Gabbiani G (1990) Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 63:21–29
Darby I, Bisucci T, Hewitson T, MacLellan D (1997) Apoptosis is increased in a model of diabetes-impaired wound healing in genetically diabetic mice. Int J Biochem Cell Biol 29:191–200
Delvoye P, Wiliquet P, Levêque JL, Nusgens BV, Lapière CM (1991) Measurement of mechanical forces generated by skin fibroblasts embedded in a three-dimensional collagen gel. J Invest Dermatol 97:898–902
Desmoulière A, Redard M, Darby I, Gabbiani G (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146:156–166
Eastwood M, MacGrouther DA, Brown RA (1994) A culture force monitor for measurement of contraction forces generated in human dermal fibroblast culture: evidence for cell-matrix mechanical signalling. Biochim Biophys Acta 1201:186–192
Eastwood M, Mudera VC, MacGrouther DA, Brown RA (1998) Effect of precise mechanical loading on fibroblast populated collagen lattices: morphological changes. Cell Motil Cytoskeleton 40:13–21
Ehrlich HP, Rajaratnam JB (1990) Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue Cell 22:407–417
Elsdale T, Bard J (1972) Cellular interaction in mass cultures of human diploid fibroblasts. Nature 236:152–155
Elsdale T, Foley R (1969) Morphogenetic aspects of multilayering in Petri dish cultures of human fetal lung fibroblasts. J Cell Biol 41:298–311
Fluck J, Querfeld C, Cremer A, Niland S, Krieg T, Sollberg S (1998) Normal human primary fibroblasts undergo apoptosis in three-dimensional contractile collagen gels. J Invest Dermatol 110:153–157
Frankfurt OS, Krishan A (2001) Identification of apoptotic cells by formamide-induced DNA denaturation in condensed chromatin. J Histochem Cytochem 49:369–378
Grinnell F (1994) Fibroblasts, myofibroblasts and wound contraction. J Cell Biol 124:401–404
Grinnell F, Zhu M, Carlson MA, Abrams JM (1999) Release of mechanical tension triggers apoptosis of human fibroblasts in a model of regressing granulation tissue. Exp Cell Res 248:608–619
Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G (2001) Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 159:1009–1020
Ishikawa O, Kondo A, Okada K, Miyachi Y, Furumura M (1997) Morphological and biochemical analyses on fibroblasts and self-produced collagens in a novel three-dimensional culture. Br J Dermatol 136:6–11
Kischer CW (1992) The microvessels in hypertrophic scars, keloids and related lesions: a review. J Submicrosc Cytol Pathol 24:281–296
Kolodney MS, Wysolmerski RB (1992) Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J Cell Biol 117:73–82
Lambert CA, Soudant EP, Nusgens BV, Lapiere CM (1992) Pretranslational regulation of extracellular matrix macromolecules and collagenase expression in fibroblasts by mechanical forces. Lab Invest 66:444–451
Lee J, Rattner A, Chepda T, Frey J, Chamson A (2002) Production of matrix metalloproteinase 2 in fibroblast reaction to mechanical stress in a collagen gel. Arch Dermatol Res 294:405–410
Lopez-Valle CA, Auger FA, Rompré P, Bouvard V, Germain L (1992) Peripheral anchorage of dermal equivalents. Br J Dermatol 127:365–371
Lucarz-Biétry A, Chapuis JF, Agache P, Humbert P (1995) A histological and ultrastructural comparative study of retracted and tense collagen lattices. Eur J Dermatol 5:524–530
Nagata M, Takenaka H, Shibagaki R, Kishimoto S (1999) Apoptosis and P53 protein expression increase in the process of burn wound healing in guinea-pig skin. Br J Dermatol 140:829–836
Nakagawa S, Pawclek P, Grinnell F (1989) Extracellular matrix organization modulates fibroblast growth and growth factor responsiveness. Exp Cell Res 182:572–582
Niland S, Cremer A, Fluck J, Eble A, Krieg T, Sollberg S (2001) Contraction dependent apoptosis of normal dermal fibroblasts. J Invest Dermatol 116:686–692
Nishiyama T, Tsunenaga M, Nakayama E, Adachi E, Hayashi T (1989) Growth rate of human fibroblasts is repressed by the culture within reconstituted collagen matrix but not by the culture on the matrix. Matrix 9:193–199
Novotny GEK, Gnoth C (1971) Variability of fibroblast morphology in vivo: a silver impregnation study on human digital dermis and subcutis. J Anat 177:195–207
Olerud JE, Chiu DS, Usui ML, Gibran NS, Ansel JC (1998) Protein gene product 9.5 is expressed by fibroblasts in human cutaneous wounds. J Invest Dermatol 111:565–572
Porter RA, Brown RA, Eastwood MA, Occleston NL, Khaw PT (1998) Ultrastructural changes during contraction of collagen lattices by ocular fibroblasts. Wound Repair Regen 6:157–166
Ryan BR, Cliff WJ, Gabbiani G, Irle C, Montandon D, Statkov PR, Majno G (1974) Myofibroblasts in human granulation tissue. Hum Pathol 5:55–67
Sarber R, Hull B, Merrill C, Sorano T, Bell E (1981) Regulation of proliferation of fibroblasts of low and high population doubling levels grown in collagen lattices. Mech Ageing Dev 17:107–117
Skalli O, Gabbiani G (1988) The biology of the myofibroblast relationships to wound contraction and fibrocontractive diseases. In: Clark RAF, Henson PM (eds) The molecular and cellular biology of wound repair. Plenum Publishing, New York, pp 373–402
Sperandio S, de Belle I, Bredesen DE (2000) An alternative non apoptotic form of programmed cell death. Proc Natl Acad Sci U S A 97:14376–14381
Tomasek JJ, Haaksma CJ, Eddy RJ, Vaughan MB (1992) Fibroblast contraction occurs on release of tension in attached collagen lattices: dependency on an organized actin cytoskeleton and serum. Anat Rec 359:68
Willingham MC (1999) Cytochemical methods for the detection of apoptosis. J Histochem Cytochem 47:1101–1109
Acknowledgements
The authors thank the “Centre de Microscopie Electronique” of Besançon (France), Isabelle Bruey and Marie-Pierre Decour for their assistance in the organization of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bride, J., Viennet, C., Lucarz-Bietry, A. et al. Indication of fibroblast apoptosis during the maturation of disc-shaped mechanically stressed collagen lattices. Arch Dermatol Res 295, 312–317 (2004). https://doi.org/10.1007/s00403-003-0438-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-003-0438-z