Abstract
Introduction
The goal for treating osteoarthritis (OA) is finding ways to decrease joint pain and dysfunction and prevent and slow the cartilage degeneration. Extracorporeal shock-wave therapy (ESWT) has been found to improve motor dysfunction and ameliorate pain with OA in animals. However, few studies have found that it can prevent and slow joint degeneration in vivo. The aim of study was to investigate the effect of ESWT on OA in rabbit.
Materials and methods
A total of 30 male New Zealand white rabbits were divided into 3 groups: control, OA induced by anterior cruciate ligament transaction (ACLT), and ALCT plus ESWT. The animals were killed at 4 and 8 weeks. Nitric oxide (NO) level was measured in the synovial cavity of knee joints, and cartilage sections were graded macroscopically by a Mankin scoring system. Chondrocyte apoptosis was investigated by flow cytometry and the expression of active caspase 3 by indirect immunohistochemistry.
Results
ESWT significantly reduced the NO level in the synovial cavity of knee joints (P < 0.05) and chondrocyte apoptosis (P < 0.05) of rabbits with OA. ESWT treatment significantly decreased the severity of cartilage lesions at both times as compared to rabbits with OA alone (P < 0.05).
Conclusion
ESWT reduced the progression of OA in rabbits. This effect may be related to decreased level of NO and is likely mediated by reduced chondrocyte apoptosis. ESWT may be a useful treatment for knee OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a chronic disease characterized by the progressive degeneration of cartilage, producing pain and loss of articular function in elderly people, with a prevalence of about 30 % in adults >60 years old [1]. The goal for treating OA is finding ways to decrease joint pain and dysfunction and prevent and slow the cartilage degeneration [2].
OA cartilage contains a high percentage of cells undergoing apoptosis than normal cartilage. Many recent studies indicated that chondrocyte apoptosis is related to the progression of OA [3]. Nitric oxide (NO) as an important inducer of apoptosis plays a considerable role in the pathogenetic mechanisms of articular diseases. It could be an important mediator of chronic articular lesions in OA. The inhibition of NO synthesis can slow cartilage degeneration [4].
Extracorporeal shock wave therapy (ESWT) was first used to break up kidney stones. Recently, this technique has been successfully used as anti-inflammatory therapy in orthopedic diseases such as pseudoarthrosis, calcifying tendinitis of the shoulder, epicondylitis, plantar fasciitis, and several inflammatory tendon diseases [5–8]. ESWT has been found to improve motor dysfunction and ameliorate pain with OA in animals [9–11]. Although ESWT has been found effective for OA in animals, few studies have found that it can prevent and slow joint degeneration in vivo.
We aimed to determine the effects of ESWT on chondrocyte apoptosis in an experimental rabbit model after 4 and 8 weeks of OA and to determine the production of NO in knee synovia. ESWT may have a protective effect on articular cartilage.
Materials and methods
Animal and surgical model
The research complied with national legislation and the US National Institutes of Health Guide for the Care and Use of Laboratory Animals and had local ethical committee approval. We used 30 male New Zealand white rabbits (8 months old; 2.5–3.5 kg; Vitalriver, China) randomly divided into 3 groups for treatment: control (n = 6), sham surgery without anterior cruciate ligament transaction (ACLT) and no ESWT; OA (n = 12), ACLT but no ESWT; and OA + ESWT (n = 12), ACLT and ESWT. All 3 animal groups were killed, half at 4 weeks and half at 8 weeks after surgery.
Osteoarthritis was induced in the left knees of rabbits by ACLT as described [12]. Briefly, rabbits were anesthetized by intramuscular administration of 50 mg/kg ketamine and 10 mg/kg xylazine. The skin was disinfected with iodine after shaving the knee joint. A parapatellar incision was made in the skin on the medial side of the left knee. The patella was dislocated laterally and the knee was placed in full flexion. Then the anterior cruciate ligament was transected with use of microdissecting scissors. Complete transection was confirmed by a manual anterior drawer test. After relocation of the patella, the joint capsule and subcutaneous tissue were closed with use of a 4/0 absorbable suture, and the skin was closed with use of a 2-0 silk surgical suture (Jiangsu, China). To prevent bacterial infection and pain, penicillin and fentanyl were given after surgery. In the control group, the knees of rabbits underwent arthrotomy, but the anterior cruciate ligament was not transacted. After surgery, rabbits were allowed to move freely in a normal cage.
