Abstract
Introduction
Continuous femoral nerve blocks for total knee arthroplasty can cause motor weakness of the quadriceps muscle and thus prevent early mobilisation. Perioperative falls may result as an iatrogenic complication. In this randomised and blinded trial, we tested the hypothesis that a continuous adductor canal block is superior to continuous femoral nerve block regarding mobilisation (‘timed up-and-go’ test and other tests) after total knee arthroplasty under general anaesthesia.
Methods
In our study, we included patients scheduled for unilateral knee arthroplasty under general anaesthesia into a blinded and randomised trial. Patients were allocated to a continuous adductor canal block (CACB) or a continuous femoral nerve block (CFNB) for three postoperative days (POD 1–3); with a bolus of 15 ml ropivacaine 0.375 %, followed by continuous infusion of ropivacaine 0.2 % and patient-controlled bolus administration. Both groups received an additional continuous sciatic nerve block as well as a multimodal systemic analgesic treatment. The primary outcome parameter was mobilisation capability, assessed by ‘timed up-and-go’ (TUG) test. Analgesic quality, need for opioid rescue and local anaesthetic consumption were also assessed.
Results
Forty-two patients were included and analysed (21 patients per group). No significant difference was noted in respect to mobilisation at POD 3 (TUG [s]: CACB 45, CFNB 51). It is worth saying that pain scores (numeric rating scale, NRS) were similar in both groups at POD 3 {rest [median (interquartile range)]: CACB 0 (0–3), CFNB 1 (0–3); stress: CACB 4 (2–5), CFNB 3 (2–4)}.
Conclusions
Concerning the mobilisation capability, we did not actually observe a superior effect of CACB compared with CFNB technique in our patients following total knee arthroplasty. Moreover, no difference was observed concerning analgesia quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total knee arthroplasty (TKA) is associated with severe postoperative pain, which can delay mobilisation and may influence functional outcome due to delayed postoperative mobilisation and physiotherapeutic capability. For the last 15 years, continuous femoral nerve block techniques—often combined with sciatic nerve blocks [1]—have been regarded as the gold standard for postoperative pain treatment [2, 3]. However, block-related motor weakness can delay mobilisation and may be associated with falls [4, 5]. To avoid such motor weakness, pain quality and motor function during femoral nerve block have been studied [6–12]. Surrogate measures of quadriceps strength such as dynamometric tests have been used rather than clinical outcome factors such as ‘timed up-and-go’ (TUG) testing or cumulated ambulation score (CAS), the use of walking aids, or incidences of falls during hospitalisation.
Accordingly, we investigated the mobilisation of patients undergoing unilateral TKA and their postoperative pain treatment by continuous femoral nerve block (CFNB) or continuous adductor canal block (CACB). Furthermore, this is the first study to analyse CACB or CFNB in combination with an anterior sciatic nerve block catheter.
Our primary hypothesis is that CACB is superior to CFNB regarding mobilisation (TUG test and other tests) after TKA under general anaesthesia.
In addition, we suggest that postoperative pain scoring and analgesic consumption are comparable in both groups (secondary outcome parameters).
Methods
Patients and design
This blinded and randomised study was performed after approval of the local ethics committee (Ethical Commission, University Hospital Marburg, AZ 06/13; clinicaltrials.gov; study identifier NCT02125903). It was carried out in accordance with the principles of the Helsinki Declaration. Data are presented according to the CONSORT statement. The study was conducted at the Philipps-University Hospital in Marburg, Germany. Between May 2013 and November 2014, patients aged 50–80 years, scheduled for unilateral TKA (excluding revision surgery) were screened for enrolment (SD, DH). Exclusion criteria were ASA status (American Society of Anesthesiology) of four or higher, known allergy to amide local anaesthetics, inability to cooperate or to give informed consent, pre-existing neuropathies or inability to perform a TUG test. Additionally, patients with significant coagulopathy, severe rheumatic disease or Parkinson’s disease were excluded. Written informed consent was obtained from all subjects prior to enrolment.
