Abstract
Introduction
This study compares survival and outcomes in four total knee arthroplasty (TKA) populations defined by baseline body mass index (BMI). We hypothesised that there would be no difference in survival between the groups.
Materials and methods
Using an initial cohort of 1059 TKAs, BMI was systematically measured prior to surgery. A retrospective study was conducted and patients were accordingly allocated to four groups: normal or underweight (BMI < 25; n = 111), overweight (25 ≤ BMI < 30; n = 417), moderately obese (30 ≤ BMI < 35; n = 330) and severely or morbidly obese (BMI ≥ 35; n = 201). The pre- and postoperative clinical and radiographical profiles of the four groups were compared, along with any postoperative complications and the survival of each group. The minimum follow-up was 24 months. All implants had an ultra-congruent cementless posterior-stabilised rotating-platform design (Amplitude®). The primary endpoint was implant survival using Kaplan–Meier analysis. Statistical analysis was conducted using Chi-squared and Kruskal–Wallis H tests to compare the data with p < 0.05.
Results
A total of 94 knees from normal weight or underweight individuals were analysed, 346 from overweight, 281 from moderately obese and 159 from severely or morbidly obese. All knees had been operated on between 2002 and 2011 with an average follow-up of 61.7 (12–146) months. A greater degree of obesity was significantly correlated with young age at intervention (p < 0.001), as well as with a low average preoperative maximum flexion angle (p < 0.001) and KSS (p < 0.001). Postoperatively, there were no significant differences between the groups in terms of patient satisfaction (p = 0.9) or mechanical axial deviation evaluated with whole-leg standing radiography (mFTA, p = 0.3; mFA, p = 0.1; mTA, p = 0.3). The greater the degree of obesity, the lower the average postoperative maximum flexion angle (p < 0.001), KSS knee score (p < 0.001) and function score (p = 0.005). There was no significant difference between the groups in terms of total rate of postoperative complications (p = 0.9) or implant revision (p = 0.9), or in terms of 10-year implant survival (p = 0.4).
Conclusions
Obesity does not affect mid-term implant survival, irrespective of BMI, but has a negative influence on functional outcomes and potential risk of postoperative complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the major risk factors of osteoarthritis is obesity, the incidence of which has been rising steadily among the general population in recent years. Performing a TKA in an obese patient appears to lead to greater postoperative complications (especially infections [1]), with poorer long-term clinical results than in patients with normal body weight [2,3,4].
There is also still some debate about the role of obesity as a risk factor for aseptic loosening of the tibial component, with different results reported in the literature [3,4,5]. In addition, the rational use of cement does not appear to alter these results [6, 7].
The controversial benefits of a mobile-bearing design for stress distribution and a theoretical reduction in aseptic loosening are therefore relevant for obese patients [8], in whom the mechanical stresses on the tibial component are considerably higher, although there is no objective published evidence of this [9, 10].
Using a mobile-bearing design with cementless implant could be an alternative to systematic cemented components in obese patients, to decrease the risk of aseptic loosening.
The aim of the present study was to conduct a retrospective comparison of implant survival and of the mid-term clinical and radiological outcomes in four TKA populations defined by the patient’s baseline body mass index (BMI), all taken from a single prospective cohort with a cementless posterior-stabilised rotating-platform design. The postoperative complications of these four groups were analysed and compared. We hypothesised that there would be no difference in implant survival between the groups.
Materials and methods
We conducted a retrospective study, from a prospective database of primary TKAs performed by two surgeons at two different centres (Polyclinique du Beaujolais and Polyclinique du Val de Saône), using the same surgical method, between 2002 and 2011. The patients underwent clinical and radiological follow-up at 2 months, 1 year and then every 2 years post-surgery. This initial cohort comprised 1059 primary TKAs.
All technical information from the procedure (surgical reports) as well as the pre- and postoperative clinical and radiological data was collated prospectively in an electronic database.
The data were collected using CliniRecord, which was approved by the CNIL (Commission Nationale de l’Informatique et des Libertés) in 2009 (No. 1355265), with permission to extend the data storage period granted in 2011, and was declared compliant with the CNIL’s reference methodology MR-003 in 2016 (No. 2007515).
Informed consent was obtained from all individual participants included in the study.