ESWT
Shock waves were applied by use of an EMS instrument (Swiss DolorClast, Switzerland) beginning immediately after OA surgery and for 3 times in 1 week. Animals were anaesthetized by a intramuscular injection of ketamine (5 mg/kg) and butorphanol (0.1 mg/kg). All ESWT applications were performed under general anesthesia. Ultrasound lubricate gel was applied to the knee, and the head of the shock wave instrument was moved around the knee avoiding the vein and nerve. Each rabbit received 600 impulses of shock waves at 1.5 × 105 Pa to the left knee once. The optimal dose of shock waves was based on previous experience in animal experiments [13] and our previous experiments in vitro (unpublished data). Immediately after shock-wave application, the left knee was examined for swelling, ecchymosis or hematoma.
NO quantification
The synovial cavity of knee joints was washed with 0.4 ml phosphate-buffered saline (PBS) before rabbits were killed. The synovial exudates were collected by aspiration and centrifuged (500 g/10 min), then supernatants were stored at −20 °C. NO production was determined as total NO2 −/NO3 − levels by the Griess reaction [14]. Briefly, NO3 − was determined in synovial exudate supernatant (0.08 ml) and converted to NO2 − by incubation of 0.01 ml nitrate reductase from Aspergillus species (1 U/ml) and 0.01 ml NADPH (1 mM) for 30 min at 37 °C. NO2 − levels were determined spectrophotometrically at 540 nm by comparing the absorbance of 0.1 ml sample after adding 0.1 ml Griess reagent [sulfanilic acid (1 % w/v) and N-(1-naphythyl)ethylenediamine (0.1 % w/v) in 5 % phosphoric acid] to a NaNO2 (1–100 μM) standard.
Grading of lesions
Immediately after collection of synovial exudates, knees were dissected. Macroscopy grading of lesions in femoral condyles and tibial plateaus was as described [15] with use of the following scales: 0, normal-appearing surface; 1, minimal fibrillation or a slight yellowish discoloration of the surface; 2, erosion extending into superficial or middle layers; 3, erosion extending into the deep layers; and 4, erosion extending to the subchondral bone.
Histology
Isolated cartilage from femurs and tibias were fixed in 10 % neutral buffered formalin for 24 h, then decalcified for 6 weeks in an EDTA solution (Sigma) and embedded in paraffin. Serial sections (5 μm) were stained with hematoxlylin and eosin/Safranin-O. The severity of OA lesions was graded on a scale of 0–14 by the histology/histochemical scale of Mankin et al. [15]. This method evaluates the severity of erosion and/or fissures of cartilage, disorganization or loss of chondrocytes, and pannus formation. The scoring was based on the most severe histologic changes within each cartilage section.
Immunohistochemistry
Indirect immunohistochemical staining was used to detect the expression of active caspase 3. Cartilage samples were deparaffinized in toluene, hydrated in a series of graded dilutions of ethanol and blocked with goat serum (Zhongshan, China), diluted 1:5 with PBS, then incubated with the primary polyclonal rabbit antibody for caspase 3 (1:200 dilution, Sigma) for 1 h, then with biotin-labelled secondary goat anti-rabbit antibody (Zhongshan, China). The reaction was developed with use of diamino-benzidine tetrahydrochloride (DAB, Sigma). Hematoxylin (Sigma) was used for counterstaining. For a negative control, the primary antibody was omitted, with PBS incubation alone. Sections were photographed under a microscope (BX-51, Olympus, Japan). Proportion of positive staining for caspase 3 was calculated by counting the number of positively and negatively stained cells in each zone, with at least 300 cells per specimen.
