Abstract
Introduction
Core decompression is the standard surgical procedure in the treatment of early stage non-traumatic osteonecrosis of the femoral head (ONFH). However, there is still a debate whether decompression in combination with supplementary augmentation by bone grafts, growth factors, or cell implementation is superior to conventional decompression alone. This study evaluated patients after core decompression combined with an augmentation by a demineralised bone matrix, and particularly aimed to report long-term conversion rates to total hip replacement (THR).
Materials and methods
14 patients with 18 hips suffering from ONFH (Ficat stage I-IIB) underwent this surgical procedure. All patients underwent radiographic and MRI investigations at baseline and at follow-up periods of 12 and 24 months. The clinical follow-up was done using the Merle d’Aubigné-score for an average period of 9 years after surgery.
Results
14 of the 18 subjects (77 %) achieved at least a good clinical result after 2 years. The Merle d’Aubigné-score improved significantly after 12 (p = 0.0001) and 24 months (p = 0.0002). However, the MRI volumetric analysis showed an increased necrotic bone volume from 3.16 ± 0.54 to 3.88 ± 0.62 cm3 (p = 0.04). Within 9 years, 13 out of 18 cases (72 %) required further surgery by THR. Only 7 out of 18 subjects (39 %) reported an ongoing postoperative clinical benefit, and would retrospectively redo the same surgical approach again. The five patients that did not require THR were still satisfied after 9 years.
Conclusions
In patients with early- stage femoral head osteonecrosis core decompression combined with the implantation of a demineralised bone matrix leads to a limited, temporary pain relief as seen in core decompression alone. However, long-term results were not encouraging with a high rate of conversion to arthroplasty. Therefore, core decompression with implantation of a demineralised bone matrix may be not appropriate to avoid THR in the long term.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteonecrosis of the femur head (ONFH) is a progressive disease of the proximal femur that unfortunately results frequently in a partial or complete collapse of the femoral head (>90 %), with a concomitant hip osteoarthritis [1]. ONFH is not uncommon, and yearly about 10,000–20,000 adults in the US suffer from this often debilitating disease [2]. Typically, younger male adults between the ages of 35–45 years develop an ONFH, and a considerable proportion of patients suffer from a bilateral hip involvement [3].
The aetiology of ONFH is multifactorial but still unclear in details. It is important to differentiate between posttraumatic and non-traumatic types of ONFH. Traumatic ONFH is caused by an acute interruption of the femoral head blood perfusion—typically after intracapsular femoral head and neck fractures. The non-traumatic, idiopathic ONFH relies on unclear pathophysiologic factors. Clinical observations have established several risk factors for idiopathic ONFH, including renal insufficiency, alcohol or steroid abuse, vascular deficiencies, fat embolisms, or coagulopathies [4]. The most accepted classifications of ONFH include the radiographic classification of Ficat [5, 6] and the MRI-based ARCO classification (Association Research Circulation Osseous) [7, 8].
The clinical picture of ONFH is variable, and it should be noted that not all patients with non-traumatic ONFH develop rapid clinical symptoms. In particular, early stages of ONFH (Ficat I) may be painless, but unfortunately patients often develop a painful limitation of active and passive hip movement, eventually leading to an inability of pain-free walking. This emphasizes the need for an early diagnosis of ONFH, for example by MRIs, which are further used for therapy monitoring. In this context, MRIs have proven a high accuracy in several studies [9]. In addition to the detailed medical history and a physical examination, the diagnostic workup includes plain X-rays, and in doubtful cases a Technetium 99 m diphosphonate bone scanning [10], or computed tomography (CT) [9]. By MRIs, a considerable amount with bilateral hip involvement can be identified, and still there is a controversy whether asymptomatic hips with diagnosed ONFH should be treated or not [11].