Anaesthesia, surgery and perioperative analgesia
All patients received midazolam (3.75–7.5 mg orally) before transport to the operating room. Standard monitoring was applied and regional anaesthesia techniques were performed under mild analgosedation using fentanyl [0.05–0.1 mg intravenously (IV)]. All patients received an anterior sciatic nerve block (ASNB) catheter in combination with either CACB or CFNB guided by ultrasound. Thereafter, catheters were fixed using sutures and sterile draping. After induction of general anaesthesia according to the predefined local standard using fentanyl (2–4 µg/kg IV) and propofol (1.5–2.5 mg/kg IV) followed by a single dose of rocuronium (0.5–0.6 mg/kg IV), endotracheal intubation was performed. Balanced anaesthesia was maintained using sevoflurane (0.6–1.7 % end-tidal concentration) and fentanyl boluses. The EEG-guided anaesthesia depth monitoring (goal: bispectral index (BIS-Index) 40–60) was as previously described [13]. Standardised prophylaxis for prevention of postoperative nausea and vomiting (PONV) was applied using two of three different drugs with regard to pre-existing contraindications (dexamethasone 4–8 mg IV, granisetron 1 mg IV or droperidol 1.25 mg IV). Before emergence from anaesthesia, all patients received metamizole as an injectable non-opioid analgesic (10–15 mg/kg IV). Elective unilateral TKA was performed by two experienced surgeons using a standardised medial parapatellar approach, in a bloodless field using a femoral tourniquet according to the local standards.
Postoperative analgesia
Patients were monitored in the post-anaesthesia care unit (PACU) until their fast-track score for discharge capability reached 12 or 14 (of a maximum 14 points) [13, 14]. Piritramide (a synthetic opioid analgesic, 0.75× potency compared with morphine IV equivalent, bolus of 3.75 mg IV) was given as an opioid whenever the numeric rating scale (NRS) was higher than five. The nerve block catheters were connected to pumps (Ambit, Teleflex Medical, Kernen, Germany) prefilled with ropivacaine 0.2 %. A standard continuous flow rate of 6 ml/h and a bolus function of 6 ml (lockout time 30 min) was used. PCA (patient-controlled analgesia) flow rates were reduced whenever optimal pain treatment (NRS < 2) was achieved, in order to reduce side effects. The standardised oral analgesic regimen was ibuprofen 400–600 mg orally ×3. Opioids (oxycodone 10 mg orally or piritramide 3.75–7.5 mg IV) served as a rescue medication on the ward, whenever NRS at rest exceeded four. Alternative comparable drugs were allowed, e.g., acetaminophen in case of allergy to ibuprofen. Patients were visited twice daily by our local acute pain staff as well as by our study assistants for mobilisation testing and score evaluation.
Randomisation and blinding
Sealed envelope randomisation was used; the block-random allocation sequence was generated on http://www.sealedenvelope.com. The envelopes were opened in the induction room, but patients were not informed as to their group. Insertion sites were occluded using a sterile draping technique covering both possible catheter insertion sites to maintain double blinding. The PCA pumps for continuous sciatic nerve block catheters were labelled with ‘back side of knee’. The PCA pump for continuous femoral or saphenous nerve block catheter technique was labelled with ‘front side of knee’ to enable the patient to selectively choose the bolus function with regard to the respective painful part of the knee.
Staff (SD, DH) performing the mobilisation tests and documenting the data were also unaware of the randomisation.