Flow cytometry
Each sample of femurs and tibias of knees was sectioned at full thickness together. Cartilage digestion was performed in a sterile chamber at 37 °C for 12 h in Dulbeco’s modified Eagle’s medium (Sigma), containing hyaluronidase (0.5 mg/ml, Sigma) and II–type collagenase (1.5 mg/ml, Sigma), for 1 ml per sample. After digestion, cells were filtered through 0.1-mm nylon mesh and washed twice with PBS, then underwent flow cytometry with the use of a kit (BD PharMingen, CA, USA). The apoptotic percentage in 10,000 cells was determined. All experiments were performed three times.
Statistics
Results are expressed as mean ± SD. Differences among groups were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. P < 0.05 was considered statistically significant.
Results
We found no device-related problems or systemic or local complications.
NO production in joint exudates
NO production was higher in OA than control knees at 4 weeks, then decreased at 8 weeks but was still higher than in control knees (Fig. 1). NO production did not differ between OA + ESWT and control knees at both times (P > 0.05).
Gross morphology
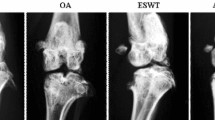
Cartilage from control group had a normal appearance. Minimal fibrillation was the greatest gross change at 4 weeks, but at 8 weeks, some knees showed fibrillation and erosion (Moran grades 3 and 4) (Fig. 2a). Cartilage lesions were less severe in OA + ESWT than OA-alone rabbits (Moran grade 3 vs. 4), with no significant differences between the 2 groups at 4 weeks (P > 0.05) but significant differences at 8 weeks (P < 0.01). Gross morphology revealed a significant therapeutic effect of ESWT at the later period.
Macroscopy and histomorphologic evaluation at 4 and 8 weeks. a Macroscopy grading of lesion changes to the femoral condyles and tibial plateaus in rabbits. b Cartilage sections were evaluated by a Mankin scoring system. Data are mean ± SD. ## P < 0.01, compared with control group. **P < 0.01, between ACLT + ESWT and ACLT group
OA histology
Grade of OA by Markin score was higher at every time in the OA and OA + ESWT than control groups (P < 0.01; Fig. 2b). The mean histologic grade of OA increased with increasing time. The histologic grade was 0.33 ± 0.52 in the control group and was 2.00 ± 0.63 after 4 weeks and 4.83 ± 1.17 after 8 weeks in the OA + EWST group as compared with 3.67 ± 0.52 after 4 weeks and 8.33 ± 0.82 after 8 weeks in the OA-alone group (all P < 0.01).
Immunohistochemistry of chondrocyte apoptosis
Percentage of apoptosis detected by caspase 3 immunohistochemistry differed between the OA + ESWT and OA-alone groups (P < 0.01) (Table 1). In the control group, only a small percentage of chondrocytes was located in each zone of cartilage that stained positive for caspase 3 (Fig. 3a). Significant differences existed between OA + ESWT and OA-alone groups at both times (p < 0.01), and chondrocytes were located almost exclusively in the superficial and upper intermediate layers (Fig. 3b–e). ESWT decreased the chondrocyte apoptosis especially at 4 weeks.
Chondrocyte apoptosis by immunohistochemical analysis of caspase 3 level (a–e) and Annexin V-FITC/PI flow cytometry (f–j). a–e Indirect immunohistochemical staining of caspase 3 activity. Positive (apoptotic) chondrocytes were stained brown, and negative chondrocytes were stained blue. Magnification ×200. f–j Flow cytometry of apoptosis rate of chondrocytes. A two-parameter cytogram of log (FL1) (Annexin V-FITC) versus log (FL2) (PI) was plotted
Flow cytometry of chondrocyte apoptosis
Apoptosis was greater in treated than untreated samples at both times (Fig. 3f–j), and apoptosis was significantly lower with OA + ESWT than OA alone (P < 0.01). ESWT could decrease the apoptosis rate in chondrocytes as compared with OA alone (P < 0.01) (Table 1).
Discussion
We tested whether ESWT protected against cartilage degradation in experimental OA in rabbits. ESWT could inhibit the production of NO in knee synovia and reduce chondrocyte apoptosis, which suggests that the therapy may have disease-modifying activity in OA.