The treatment of painful non-traumatic ONFH includes various non-operative and operative procedures, including recent innovative therapeutic approaches such as the application of mesenchymal and bone marrow mononuclear stem cells (MSCs or BMCs), or growth factors (for example bone morphogenetic proteins) [12–15]. Such approaches are typically combined with the classical decompression surgery. This procedure has been introduced by Ficat and Arlet [6] and relies on the assumption that an increased intra-medullary pressure is involved in the pathogenesis of ONFH. The original operative procedure uses a 8–10 mm cannula which is inserted in the osteonecrotic lesion under fluoroscopic guidance [16]. Alternatively, a procedure with multiple smaller drillings has been introduced recently, which results in fewer complications such as femoral fractures [17]. Other surgical strategies include bone grafting (autologous bone grafts from iliac crest or tibia, allograft cancellous grafts, non-vascularized cortical grafts, or tantalum implants), or intertrochanteric de-rotating or flexion/extension osteotomies [18]. None of these surgical options was found to be superior to any other treatment, as determine by randomized studies, and some of these surgical procedures are technically demanding. Non-operative treatment options include a temporary partial weight bearing and medical therapies with lipid-lowering agents (statins), vasodilators, anticoagulants, bisphosphonates, hyperbaric oxygen therapy, or biophysical modalities, such as pulsed electromagnetic field stimulation [18].
Here we report long-term results in patients with painful non-traumatic ONFH. All patients presented with persisting pain at a relatively early stage oft the diseases (Ficat I–II). All patients were surgically treated by decompression in combination with implantation of a demineralised bone matrix. Patients were followed for an average of 9 years after surgery, and clinical outcome was compared with historic data from the literature. One specific aim of this study was to find out whether a conversion to total hip replacement (THR) can be avoided in the long term.
Materials and methods
Study design and inclusion criteria
Patient data were consecutively collected from patients with avascular osteonecrosis of the femoral neck. ONFH was diagnosed according to the radiographic criteria by Ficat [5]. In details, patients were included according to the following criteria:
-
No history of trauma, no malignancies
-
Radiographic criteria of Ficat stage I–II without collapse of the femoral head
-
Persisting hip pain for at least 9 months without significant improvement after conservative treatment
-
Informed consent for this study
-
Patient age below 55 years.
The exclusion criteria for this study were as follows:
-
Traumatic intracapsular hip fracture in patient history
-
Radiographic criteria of Ficat stage III–IV with collapsed femoral head
-
Inability to walk.
Study population
From 1/2001 through 2/2003, all consecutive patients with ONFH were included in case of fulfilling the study inclusion criteria. No patients with posttraumatic osteonecrosis were included. Each patient failed a conservative therapy (physiotherapy, pain medication). Patient demographics were summarized in Table 1.
Surgical and postoperative procedure
A standard lateral approach to the proximal femur was done, extending from the greater trochanter along the femoral shaft. By intraoperative fluoroscopy the ONFH area was identified. A k-wire was drilled along the femoral neck axis towards the ONFH area until reaching the subchondral lamella of the femoral head. Again, fluoroscopy was used to verify correct placement of the k-wire within the necrosis area. Then two different sized cannulated drillers (4.5–6 mm) were inserted via the k-wire, to obtain a canal of 6 mm in diameter. The k-wire was removed. 3 ml of demineralised bone matrix was injected through the canal according to the manufacture’s protocol. Before wound closure, a careful washing of the soft tissue was performed to avoid extensive postoperative ossifications. Postoperative antiphlogistic medication was mandatory.
Postoperatively, patients were allowed partial weight bearing with approximately 15 kg for 6 weeks. After hospital discharge around postoperative day 4–6, outpatient physiotherapy was continued throughout several months. After 6 weeks, the partial weight bearing was continuously increased to achieve full body weight within 12 postoperative weeks. Postoperative pain medicine included diclofenac or ibuprofen.
Grafton® demineralised bone matrix
The Grafton® product line (Netherlands Bone bank Foundation, Leiden, Netherlands) consists of a flowable gel form, a pliable form with dimensional integrity, and a putty form that maintains cohesiveness [19]. Here the flowable form of the demineralised allograft bone was chosen to allow easy injection through the decompression canal. The application of this osteoconductive device was done according to the manufacture’s advice. The study was performed in compliance with the local ethics committee, and Grafton® was used as an approved drug. Patients underwent no additional exposure to radiation (X-rays, CT scans) as compared to the routine patient follow-up in other patients with ONFH.