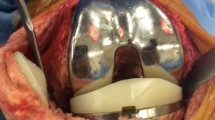
The surgical technique used a posterior reference with a first tibial cut and a second distal femoral cut. There was no patella resurfacing in 690 of the cases (62.4%). The implant used was the Score posterior-stabilised (ultra-congruent), rotating-platform implant from Amplitude®, with a cementless design and hydroxyapatite coating (Fig. 1).
BMI was routinely measured before surgery, using the patient’s weight (in kg) and height (in m). This figure was then used to define four preoperative groups:
-
Normal or underweight (BMI < 25; n = 111);
-
Overweight (25 ≤ BMI < 30; n = 417);
-
Moderately obese (30 ≤ BMI < 35; n = 330);
-
Severely or morbidly obese (BMI ≥ 35; n = 201).
The clinical follow-up used the Knee Society Score (KSS) questionnaire for calculating the KSSs at each visit [11]. The Knee Society radiology assessment comprised, for each visit, a frontal and lateral X-ray of the operated knee, a skyline projection of the patella with 45° knee flexion, and a whole-leg standing X-ray [12]. It permitted to calculate mechanical femorotibial angle (mFTA), mechanical femoral angle (mFA) and mechanical tibial angle (mTA). All local and general complications secondary to the arthroplasty were noted and recorded in the database, using the standardised list and definitions of the Knee Society [13].
Any knees for which the clinical and radiological follow-up was less than 24 months were excluded: lost to follow-up (n = 177 knees), deceased (n = 2 knees). A total of 880 knees were included in the postoperative analysis (94 normal or underweight, 346 overweight, 281 moderately obese and 159 severely or morbidly obese) (Fig. 2), with an average follow-up of 61.7 (24–146) months. Thirty-five patients (4% of the followed cohort) had 10 years of follow-up (4 normal or underweight, 15 overweight, 11 moderately obese and 5 severely or morbidly obese).
Statistical analysis
The data were analysed using a Chi-squared test for comparing distributions between the four groups, and a Kruskal–Wallis H test for quantitative data (large samples and variables following a normal distribution), using the software package R© (The R Project for Statistical Computing, http://www.R-project.org). Survival curves were produced using the Kaplan–Meier method and compared using a log-rank test. The results were considered statistically significant if p < 0.05.
Results
Preoperative data (Table 1)
There was no significant difference between the groups in terms of past medical history (meniscectomy, ligamentoplasty, osteotomy, anterior tibial tubercle (ATT) transfer, injections; p = 0.4), preoperative stage of osteoarthritis (p = 0.5), preoperative flexion (p = 0.7), mean KSS knee score (p = 0.06), mechanical axial deviation on the whole-leg X-ray (mFTA, p = 0.2; mFA, p = 0.06; mTA, p = 0.2), or preoperative patellar positioning in the frontal (p = 0.02) or sagittal (p = 0.9) planes.
There was a significant correlation between a greater degree of obesity and a younger age at intervention (p < 0.001). Likewise, the greater the degree of obesity, the lower the mean maximum flexion angle (p < 0.001) and KSS function score (p < 0.001).
Finally, a BMI < 25 was more commonly associated with lateral femorotibial erosion than the groups with BMI > 25 (p = 0.002).
Postoperative data (Table 2)
There were no significant differences between the groups in terms of average follow-up (p = 0.1), patient satisfaction (p = 0.1), KSS knee score improvement (p = 0.08) and function score improvement (p = 0.02) or mechanical axial deviation evaluated with whole-leg radiography (mFTA, p = 0.3; mFA, p = 0.1; mTA, p = 0.3).
The greater the degree of obesity, the lower the average maximum flexion angle (p < 0.001), KSS knee score (p < 0.001) and function score (p = 0.005).
Complications and survival (Table 3)
There was no significant difference between the groups in terms of total rate of postoperative complications (p = 0.9). However, the incidence of unexplained pain and patellofemoral complications (patellar instability, patellar fracture, or clunk syndrome) was significantly higher in the BMI ≥ 35 group, whereas the BMI < 35 groups experienced a significantly higher rate of stiffness (p = 0.02).
There was no significant difference between the groups in terms of implant revision comprising the replacement of one or more of the components (tibia, femur, patellar button, polyethylene) (p = 0.9).