Many elderly people have chronic pain while walking and functional limitation in normal life due to OA. The goal of OA treatment is to relieve pain and improve function limitation. The initial treatment of knee OA should be conservative before surgery is considered. Veterinarians first began to use ESWT to treat OA in horses [9]. Recently, the therapy was reported to have positive effects for pain relief and regressing OA development of different joints in animals [9–11]. ESWT seemed to be a valuable adjunct for management of equine OA [10]. In a rat OA knee model, ESWT improved rat walking ability and reduced CGRP-positive neurons in dorsal root ganglia [11]. The authors recommended ESWT for knee OA before surgical treatment. The degree of lameness in horses improved significantly with ESWT, although a disease-modifying effect of ESWT was not detected [9].
Although ESWT can relieve OA symptoms and improve function, the mechanism of action was not well demonstrated. We showed that ESWT reduced the histologic grading of cartilage lesions in rabbits with OA. Although the macroscopy grading of cartilage lesions was not significantly improved with ESWT, the reduced histology grading represents a structure-modifying effect. This finding agrees with that of Wang et al., who found that application of ESWT to the subchondral bone of the medial tibia condyle resulted in regressed OA of rat knees with changes in bone density, bone strength, biomarkers of the bone, and cartilage remodeling.
ESWT did not cause damage to chondrocytes or joint cartilage in in vivo studies [16, 17]. With ESWT to joint cartilage in immature New Zealand White rabbits, macroscopy, radiology and histology at 0, 3, 12 and 24 weeks revealed no pathological changes in joint cartilage [16]. Long-term survival of human chondrocytes in fluid suspension or viscous alginate substance was not affected 3 weeks after ESWT [17]. Chondrocyte DNA was not affected even at high-energy levels. The authors assumed that side effects of ESWL would not occur in a clinical setting. Moreover, radial shock waves do not seem to structurally damage articular cartilage in vitro [18]. In addition, our study did not reveal obvious side effects of ESWT on joints. Therefore, side effects of ESWT may be rare, but caution should be exercised when extremely high energy is used [19]. ESWT may be a viable option for OA before surgical treatment. However, before ESWT can be used as therapy for OA in the clinic, the effects of ESWT in a larger OA animal model should be researched. Moreover, we need to verify the optimal dosage and frequency of ESWT we used in cells and animals to determine the safety of ESWT and the best treatment strategy. In addition, the major clinical guidelines for management of OA should involve a combination of nonpharmacologic and pharmacologic therapies because a single therapy may not be adequate. Whether ESWT combined with pharmacologic therapies has better effect on OA needs further study.
The exact action mechanism of shock waves on regressing knee OA remains relatively unknown. However, a recent study showed that ESWT to the subchondral bone of the medial tibia condyle produced OA regression in rat knee. Local ESWT application affects the entire knee joint [20]. In addition, several studies reported that ESWT acts through a possible anti-inflammatory mechanism by downregulating NF-kappaB activation and NF-kappaB-dependent gene expression [21–23]. With ESWT, intracellular levels of TNF-alpha and IL-10 were downregulated in chondrocytes, which might restore TNF-alpha and IL-10 production by OA chondrocytes to normal levels [23]. Our results demonstrated that NO production with ESWT was lower than that of the control with both short- and long-term OA. ESWT may reduce the production of NO in OA. NO plays a catabolic role in the development of OA and mediates the inflammatory response; it is involved in the degradation of matrix metalloproteinases, inhibits the synthesis of both collagen and proteoglycans, and helps to mediate apoptosis [24]. Moreover, NO is involved in perception and reduction of pain, which could be a target for the management of pain in OA [25]. Therefore, reduced NO production by ESWT could be a factor explaining reduced pain in OA and improved function.
Osteoarthritis cartilage contains a higher percentage of cells undergoing apoptosis than normal cartilage. Recent studies have demonstrated increased cell apoptosis as a feature of OA cartilage in humans and in animal models. The aim is to inhibit chondrocyte apoptosis as potential therapy in the prevention and the initiation of OA changes [26]. We used flow cytometry and caspase 3 immunohistochemistry and revealed that regardless of the time examined, apoptosis was significantly lower in OA knees with than without ESWT. However, the rate of chondrocyte apoptosis detected by caspase-3 immunohistochemistry was higher than that detected by flow cytometry. The specificity of binding may be questionable with anti-rabbit antibodies against polyclonal rabbit active caspase-3. The results obtained by the flow cytometry method may be more reliable. However, distribution of chondrocyte apoptosis can be observed by caspase 3 immunohistochemistry. Collectively, these observations support that ESWT is associated with reduced chondrocyte apoptosis in OA-affected cartilage.