Interventions
Catheters were inserted using a linear ultrasound probe (Sonosite S series, Sonosite, Bothell, Washington, USA). Catheters were placed using stimulation catheter sets (Stimucath, Teleflex Medical, Kernen, Germany) under an aseptic technique according to the local guidelines. CFNB catheters were inserted using an out-of-plane approach under a dual guidance technique (i.e., ultrasound for visualisation of needle tip position and nerve stimulation for verification of correct muscle twitch) above the bifurcation of the femoral artery. After identification of the femoral nerve, the needle was carefully positioned next to the nerve under the fascia and verified using a nerve stimulation technique (0.5–0.8 mA, 0.1 ms; HNS 12, B. Braun Medical, Melsungen, Germany). Afterwards, a stimulating catheter was inserted 3–5 cm over the needle under nerve stimulation. Nerve stimulation via the indwelling catheter was used to verify correct catheter positioning next to the nerve. For details, see Morin et al. [15] and Mariano et al. [16]. After removal of the needle, the catheter was fixed using suturing and sterile draping. The initial bolus volume of 15 ml of ropivacaine 0.375 % was injected via the catheter. The PCA pump filled with ropivacaine 0.2 % was connected afterwards with a continuous flow rate of 6 ml/h and a bolus function of 6 ml.
CACB catheterisation was performed using an in-plane approach under ultrasound guidance at the distal thigh. After identification of the adductor canal and the femoral artery posterior to the sartorius muscle, the needle was successfully inserted next to the saphenous nerve. The insertion point was 20 cm proximal to the cranial margin of the patella as measured by a ruler. After identification of the correct needle tip position, local anaesthetic (15 ml of ropivacaine 0.375 %) was injected and the catheter was subsequently inserted. All patients received an additional ASNB under dual guidance technique using an out-of-plane approach. After correct identification of the sciatic nerve using nerve stimulation (0.5–1.2 mA, 0.1 ms, with a foot flexion or a foot extension response), a stimulating catheter was inserted under continuous nerve stimulation. Initial bolus of 15 ml of ropivacaine 0.375 % was followed by a standardised PCA technique as mentioned above. All patients received daily physiotherapy starting at the first postoperative day (POD 1) with passive mobilisation of the leg and active mobilisation starting at POD 2.
Outcome parameters and assessment
Only two trained study assistants assessed patients’ baseline parameters and test results to reduce potential bias factors.
Mobilisation capability
The TUG test is an established mobilisation ability test measuring the time it takes a person to get up from a chair, walk 3 m, and return to the chair [17–19]. Patients in our study were not allowed to use any devices but walked arm in arm with one study assistant for safety. They were not allowed to put any weight on the assistant while walking, and were thus forced to achieve full weight-bearing on both feet. Measurements were repeated postoperatively at POD 1, 2 and 3.
In addition, we calculated the postoperative CAS [20, 21] for POD 1–3. In brief, the CAS is the result of a daily assessment of the patient’s ability to perform three basic functions of transfer and ambulation (ability to get in and out of a bed, sit-to-stand from a chair, and walk with an appropriate aid). There are three levels: unable to perform, perform aided and perform without additional aid. Thus, a maximum of 6 points can be reached for each assessment time point, to a maximum of 18 points. Subjective quadriceps muscle function was assessed by the study assistant at each time point according to Medical Research Council criteria on a 6-point scale from 0 (none) to 5 (normal strength) [22].
The mobilisation score (MoSc) was rated on a 6-point scale from 0 to 5 (0 = none, 1 = passive mobilisation, 2 = ability to sit in a chair, 3 = ability to stand, 4 = ability to walk with a walking frame, 5 = ability to walk with crutches or without device).
Assessment of pain, pain therapy and patient satisfaction
Pain at rest and stress (movement) in the knee was evaluated on a numeric rating scale (NRS rest/NRS stress, 0–10, 0 = no pain, 10 = worst imaginable pain) at each time point. To identify coexisting painful comorbidities, we evaluated an NRS body for pain in any other body part (rest and stress, 0–10). Overall patient satisfaction with postoperative therapy was evaluated using an NRS from 0 (completely unsatisfied) to 10 (totally satisfied). Local anaesthetic consumption was recorded including the bolus amounts for each specific nerve block catheter. Non-opioid analgesics (ibuprofen in gram per day, metamizole in gram per day) and opioid rescue therapy (yes/no) were recorded.