Radiological and clinical outcome parameters and follow-up
Within the first postoperative year, patients underwent both clinical and radiographic follow-up examinations after 6, 12, and 26 weeks. Afterwards, patients were re-evaluated after 1, 1.5, 2, and 9 years to obtain mid- and long-term results. In details, the following radiographic follow-up was performed.
Radiographic follow-up
-
X-rays: axial view and anterior-posterior view of the pelvis (6, 12, 26 weeks, and 1, 1.5, 2 years after surgery)
-
MRI (6, 12, 26 weeks, and 1, 1.5, 2 years after surgery).
MRIs were done with a 1.0 Tesla device (Philips Einthoven, Netherlands, 1.0 Tesla). T1 (with or without contrast agent; 0.1 mmol/kg body weight; Gadolinium+-DPTA, Magnevist ®, Schering, Berlin, Germany) and T2-weighted images were done.
The volumetric analysis of the necrotic bone area was determined by measuring the necrotic area (cm2) per MRI slide. The necrotic area was determined in at least 8–13 single different MRI slides; then the respective area was multiplied by the thickness of the MRI slide (usually 0.36 cm), and the respective volumes were added to the total volume of the necrosis.
The clinical outcome was investigated by a detailed physical examination of the range of motion (extension, flexion, rotation), as well as by standardised scores including the Merle d’Aubigné score, and the visual analogue scale for pain (VAS) (0: no pain-10: maximal pain). The Merle d’Aubigné score ranges from poor (0–9 points), satisfactory (9–12 points), good (13–16) to very good (17–18) [20]. The follow-up periods were similar to those above mentioned. Surgical and non-surgery related complications were determined. Particular emphasis was put on the fact whether a conversion to THR was required within the first 9 years after surgery. These data were collected retrospectively.
Statistics
For descriptive data analysis, the mean, standard deviation and range were determined. To find out whether patients improved clinically from the performed operation, a paired samples t test was applied. All tests were two-sided and a p value ≤0.05 was considered significant. The clinical outcome was determined by the Merle d’Aubigné score to find out whether the mean postoperative values for the Merle d’Aubigné score were different from the preoperative examinations. Data analysis was performed with SPSS for Windows 12.0 (SPSS inc. Chicago, Illinois, USA).
Results
Study population and risk factors for femoral head osteonecrosis
From 1/2001 through 2/2003 14 patients were included for this study (11 males, 3 females). Four patients had a bilateral avascular osteonecrosis of the femoral head, therefore, a total of 18 ONFH hips were included. Mean patient age at the time of surgery was 39.6 ± 8.6 years. The patients were followed for an average period of 9 years after surgery. Patient data were carefully collected at the above-mentioned pre-defined intervals. 17 out of 18 hips could be followed throughout the follow-up period. One patient was lost to follow-up due to moving abroad. Patients with bilateral ONFH were not operated simultaneously due to the partial weight bearing regime postoperatively, thus, a second operation on the contralateral hip was performed within 3–6 months after the previous operation.
The risk factors for the development of an ONFH are shown in Fig. 1. Eight patients showed typical risk factors (steroid, alcohol, or nicotine abuse). The other subjects showed no risk factors, and were therefore classified as idiopathic (Fig. 1).
Pre- and postoperative clinical classification by the visual analogue scale for pain (VAS) and the Merle d’Aubigné score
The mean preoperative VAS was 6.3 ± 1.4, which improved to 3.1 ± 1.8 after 1 year (p < 0.001). However, the VAS deteriorated to 4.1 ± 2.7 after 2 years (Fig. 2). A likewise result was found for the Merle d’Aubigné score; here, the initial preoperative score was 12,5 ± 1.2, to be improved to 15.3 ± 1.7 after 1 year (p < 0.001) and to be slightly decreased to 14.8 ± 1.9 after 2 years postoperatively (p < 0.001) (Fig. 3). Noteworthy, both scores demonstrated deterioration between the first and second postoperative year after initial clinical improvement. The walking ability showed an improved score after 1–2 years after surgery (p = 0.014; p = 0.02, respectively).