In summary, we show that ESWT reduces the progression of experimental OA in rabbit. This effect could be related to decreased level of NO and is likely mediated by reduced level of chondrocyte apoptosis. ESWT may be a useful treatment for knee OA, which can represent a viable option in the treatment of OA before surgery. Nonetheless, the optimal dosage and frequency of ESWT and optimal stage of OA for treatment need to be determined, as does whether ESWT combined with pharmacologic therapies has better effect on OA.
References
Shirai T, Kobayashi M, Nishitani K, Satake T, Kuroki H, Nakagawa Y, Nakamura T (2011) Chondroprotective effect of alendronate in a rabbit model of osteoarthritis. J Orthop Res 29(10):1572–1577. doi:10.1002/jor.21394
Hawker GA, Mian S, Bednis K, Stanaitis I (2011) Osteoarthritis year 2010 in review: non-pharmacologic therapy. Osteoarthr Cartil 19(4):366–374. doi:10.1016/j.joca.2011.01.021
Kim HA, Blanco FJ (2007) Cell death and apoptosis in osteoarthritic cartilage. Curr Drug Targets 8(2):333–345
van der Kraan PM, van den Berg WB (2012) Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthr Cartil 20(3):223–232. doi:10.1016/j.joca.2011.12.003
Endres S, Weiskirch M, Hinz C, Hutter F, Wilke A (2008) Extracorporeal shock-wave therapy in the treatment of pseudoarthrosis: a case report. Cases J 1(1):276. doi:10.1186/1757-1626-1-276
Albert JD, Meadeb J, Guggenbuhl P, Marin F, Benkalfate T, Thomazeau H, Chales G (2007) High-energy extracorporeal shock-wave therapy for calcifying tendinitis of the rotator cuff: a randomised trial. J Bone Joint Surg Br 89(3):335–341. doi:10.1302/0301-620X.89B3.18249
Staples MP, Forbes A, Ptasznik R, Gordon J, Buchbinder R (2008) A randomized controlled trial of extracorporeal shock wave therapy for lateral epicondylitis (tennis elbow). J Rheumatol 35(10):2038–2046
Metzner G, Dohnalek C, Aigner E (2010) High-energy Extracorporeal Shock-Wave Therapy (ESWT) for the treatment of chronic plantar fasciitis. Foot Ankle Int 31(9):790–796. doi:10.3113/FAI.2010.0790
Frisbie DD, Kawcak CE, McIlwraith CW (2009) Evaluation of the effect of extracorporeal shock wave treatment on experimentally induced osteoarthritis in middle carpal joints of horses. Am J Vet Res 70(4):449–454. doi:10.2460/ajvr.70.4.449
Revenaugh MS (2005) Extracorporeal shock wave therapy for treatment of osteoarthritis in the horse: clinical applications. Vet Clin North Am Equine Pract 21(3):609–625. doi:10.1016/j.cveq.2005.09.001
Ochiai N, Ohtori S, Sasho T, Nakagawa K, Takahashi K, Takahashi N, Murata R, Moriya H, Wada Y, Saisu T (2007) Extracorporeal shock wave therapy improves motor dysfunction and pain originating from knee osteoarthritis in rats. Osteoarthr Cartil 15(9):1093–1096. doi:10.1016/j.joca.2007.03.011
Li X, Li J, Cheng K, Lin Q, Wang D, Zhang H, An H, Gao M, Chen A (2011) Effect of low-intensity pulsed ultrasound on MMP-13 and MAPKs signaling pathway in rabbit knee osteoarthritis. Cell Biochem Biophys 61(2):427–434. doi:10.1007/s12013-011-9206-4
Wang FS, Yang KD, Kuo YR, Wang CJ, Sheen-Chen SM, Huang HC, Chen YJ (2003) Temporal and spatial expression of bone morphogenetic proteins in extracorporeal shock wave-promoted healing of segmental defect. Bone 32(4):387–396 pii: S8756328203000292