Sample size calculation
According to published literature, we assumed that patients with CFNB would perform the TUG test at POD 3 within 30 s compared with CACB patients performing it within 20 s; this would need 22 patients for each group (accepted Beta 0.90, Alpha 0.05, G*Power, Release 3.1, F. Faul, University of Düsseldorf, Germany [23]). Considering a potential dropout rate of 5 % we aimed to include 46 patients in this study.
Statistical analysis
Statistical analysis (as an intention-to-treat analysis) was performed using Excel for Mac Release 14 (Microsoft Corp., Redmond, VA, USA) and SPSS (SPSS Release 20.0, IBM, Armond, NY, USA). Data is presented as median (25th–75th interquartile range) for non-normally distributed data or mean (standard deviation) for normally distributed data. Pairwise comparisons were performed using Mann–Whitney, Chi square or t tests when appropriate. The significance level was set at p < 0.05.
Primary outcome parameters were mobilisation capability using TUG testing, CAS and MoSc. Secondary outcome parameters included analgesic quality using NRS at rest and stress, analgesic dosages, need for opioid therapy as well as patient satisfaction.
Results
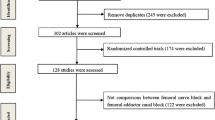
Forty-four patients, 22 in each group, were randomised (Fig. 1). Two patients, one in each group, were excluded due to non-performance of preoperative TUG tests. Intention-to-treat analysis was used. Protocol violations were observed in one patient in each group due to accidental catheter dislocation or leakage. In addition, five sciatic catheters dislocated or were removed before POD 3 (Table 1). Postoperatively, two patients of the CFNB group had falls during their hospital stay, not associated with study-related mobilisation testing. The demographic data revealed no differences between groups. The trial ended according to the predefined sample size.
TUG test
None of our patients were able to perform a TUG test at POD 1. Sixteen patients of the CACB group and 11 patients of the CFNB group were unable to perform a TUG test according to our criteria at POD 2. Twelve patients having had CACB and six who had CFNB were unable to perform the TUG test at POD 3 (Fig. 1); major reasons were dizziness and impaired cardiovascular status. No patient refused because of pain. In the remaining patients, there were no significant differences between groups in preoperative testing or at POD 3 (Table 2). Preoperative times were significantly shorter for POD 3 testing in both groups.
Other mobility data
CAS showed no significant differences between groups (11/18 vs 12/18 points, Table 2). Subjective quadriceps strength was not significantly different between groups at POD 0, POD 1, POD 2 and POD 3 (Table 2). Overall MoSc showed no differences between groups at any time (Table 2). Preoperatively, the patients were able to walk without any walking aids [MoSc 0 (0–0), median (25th–75th IQR)] whereas at POD 3 the median (25th–75th IQR) MoSc in both groups was 5 (4–5) (Table 2).
Patient falls (unrelated to study testing) occurred in two patients of the CFNB group whereas no falls occurred in the CACB group (p = 0.15).
Pain at rest and stress
There were no differences in preoperative baseline values for pain at rest and stress revealed between groups [NRS score 4 (2–5) vs 2 (0–3), CACB vs CFNB group, Fig. 2]. Postoperatively, the only differences were for NRS stress at POD 0 [NRS score 3 (1–5) vs 2 (0–3), p = 0.0037 for CACB vs CFNB group, Fig. 3] (Table 3).
Local anaesthetic consumption
There were no significant differences in local anaesthetic consumption between CACB and CFNB groups (465 vs 525 ml ropivacaine 0.2 %). The differences in bolus amounts at POD 3 were obviously significant (p = 0.004), but the total bolus amounts between CACB and CFNB catheter groups (48 vs 54 ml ropivacaine 0.2 %, Table 4) did not differ. Ropivacaine 0.2 % consumption of the sciatic nerve block catheters did not differ between groups.
Systemic analgesic therapy
Table 4 shows that there were no differences between groups in intraoperative fentanyl, the need for additional piritramide in PACU, postoperative consumption of ibuprofen and metamizole, or rescue opioid therapy.