Preoperative and postoperative radiographic evaluation
The radiographic evaluation was done by a radiologist and a surgeon, according to the Ficat [21] and ARCO classification [8, 22]. Preoperatively, the Ficat scores were distributed as follows: no patient showed a Ficat stage III or IV, while one patient showed Ficat stage I, n = 6 hips a Ficat stage IIA, and n = 7 hips a Ficat stage IIB. The preoperative ARCO classification showed n = 1 patient with stage I, and n = 13 hips with ARCO stage II. Within the first year after surgery, radiographs at 6, 12, and 26 weeks demonstrated unchanged ARCO and Ficat classifications. However, after 1 year postoperatively, the MRIs and radiographs showed a considerable deterioration. In detail, 2 years after surgery the ARCO classification showed following results: n = 1 patient with ARCO I (7 %), n = 11 hips with ARCO II (72 %), and n = 2 with ARCO III (21 %).
Pre-and postoperative MRI evaluation
In 16 cases the preoperative MRI showed a relevant hip joint effusion in T2-weighted images. Interestingly, the preoperatively existing hip joint effusion was postoperatively not found anymore in eight cases, or at least considerably reduced in three other cases.
The results from the volumetric analysis showed an preoperative necrotic volume of 3.16 ± 0.54 cm3. The necrosis volume increased 3.46 ± 0.62 cm3 after 1 year (p = 0.14), and to 3.88 ± 0.64 cm3 (p = 0.004) after 2 years, respectively.
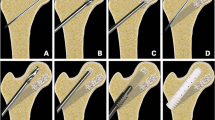
A typical radiographic case presentation is shown in Fig. 4a (X-rays) and b (MRIs), showing no change as compared to the preoperative radiographs and MRIs.
a X-rays of a 36-year-old patient with ONFH stage II according FICAT immediate postoperatively, 52 weeks and 104 weeks postoperatively (left to right). The stage of necrosis is unchanged with time, and progression is not shown, b T1-FFE/M-weighted MRIs of the same patient, demonstrating an unchanged size of the of necrosis area. Note that the drill channel reached the area of necrosis (middle picture)
Long-term clinical follow-up examination
The long-term clinical follow-up examination was done at an average of 9 years after decompression and Grafton® implantation. At this point of time, 13 out of 18 (72 %) hips had undergone a further operative intervention by THR, indicating that the current surgical procedure failed to prevent THR (Fig. 5). Only five hips did not require further operative intervention. After 9 years, the patients were asked whether they were satisfied or not with the decompression and Grafton® injection. The five patients who did not require further hip surgery were still satisfied with the outcome even after 9 years postsurgery. Two of the patients who required THR were satisfied despite conversion to THR, as in their opinion they achieved some delay of THR operation. Although all patients acknowledged initial pain alleviation, the majority of patients with conversion would not undergo the similar operative approach in retrospective.
In patients with conversion to THR, a progressive hip joint effusion, or deterioration of the radiographic stage of the disease was present. Retrospectively, each patient reported an increase in the VAS, and a deterioration of the functional ability, which lead to the decision of THR implantation. In the remaining patients without conversion to THR, the mean VAS was 3.67 ± 2.25 and the Merle d’Aubigné score was 15.3 ± 1.5 (Figs. 2, 3).
Interestingly, no complications were reported after standard unconstrained THR replacement after an average of 54 months, despite the presence of risk factors for infection (alcohol or steroid abuse, for example), or component loosening by osteonecrosis.