There was no significant difference between the groups in terms of 10-year implant survival (the survival endpoint being the replacement of one or more components): 98 ± 3% for the group with BMI < 25, 96 ± 3% for the group with 25 ≤ BMI < 30, 99 ± 2% for the group with 30 ≤ BMI < 35 and 95 ± 7% for the group with BMI ≥ 35 (p = 0.4). Figure 3 shows the survival curves before revision surgery for each of the groups.
For the 35 patients with 10 years of follow-up, no component replacement was reported, with a survival rate of 100%.
Discussion
The main contribution of this study is evidence of equivalent survival of the same posterior-stabilised, cementless, rotating-platform implant in different patient populations with varying body weight (normal, overweight, moderately obese, morbidly obese), as defined using the WHO classification, taken from an initial uniform cohort.
Obesity has for a long time been a confirmed risk factor for osteoarthritis of the knee [14, 15]. Furthermore, Derman et al. [16] found that the increase in the number of TKAs in the USA was correlated to a BMI > 25 in the total population, much more so than hip replacement surgery. This means, as we have found, that the higher the BMI, the earlier the onset of osteoarthritis and the younger the patient at intervention.
In addition, obese patients with a high BMI experience greater osteoarthritis-related pain, meaning they need an implant sooner. It has also been observed that obesity negatively affects preoperative function of the knee, in terms of maximum flexion angle or clinical score. This has also been demonstrated by Marks [17], who found that obesity has a negative impact on both walking distance and pain.
Likewise, obesity appears to encourage medial erosion of the knee, thus not only aggravating any pre-existing varus but also the varus moment of these patients when walking [18].
Several authors have discovered the harmful effects of obesity on postoperative clinical results, with a fall in mid-term functional scores [3, 4]. However, according to Torres-Claramunt et al. [19] and Baker et al. [20], the gains in terms of functionality and quality of life are similar irrespective of the degree of obesity, with comparable levels of satisfaction among obese and non-obese patients.
Our results corroborate these findings, showing that mean KSSs and mean maximum flexion angle decreased with the degree of obesity, but that obesity did not influence postoperative patient satisfaction and mean KSSs improvement, which was the same between the groups.
Similar to Ayyar et al. [21], we found no link in our series between the global rate of postoperative complications (including infection, patellofemoral complications, fractures, unexplained pain and revisions) and the degree of obesity. Nevertheless, the more obese patients in our series were more vulnerable to sepsis, which supports the findings of Electricwala et al. [1] and Kerkhoffs et al. [22], as well as to patellofemoral complications (such as instability or clunk) and unexplained pain. On the other hand, they were less susceptible to postoperative stiffness in flexion (this is certainly linked to a functional requirement in terms of less flexion than in a non-obese patient).
Our findings for postoperative alignment, irrespective of the degree of obesity, can explain the absence of any effect of weight on aseptic loosening of the implants in our series. In contrast to Estes et al. [23], we found no correlation between weight and postoperative implant alignment in the follow-up whole-leg X-rays.
Like Ayyar et al. [21] and Bordini et al. [24], we found no negative effect of obesity on the rate of revision, all causes combined (septic or aseptic loosening, or other mechanical cause).
Finally, the 10-year survival results from our series were the same for all groups, this being the conclusion drawn only recently by Chen et al. [2] in a large population of over 7000 patients, as well as by Issa et al. [25] in 2013.
Many studies showed similar survival rate between cemented and cementless implants in uni knee arthroplasties [26] or TKA [6, 7] for general population. However, there is a lack of data on the effects of a cementless implant design on mid- and long-term survival in obese patients. Jackson et al. [27] are the only ones to have studied 10-year survival in a population of 535 cementless implants in obese and non-obese patients. They found no difference between the groups, and survival rates were comparable to the studies cited above, with values around 97%.
Moreover, cementless fixation could be enhanced using metaphyseal tibial sleeves [28] or hybrid fixation with polyaxial locking screws [29], showing excellent primary stability of implants in recent studies. Their rational use may additionally reduce the residual risk of loosening in cementless implants.
Despite regular contact, many patients were still lost to follow-up (17% of the initial series). This limitation may bias our results. In addition, the short average follow-up duration (5 years) remains low, introducing a positive bias for the survival analysis. Nevertheless, due to the large size of each group, the power of our study is satisfactory.