Patient satisfaction
Overall patient satisfaction was similar in the two groups (Table 3).
Discussion
Our study of TKA found no difference in TUG test results between CACB and CFNB. The quality of analgesia, local anaesthetic consumption, the need for additional opioids and patient satisfaction were also similar, as has been shown recently elsewhere [8, 10–12, 24, 25]. Memtsoudis and colleagues applied both blocks as single-injection blocks [6], but also added continuous epidural anaesthesia to spinal anaesthesia. They found a similar analgesic quality, but the expected difference in motor function did not materialise. Obviously, the simultaneous epidural and spinal anaesthesia/analgesia affected the assessment of analgesic quality and motor function following the peripheral nerve blocks. Thus, improvements in analgesia or reduction of opioid consumption in their study should be discussed carefully. The same may be true of the study of Kim and co-workers [25], who also used single-injection nerve blocks prior to surgery; there was a significant difference in quadriceps strength in favour of adductor canal block at 6–8 h, but ambulation was not measured. The assessment of motor function in the first hours of the postoperative phase should be considered carefully since it has been shown that it can be decreased, regardless of the application of local anaesthetics to peripheral nerves [26, 27]. Grevstad and co-workers applied both peripheral blocks as single-injection blocks in patients with severe pain undergoing TKA (VAS ≥ 6) [24]. They evaluated analgesic quality and effects on muscle strength after 120 min, whereas our assessment was after three postoperative days. Jaeger’s group, using continuous catheter techniques for about 24 h [9] found similar analgesia quality as noted elsewhere, but no difference in mobilisation between the two blocks. The correct terminology of ‘adductor canal blocks’ or ‘saphenous nerve blocks’ is currently being discussed [28–30]. We chose a relatively distal injection to avoid cranial spread of local anaesthetics to the nerves innervating quadriceps muscles.
The present investigation has a number of limitations. First, the TUG test we used as the primary endpoint was inapplicable in about half of the patients postoperatively. Almost 30 % of patients were unable to perform a TUG test on POD 1 after TKA and about 9 % on POD 2 [21]; this study appeared after we had started ours. Grevstad et al., comparing single-injection adductor canal blockade and femoral nerve blocks, were unable to perform the TUG tests 120 min postoperatively in 28 % of patients with femoral nerve block alone [24]. This problem became obvious in our study at the third postoperative day (POD 3). Our subjects were required to walk arm in arm without any walking aid, and were thus fully bearing weight on both legs, whereas Grevstad’s used a high four-wheel walker with arm support [24]. Remarkably, the original description of the TUG test [17] asked for predefined walking aids (‘none, cane or walker’) whereas subsequent studies allowed several different walking aids for each patient such as a high walker with arm support [12], no predefined walking aids [13, 23], or ‘customary cane or walker’ [19]. It is thus difficult to compare TUG test results or the general capability to perform a TUG test between studies. Taken with the problems of performing tests shortly after a TKA, we believe that TUG is a poor primary outcome parameter for early postoperative endpoints. Potential bias factors that explain these problems with postoperative muscle weakness are the continuous sciatic nerve block. Use of a tourniquet during TKA is a separate risk factor for muscle weakness [27]. We therefore adopted the assessment of ambulation (MoSc, CAS) by considering other relevant criteria for discharge [17] such as transfer capability: e.g., to get in and out of a bed, transfer to a chair, to be able to walk alone or with crutches [21].
Second, the combination of femoral nerve and adductor canal blocks with a continuous sciatic nerve block may interfere with mobility and the evaluation of pain. However, this topic is poorly investigated. The published studies suggest a benefit of an additional single-injection sciatic nerve blockade compared to femoral nerve blockade alone for TKA [31, 32].