Discussion
This study shows long-term results of patients with early and mid-stage avascular necrosis of the femoral neck (Ficat stage I–II) after decompression surgery in combination with an injection of Grafton®, an osteoinductive and osteoconductive allogenic demineralised bone matrix [19]. The clinical long-term study results were dis-encouraging with a high conversion rate to THR of 72 % after 9 years, while the data demonstrate some clinical improvement within the first 2 years postsurgery, and MRIs and radiographs temporarily showed no significantly disease progression. This is supported by MRI measurements of the necrotic bone volume within 2 years after surgery and appears to be in line with historic clinical results from the current gold standard treatment by decompression alone, as well as with recent results using stems cells, or growth factors in addition to decompression (see paragraph below).
This study population showed the typical distribution of risk factors for femoral head osteonecrosis (see Fig. 1). Similar to other studies, patients with early stage (Ficat I–IIB) ONFH were included [23], though in opposite to recent studies, it should be considered that more than half of included hips were Ficat stage IIB. This might be important in evaluating the current study results, as there is increasing evidence that decompression with additional matrix or cell injections is predominantly beneficial in early stages of the disease. Thus, a general trend of selecting patients with Ficat stage I can be observed, rather than choosing patients with Ficat stage II, or higher [24].
Comparison with standard core decompression
Core decompression is still the gold standard of surgical ONFH treatment, and it is required to compare new approaches with core decompression. As a major study limitation, no control group of decompression alone, or conservative treatment was included, thus the current data were compared with historic decompression data. The principle of core decompression relies on a decrease of the intramedullary pressure with a subsequent increase of femoral head blood perfusion. A rabbit study of core decompression demonstrated that the glucocorticoid-induced decrease in femoral head perfusion was enhanced after core decompression [25]. Ficat and Arlet [6] showed initial data after core decompression in stage I or II lesions. In 1996, a meta-analysis by Mont et al. [26] showed an overall success rate of 59 % after decompression, and after 65 months postoperatively, these patients remained without further operative interventions.
A more recent meta-analysis by Marker et al. [23] collected data from a total of 1,268 hips after decompression (from 1992 to 2007) and found a clinical success rate of 70 % without requiring additional surgery after 63 months. Compared to the earlier meta-analysis by Mont et al., this indicates a trend of better results, as probably less patients with advanced ONFH stages were included. Our current results found a success rate of 86 % after 24 months, which were slightly better than the results from the meta-analyses.
Comparison with demineralised bone matrix
Only limited data are available on injection of Grafton® after core decompression. Similar to our study, Aaron et al. investigated Grafton® in combination with decompression in a group of 21 patients (28 hips) with Ficat stage II or III, and evaluated the hip survival rate by the subsequently required THR operation after a follow-up period of 3 years. In Ficat stage II, the survival rate was 83 % as compared to 69 of decompression alone. In Ficat stage III, the survival rate was 69 % after Grafton®, and only 28 % after decompression alone. The authors concluded that using Grafton® leads to significantly better hip survival rates, irrespective of the Ficat stage (p < 0.005) [19, 27]). Whang et al. investigated another available demineralised bone matrix in combination with auto-iliac bone in 138 hips with stage IIA-IIIA. After a follow-up period of 25 months a clinical success rate of 68 % was reported. The radiographic success was dependant on the preoperative stage, and varied between 100 % (IIA) and approximately 50 % (IIB–IIIA) [28].
Comparison with bone grafting
Autologous iliac crest grafting, fibular grafts, or vascularized bone grafts have been performed. Rosenwasser et al. [29–31] introduced a curettage with bone grafting from the iliac crest and found good results with asymptomatic hips in 13 out of 15 patients after 12 years. Mont e al reported results of bone grafting after decompression and showed a success rate of 73 % good or excellent clinical results after an average period of 56 months [32] Cortical, non-vascularized bone graft, such as tibia graft, and non-vascularized fibular grafts allow predominantly mechanical stability with doubtful clinical outcome [33]. Buckley et al. [34] used a tibial autogenous graft in three hips, a fibular autogenous graft in seven hips, and a fibular allograft, in ten hips, and found good clinical results in 18 out of 20 cases. Another advantage is the technically easier procedure as compared to vascularized bone grafts, and it should be noted that the bone grafts can be enhanced using growth and differentiation factors such as bone-morphogenetic proteins [35]. Recently, the free-vascularized fibula graft was recommended especially for younger patients in the pre-collapse state of the femoral head, particularly with the progress of microsurgical techniques [36, 37].