Our cohort constitutes the only analysis of survival among different groups of patients with increasing degrees of obesity within a single uniform population of cementless, rotating-platform, posterior-stabilised implants, and of the impact of obesity.
We have also shown that obesity does not negatively affect the survival of cementless rotating-platform implants, although it does remain a harmful risk factor in terms of functional score, albeit without affecting patient satisfaction or quality of life.
Conclusions
Obesity does not affect mid-term implant survival irrespective of BMI. In addition, a cementless implant is entirely suitable provided the orthogonal positioning of the tibial plateau is respected. Nevertheless, overweight patients remain at risk of additional complications, especially sepsis, and they must be advised to lose weight after the arthroplasty.
Abbreviations
- TKA:
-
Total knee arthroplasty
- BMI:
-
Body mass index
- KSS:
-
Knee Society Score
- ATT:
-
Anterior tibial tubercle
- mFTA:
-
Mechanical femorotibial angle
- mFA:
-
Mechanical femoral angle
- mTA:
-
Mechanical tibial angle
References
Electricwala AJ, Jethanandani RG, Narkbunnam R et al (2016) Elevated body mass index is associated with early total knee revision for infection. J Arthroplasty. doi:10.1016/j.arth.2016.05.071
Chen JY, Lo NN, Chong HC et al (2016) The influence of body mass index on functional outcome and quality of life after total knee arthroplasty. Bone Jt J 98-B:780–785. doi:10.1302/0301-620X.98B6.35709
Si H, Zeng Y, Shen B et al (2015) The influence of body mass index on the outcomes of primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 23:1824–1832. doi:10.1007/s00167-014-3301-1
Collins RA, Walmsley PJ, Amin AK et al (2012) Does obesity influence clinical outcome at nine years following total knee replacement? J Bone Joint Surg Br 94:1351–1355. doi:10.1302/0301-620X.94B10.28894
Abdel MP, Bonadurer GF, Jennings MT, Hanssen AD (2015) Increased aseptic tibial failures in patients with a BMI ≥ 35 and well-aligned total knee arthroplasties. J Arthroplasty 30:2181–2184. doi:10.1016/j.arth.2015.06.057
Bagsby DT, Issa K, Smith LS et al (2016) Cemented vs cementless total knee arthroplasty in morbidly obese patients. J Arthroplasty 31:1727–1731. doi:10.1016/j.arth.2016.01.025
Lizaur-Utrilla A, Miralles-Muñoz FA, Sanz-Reig J, Collados-Maestre I (2014) Cementless total knee arthroplasty in obese patients: a prospective matched study with follow-up of 5–10 years. J Arthroplasty 29:1192–1196. doi:10.1016/j.arth.2013.11.011
Huang Z, Ouyang G, Xiao L (2011) Rotating-platform knee arthroplasty: a review and update. Orthop Surg 3:224–228. doi:10.1111/j.1757-7861.2011.00156.x
Bo Z, Liao L, Zhao J et al (2014) Mobile bearing or fixed bearing? A meta-analysis of outcomes comparing mobile bearing and fixed bearing bilateral total knee replacements. Knee 21:374–381. doi:10.1016/j.knee.2013.10.002
van der Voort P, Pijls BG, Nouta KA et al (2013) A systematic review and meta-regression of mobile-bearing versus fixed-bearing total knee replacement in 41 studies. Bone Jt J 95-B:1209–1216. doi:10.1302/0301-620X.95B9.30386
Lingard EA, Katz JN, Wright RJ et al (2001) Validity and responsiveness of the Knee Society Clinical Rating System in comparison with the SF-36 and WOMAC. J Bone Joint Surg Am 83-A:1856–1864
Ewald FC (1989) The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop 248:9–12
Healy WL, Della Valle CJ, Iorio R et al (2013) Complications of total knee arthroplasty: standardized list and definitions of the Knee Society. Clin Orthop 471:215–220. doi:10.1007/s11999-012-2489-y
Coggon D, Reading I, Croft P et al (2001) Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord J Int Assoc Study Obes 25:622–627. doi:10.1038/sj.ijo.0801585
Hartz AJ, Fischer ME, Bril G et al (1986) The association of obesity with joint pain and osteoarthritis in the HANES data. J Chronic Dis 39:311–319