Nonetheless, our aim was to better discern the benefits of CACB and CFNB techniques, by excluding pain from dorsal areas of the thigh and the popliteal fossa, which are common after knee arthroplasty [33]. In patients undergoing TKA, pain control was better with combined femoral and sciatic catheters than a single femoral nerve block catheter. The analgesic effects of adductor canal or femoral nerve blocks can be more easily quantified when the pain bias of the knee is systematically avoided using a sciatic nerve block catheter.
Third, our study was conducted as a randomised and double-blinded study. Those who acquired the postoperative data did not participate in catheter insertion and had no access to the patients’ documentation pre- or postoperatively. Sterile draping was designed to conceal the identity of the catheter insertion site. However, as in any other studies, we cannot exclude the possibility that the insertion sites may have been accidentally identified in some patients.
Our study was planned without a control group with placebo infusion of local anaesthetics via the indwelling catheter or even without any peripheral nerve block. However, due to ethical reasons this was not allowed by our local ethical committee as peripheral nerve blocks are postoperative gold standards for pain treatment. This lack of a real control group with a multimodal systemic analgesic treatment is comparable to the majority of published studies investigating benefits of peripheral nerve blocks.
Our study showed no relevant differences in pain scores, consumption of local anaesthetics or need for opioid rescue. We did not expect to find any differences in pain scores or need for systemic opioid therapy. This may be explainable by comparable effectiveness of continuous nerve blocks and a multimodal systemic analgesic treatment including non-opioids (e.g., ibuprofen) as well as opioids during PACU stay or as a rescue medication for severe pain during ward stay. Therefore, secondary outcome parameters for pain scores should not be interpreted as a sign of comparable analgesic quality of both femoral and saphenous nerve blocks alone. Potentially, differences of analgesic effectiveness and resulting pain score levels might have been diminished by our systemic multimodal analgesic treatment. However, this represents common clinical practice and represents a strength of our study when transferring study results to daily practice.
Two patient falls occurred in the CFNB group but none occurred in the CACB group. This relevant aspect showed no statistical significance but might be significant in other studies with higher patient numbers. No causation can definitively be made regarding peripheral continuous nerve blocks, but some authors found a correlation of continuous nerve blocks and patient falls [4, 34]. However, peripheral regional anaesthesia is just one factor among others for postoperative quadriceps dysfunction. Several surgical factors (e.g., use of tourniquet) do also have relevant impact on muscle function [28–30.
Conclusions
We found no difference between CACB and CFNB as regards postoperative mobilisation in patients undergoing TKA, nor did we observe any difference in quality of analgesia. We conclude that, in the clinical setting, the motor-sparing effect of adductor canal block is less than has been assumed.
References
Ilfeld BM, Madison SJ (2011) The sciatic nerve and knee arthroplasty: to block, or not to block—that is the question. Reg Anesth Pain Med 36:421–423. doi:10.1097/AAP.0b013e31822940d2
Bauer MCR, Pogatzki-Zahn EM, Zahn PK (2014) Regional analgesia techniques for total knee replacement. Curr Opin Anaesthesiol 27:501–506. doi:10.1097/ACO.0000000000000115