Comparison with tantalum beads (trabecular metal) stem cells, or growth factors
The use of porous tantalum beads may provide mechanical stability without the donor-site disadvantages of autologous bone grafting, or infectious complications after allogenic transplants. Veillette reports about 60 hips (ONFH stage II–III) with a conversion rate to THR of 15.5 % after 48 months. Another prospective study found a survival rate of 86 % in stage I–II after 39 months [38]. However, no long-term data are yet available on the implantation of tantalum beads in ONFH. In the future, porous trabecular metal tantalum implants, or calcium pyrophosphate cement may be combined with mesenchymal stem cells (MSCs) [30, 31, 39, 40].
Not much data also are available concerning augmentation with MSCs after decompression. In 2005, Gangij performed a controlled randomized study in 10 patients with ONFH and found a significant pain reduction within 24 months, as compared to controls [18]. A similar clinical benefit and safety was reported by the usage of bone marrow mononuclear cell (BMMCs) [14, 15]
Long-term results and conversion rates to THR
The reported short-term results of decompression with Grafton® injection show some improvement within 1–2 years after surgery, in agreement with previous data from Aaron et al. However, after the first year postsurgery, deterioration occurred, and after 9 years, a 72 % conversion rate to THR was found as a considerable decrease of functionality of the hips had occurred. Although patients without conversion to THR showed a good function, the high conversion rates indicated that the currently investigated operative procedure is not an appropriate method to achieve long-term improvement and avoidance of THR. Therefore, the current study results do not support previous encouraging results from Aaron et al. [41].
Despite the typical risk profile of ONFH patients, no complications such as wound healing and component loosening were noted after THR. A comparison of the current long-term data with other studies is not possible as in most studies the follow-up period was considerably shorter. A meta-analysis reported 5-year data, and showed a rate of additional surgery in 30 % of all patients after 63 months, ranging from 20 % in Ficat stage I, to 66 % in stage III. Interestingly, after initial non-operative treatment, the conversion rate to THR was 63 % [23], indicating the significance of a conservative treatment in some cases.
Study limitations
As a major limitation this data collection was not a controlled study, and no control groups of decompression and conservative treatment were included. Therefore, a comparison of the current study results with historic data was necessary. Another limitation relies on the fact that the current study population was relatively small compared to others; this limitation might be outweighed by an outstanding long-study follow-up period.
Conclusions
The combination of a decompression surgery with injection of an osteoconductive demineralised bone matrix (Grafton®) shows initial comparable results to decompression alone, and to other augmentation strategies with stem cells or growth factors. The current data, however, clearly shows that the current surgical approach fails to achieve long-term improvement, and eventually cannot prevent hip arthroplasty in these difficult to treat patients.