Derman PB, Fabricant PD, David G (2014) The role of overweight and obesity in relation to the more rapid growth of total knee arthroplasty volume compared with total hip arthroplasty volume. J Bone Joint Surg Am 96:922–928. doi:10.2106/JBJS.L.01731
Marks R (2007) Obesity profiles with knee osteoarthritis: correlation with pain, disability, disease progression. Obes Silver Spring Md 15:1867–1874. doi:10.1038/oby.2007.221
Sharma L, Lou C, Cahue S, Dunlop DD (2000) The mechanism of the effect of obesity in knee osteoarthritis: the mediating role of malalignment. Arthritis Rheum 43:568–575. doi:10.1002/1529-0131(200003)43:3<568:AID-ANR13>3.0.CO;2-E
Torres-Claramunt R, Hinarejos P, Leal-Blanquet J et al (2016) Does obesity influence on the functional outcomes of a total knee arthroplasty? Obes Surg. doi:10.1007/s11695-016-2233-x
Baker P, Petheram T, Jameson S et al (2012) The association between body mass index and the outcomes of total knee arthroplasty. J Bone Joint Surg Am 94:1501–1508. doi:10.2106/JBJS.K.01180
Ayyar V, Burnett R, Coutts FJ et al (2012) The influence of obesity on patient reported outcomes following total knee replacement. Arthritis 2012:185208. doi:10.1155/2012/185208
Kerkhoffs GMMJ, Servien E, Dunn W et al (2012) The influence of obesity on the complication rate and outcome of total knee arthroplasty: a meta-analysis and systematic literature review. J Bone Joint Surg Am 94:1839–1844. doi:10.2106/JBJS.K.00820
Estes CS, Schmidt KJ, McLemore R et al (2013) Effect of body mass index on limb alignment after total knee arthroplasty. J Arthroplasty 28:101–105. doi:10.1016/j.arth.2013.02.038
Bordini B, Stea S, Cremonini S et al (2009) Relationship between obesity and early failure of total knee prostheses. BMC Musculoskelet Disord 10:29. doi:10.1186/1471-2474-10-29
Issa K, Pivec R, Kapadia BH et al (2013) Does obesity affect the outcomes of primary total knee arthroplasty? J Knee Surg 26:89–94. doi:10.1055/s-0033-1341408
Panzram B, Bertlich I, Reiner T et al (2017) Cementless Oxford medial unicompartmental knee replacement: an independent series with a 5-year-follow-up. Arch Orthop Trauma Surg 137:1011–1017. doi:10.1007/s00402-017-2696-9
Jackson MP, Sexton SA, Walter WL et al (2009) The impact of obesity on the mid-term outcome of cementless total knee replacement. J Bone Joint Surg Br 91:1044–1048. doi:10.1302/0301-620X.91B8.22129
Gøttsche D, Lind T, Christiansen T, Schrøder HM (2016) Cementless metaphyseal sleeves without stem in revision total knee arthroplasty. Arch Orthop Trauma Surg 136:1761–1766. doi:10.1007/s00402-016-2583-9
Benzing C, Skwara A, Figiel J, Paletta J (2016) Initial stability of a new cementless fixation method of a tibial component with polyaxial locking screws: a biomechanical in vitro examination. Arch Orthop Trauma Surg 136:1309–1316. doi:10.1007/s00402-016-2517-6
Author information
Authors and Affiliations
Contributions
RG participated in the design of the study and performed the statistical analysis. TG collected data of the study. SD collected data of the study. SL conceived of the study, and participated in its design and coordination.
Corresponding author
Ethics declarations
Conflict of interest
RG: no conflict of interest. TG: royalties from Amplitude®. SD: royalties from Amplitude®. SL: fees from Smith & Nephew® and Medacta®, research funding from Tornier-Wright® and Amplitude®.
Ethical approval
The data were collected using CliniRecord, which was approved by the CNIL (Commission Nationale de l’Informatique et des Libertés) in 2009 (No. 1355265), with permission to extend the data storage period granted in 2011, and was declared compliant with the CNIL’s reference methodology MR-003 in 2016 (No. 2007515).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Gaillard, R., Gaillard, T., Denjean, S. et al. No influence of obesity on survival of cementless, posterior-stabilised, rotating-platform implants. Arch Orthop Trauma Surg 137, 1743–1750 (2017). https://doi.org/10.1007/s00402-017-2801-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-017-2801-0