Chan E-Y, Fransen M, Parker DA, Assam PN, Chua N (2014) Femoral nerve blocks for acute postoperative pain after knee replacement surgery. Cochrane Database Syst Rev 5:CD009941. doi:10.1002/14651858.CD009941.pub2
Wasserstein D, Farlinger C, Brull R, Mahomed N, Gandhi R (2013) Advanced age, obesity and continuous femoral nerve blockade are independent risk factors for inpatient falls after primary total knee arthroplasty. J Arthroplasty 28:1121–1124. doi:10.1016/j.arth.2012.08.018
Ilfeld BM (2011) Continuous peripheral nerve blocks: a review of the published evidence. Anesth Analg 113:904–925. doi:10.1213/ANE.0b013e3182285e01
Patterson ME, Bland KS, Thomas LC, Elliott CE, Soberon JR, Nossaman BD, Osteen K (2014) The adductor canal block provides effective analgesia similar to a femoral nerve block in patients undergoing total knee arthroplasty-a retrospective study. J Clin Anesth. doi:10.1016/j.jclinane.2014.08.005
Jenstrup MT, Jæger P, Lund J, Fomsgaard JS, Bache S, Mathiesen O, Larsen TK, Dahl JB (2012) Effects of adductor-canal-blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta Anaesthesiol Scand 56:357–364. doi:10.1111/j.1399-6576.2011.02621.x
Memtsoudis SG, Yoo D, Stundner O, Danninger T, Ma Y, Poultsides L, Kim D, Chisholm M, Jules-Elysee K, Valle AGD, Sculco TP (2014) Subsartorial adductor canal vs femoral nerve block for analgesia after total knee replacement. Int Orthop. doi:10.1007/s00264-014-2527-3
Hanson NA, Allen CJ, Hostetter LS, Nagy R, Derby RE, Slee AE, Arslan A, Auyong DB (2014) Continuous ultrasound-guided adductor canal block for total knee arthroplasty. Anesth Analg 118:1370–1377. doi:10.1213/ANE.0000000000000197
Grevstad U, Mathiesen O, Lind T, Dahl JB (2014) Effect of adductor canal block on pain in patients with severe pain after total knee arthroplasty: a randomized study with individual patient analysis. Br J Anaesth 112:912–919. doi:10.1093/bja/aet441
Jaeger P, Koscielniak-Nielsen ZJ, Schrøder HM, Mathiesen O, Henningsen MH, Lund J, Jenstrup MT, Dahl JB (2014) Adductor canal block for postoperative pain treatment after revision knee arthroplasty: a blinded, randomized, placebo-controlled study. PLoS One 9:e111951. doi:10.1371/journal.pone.0111951
Jaeger P, Zaric D, Fomsgaard JS, Hilsted KL, Bjerregaard J, Gyrn J, Mathiesen O, Larsen TK, Dahl JB (2013) Adductor canal block versus femoral nerve block for analgesia after total knee arthroplasty: a randomized, double-blind study. Reg Anesth Pain Med 38:526–532. doi:10.1097/AAP.0000000000000015
Wiesmann T, Steinfeldt T, Wagner G, Wulf H, Schmitt J, Zoremba M (2014) Supplemental single shot femoral nerve block for total hip arthroplasty—impact on early postoperative care, pain management and lung function. Minerva Anestesiol 80(1):48–57
White PF, Song D (1999) New Criteria for Fast-tracking after outpatient anesthesia: a comparison with the modified Aldrete’s scoring system. Anesth Analg 88(5):1069–1072
Morin AM, Eberhart LHJ, Behnke HKE, Wagner S, Koch T, Wolf U, Nau W, Kill C, Geldner G, Wulf H (2005) Does femoral nerve catheter placement with stimulating catheters improve effective placement? A randomized, controlled, and observer-blinded trial. Anesth Analg 100(5):1503–1510. doi:10.1213/01.ANE.0000151160.93288.0A
Mariano ER, Cheng GS, Choy LP, Loland VJ, Bellars RH, Sandhu NS, Bishop ML, Lee DK, Maldonado RC, Ilfeld BM (2009) Electrical stimulation versus ultrasound guidance for popliteal-sciatic perineural catheter insertion: a randomized controlled trial. Reg Anesth Pain Med 34:480–485. doi:10.1097/AAP.0b013e3181ada57a
Podsiadlo D, Richardson S (1991) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39:142–148
Yeung TSM, Wessel J, Stratford PW, MacDermid JC (2008) The timed up and go test for use on an inpatient orthopaedic rehabilitation ward. J Orthop Sports Phys Ther 38:410–417. doi:10.2519/jospt.2008.2657