Iwase Y, Kato J, Ohtaguro K (1989) Clinical experiences of Medstone 1050 ST on extracorporeal shock wave lithotripsy. Nihon Rinsho 47(12):2777–2780
Yoshioka M, Coutts RD, Amiel D, Hacker SA (1996) Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthr Cartil 4(2):87–98 pii: S1063-4584(05)80318-8
Vaterlein N, Lussenhop S, Hahn M, Delling G, Meiss AL (2000) The effect of extracorporeal shock waves on joint cartilage—an in vivo study in rabbits. Arch Orthop Trauma Surg 120(7–8):403–406
Renz H, Rupp S (2009) Effects of shock waves on chondrocytes and their relevance in clinical practice. Arch Orthop Trauma Surg 129(5):641–647. doi:10.1007/s00402-008-0668-9
Benson BM, Byron CR, Pondenis H, Stewart AA (2007) The effects of radial shock waves on the metabolism of equine cartilage explants in vitro. N Z Vet J 55(1):40–44. doi:10.1080/00480169.2007.36733
Mayer-Wagner S, Ernst J, Maier M, Chiquet M, Joos H, Muller PE, Jansson V, Sievers B, Hausdorf J (2010) The effect of high-energy extracorporeal shock waves on hyaline cartilage of adult rats in vivo. J Orthop Res 28(8):1050–1056. doi:10.1002/jor.21074
Wang CJ, Weng LH, Ko JY, Wang JW, Chen JM, Sun YC, Yang YJ (2011) Extracorporeal shockwave shows regression of osteoarthritis of the knee in rats. J Surg Res 171(2):601–608. doi:10.1016/j.jss.2010.06.042
Ciampa AR, de Prati AC, Amelio E, Cavalieri E, Persichini T, Colasanti M, Musci G, Marlinghaus E, Suzuki H, Mariotto S (2005) Nitric oxide mediates anti-inflammatory action of extracorporeal shock waves. FEBS Lett 579(30):6839–6845. doi:10.1016/j.febslet.2005.11.023
Mariotto S, Cavalieri E, Amelio E, Ciampa AR, de Prati AC, Marlinghaus E, Russo S, Suzuki H (2005) Extracorporeal shock waves: from lithotripsy to anti-inflammatory action by NO production. Nitric Oxide 12(2):89–96. doi:10.1016/j.niox.2004.12.005
Moretti B, Iannone F, Notarnicola A, Lapadula G, Moretti L, Patella V, Garofalo R (2008) Extracorporeal shock waves down-regulate the expression of interleukin-10 and tumor necrosis factor-alpha in osteoarthritic chondrocytes. BMC Musculoskelet Disord 9:16. doi:10.1186/1471-2474-9-16
Abramson SB (2008) Nitric oxide in inflammation and pain associated with osteoarthritis. Arthr Res Ther 10(Suppl 2):S2. doi:10.1186/ar2463
Hancock CM, Riegger-Krugh C (2008) Modulation of pain in osteoarthritis: the role of nitric oxide. Clin J Pain 24(4):353–365. doi:10.1097/AJP.0b013e31815e5418
Li D, Wu Z, Duan Y, Hao D, Zhang X, Luo H, Chen B, Qiu G (2011) TNFalpha-mediated apoptosis in human osteoarthritic chondrocytes sensitized by PI3K-NF-kappaB inhibitor, not mTOR inhibitor. Rheumatol Int. doi:10.1007/s00296-011-1929-4
Acknowledgments
Project was supported by National Natural Science Foundation of China (No 31172169).
Author information
Authors and Affiliations
Corresponding author
Additional information
Z. Zhao and H. Ji contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhao, Z., Ji, H., Jing, R. et al. Extracorporeal shock-wave therapy reduces progression of knee osteoarthritis in rabbits by reducing nitric oxide level and chondrocyte apoptosis. Arch Orthop Trauma Surg 132, 1547–1553 (2012). https://doi.org/10.1007/s00402-012-1586-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-012-1586-4