Barry E, Galvin R, Keogh C, Horgan F, Fahey T (2014) Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta-analysis. BMC Geriatr 14:1–14. doi:10.1186/1471-2318-14-14
Foss NB, Kristensen MT, Kehlet H (2006) Prediction of postoperative morbidity, mortality and rehabilitation in hip fracture patients: the cumulated ambulation score. Clin Rehabil 20:701–708
Holm B, Kristensen MT, Myhrmann L, Husted H, Andersen LØ, Kristensen B, Kehlet H (2010) The role of pain for early rehabilitation in fast track total knee arthroplasty. Disabil Rehabil 32:300–306. doi:10.3109/09638280903095965
Paternostro-Sluga T, Grim-Stieger M, Posch M, Schuhfried O, Vacariu G, Mittermaier C, Bittner C, Fialka-Moser V (2008) Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med 40:665–671. doi:10.2340/16501977-0235
D’Alessio JG, Faul F, Rosenblum M, Erdfelder E, Shea KP, Lang A-G, Freitas DG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Meth 39:175–191
Grevstad U, Mathiesen O, Valentiner LS, Jaeger P, Hilsted KL, Dahl JB (2015) Effect of adductor canal block versus femoral nerve block on quadriceps strength, mobilization, and pain after total knee arthroplasty. Reg Anesth Pain Med 40:3–10. doi:10.1097/AAP.0000000000000169
Kim DH, Lin Y, Goytizolo EA, Kahn RL, Maalouf DB, Manohar A, Patt ML, Goon AK, Lee Y-Y, Ma Y, Yadeau JT (2014) Adductor canal block versus femoral nerve block for total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesiology 120:540–550. doi:10.1097/ALN.0000000000000119
Wulf H, Löwe J, Gnutzmann K-H, Steinfeldt T (2010) Femoral nerve block with ropivacaine or bupivacaine in day case anterior crucial ligament reconstruction. Acta Anaesthesiol Scand 54:414–420. doi:10.1111/j.1399-6576.2009.02200.x
Liu D, Graham D, Gillies K, Gillies RM (2014) Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res 26:207–213. doi:10.5792/ksrr.2014.26.4.207
Petterson SC, Barrance P, Marmon AR, Handling T, Buchanan TS, Snyder-Mackler L (2011) Time course of quad strength, area, and activation after knee arthroplasty and strength training. Med Sci Sports Exerc 43:225–231. doi:10.1249/MSS.0b013e3181eb639a
Mizner RL, Petterson SC, Snyder-Mackler L (2005) Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther 35:424–436. doi:10.2519/jospt.2005.35.7.424
Boezaart AP, Parvataneni HK (2014) Adductor canal block may just be an (unreliable) indirect femoral nerve block. Reg Anesth Pain Med 39:556. doi:10.1097/AAP.0000000000000137
Benthien JP, Huebner D (2015) Efficacy of continuous catheter analgesia of the sciatic nerve after total knee arthroplasty. Swiss Med Wkly 145:w14119. doi:10.4414/smw.2015.14119
Sato K, Adachi T, Shirai N, Naoi N (2014) Continuous versus single-injection sciatic nerve block added to continuous femoral nerve block for analgesia after total knee arthroplasty: a prospective, randomized, double-blind study. Reg Anesth Pain Med 39:225–229. doi:10.1097/AAP.0000000000000076
Wegener JT, van Ooij B, van Dijk CN, Hollmann MW, Preckel B, Stevens MF (2011) Value of single-injection or continuous sciatic nerve block in addition to a continuous femoral nerve block in patients undergoing total knee arthroplasty: a prospective, randomized, controlled trial. Reg Anesth Pain Med 36:481–488. doi:10.1097/AAP.0b013e318228c33a
Ilfeld BM, Duke KB, Donohue MC (2010) The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg 111:1552–1554. doi:10.1213/ANE.0b013e3181fb9507
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wiesmann, T., Piechowiak, K., Duderstadt, S. et al. Continuous adductor canal block versus continuous femoral nerve block after total knee arthroplasty for mobilisation capability and pain treatment: a randomised and blinded clinical trial. Arch Orthop Trauma Surg 136, 397–406 (2016). https://doi.org/10.1007/s00402-015-2403-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-015-2403-7