References
Arlet J (1992) Nontraumatic avascular necrosis of the femoral head. Past, present, and future. Clin Orthop Relat Res 277:12–21
Hungerford DS, Jones LC (2004) Asymptomatic osteonecrosis: should it be treated? Clin Orthop Relat Res 429:124–130
Jacobs B (1978) Epidemiology of traumatic and nontraumatic osteonecrosis. Clin Orthop Relat Res 130:51–67
Steinberg ME (1995) Core decompression of the femoral head for avascular necrosis: indications and results. Can J Surg 38(Suppl 1):18
Ficat RP (1985) Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br 67:3
Ficat RP, Arlet J (1980) Treatment of bone ischemia and necrosis. In: Hungerford DS (ed) Ischemia and necrosis of bone. Williams and Wilkins, Baltimore, pp 171–182
Mont MA, Marulanda GA, Jones LC, Saleh KJ, Gordon N, Hungerford DS, Steinberg ME (2006) Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am 88(Suppl 3):16–26
Sugano N, Atsumi T, Ohzono K, Kubo T, Hotokebuchi T, Takaoka K (2002) The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci 7:601–605
Steinberg ME, Larcom PG, Strafford B, Hosick WB, Corces A, Bands RE, Hartmann KE (2001) Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res 71–78
Hungerford MW, Hungerford DS, Khanuja HS, Pietryak BP, Jones LC (2006) Survivorship of femoral revision hip arthroplasty in patients with osteonecrosis. J Bone Joint Surg Am 88(Suppl 3):126–130
Mont MA, Zywiel MG, Marker DR, Mc Grath MS, Delanois RE (2010) The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am 92:2165–2170
Gangji V, Hauzeur JP. Treating osteonecrosis with autologous bone marrow cells. Skeletal Radiol 39:209
Gangji V, Toungouz M, Hauzeur JP (2005) Stem cell therapy for osteonecrosis of the femoral head. Expert Opin Biol Ther 5:437
Yan ZQ, Chen YS, Li WJ, Yang Y, Huo JZ, Chen ZR, Shi JH, Ge JB (2006) Treatment of osteonecrosis of the femoral head by percutaneous decompression and autologous bone marrow mononuclear cell infusion. Chin J Traumatol 9:3
Hernigou P, Daltro G, Filippini P, Mukasa MM, Manicom O (2008) Percutaneous implantation of autologous bone marrow osteoprogenitor cells as treatment of bone avascular necrosis related to sickle cell disease. Open Orthop J 2:62
Hougaard K, Kuur E (1998) 99mTc-SN-pyrophosphate scintigraphy following traumatic posterior dislocation of the hip. Injury 19:389
Mont MA, Ragland PS, Etienne G (2004) Core decompression of the femoral head for osteonecrosis using percutaneous multiple small-diameter drilling. Clin Orthop Relat Res 429:131–138
Sen RK (2009) Management of avascular necrosis of femoral head at pre-collapse stage. Indian J Orthop 43:6
Russell JL (2000) Grafton demineralized bone matrix: performance consistency, utility, and value. Tissue Eng 6:435
Merle d’Aubigné R, Postel M, Mazabraud A, Massias P, Gueguen J, France P (1965) Idiopathic necrosis of the femoral head in adults. J Bone Joint Surg Br 47:612–633
Ficat RP (1983) Treatment of avascular necrosis of the femoral head. Hip 2:279–295
Mont MA, Jones LC, Hungerford DS (2006) Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am 88:1117
Marker DR, Seyler TM, Ulrich SD, Srivastava S, Mont MA (2008) Do modern techniques improve core decompression outcomes for hip osteonecrosis? Clin Orthop Relat Res 466:1093–1103
Marker DR, Seyler TM, McGrath MS, Delanois RE, Ulrich SD, Mont MA (2008) Treatment of early stage osteonecrosis of the femoral head. J Bone Joint Surg Am 90(Suppl 4):175–187
Wang GJ, Dughman SS, Reger SI, Stamp WG (1985) The effect of core decompression on femoral head blood flow in steroid-induced avascular necrosis of the femoral head. J Bone Joint Surg Am 67:121
Mont MA, Carbone JJ, Fairbank AC (1996) Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res 324:169–178
Scully SP, Aaron RK, Urbaniak JR (1998) Survival analysis of hips treated with core decompression or vascularized fibular grafting because of avascular necrosis. J Bone Joint Surg Am 80:1270
Wang BL, Sun W, Shi ZC, Zhang NF, Yue DB, Guo WS, Shi SH, Li ZR (2010) Treatment of nontraumatic osteonecrosis of the femoral head using bone impaction grafting through a femoral neck window. Int Orthop 34:635–639
Rosenwasser MP, Garino JP, Kiernan HA, Michelsen CB (1994) Long term followup of thorough debridement and cancellous bone grafting of the femoral head for avascular necrosis. Clin Orthop Relat Res 306:17–27
Wan J, Zhang XS (2010) Percutaneous femoral head arthroplasty using calcium phosphate cement maybe a more feasible way to cure avascular necrosis of femoral head: comment on “Calcium phosphate cement to prevent collapse in avascular necrosis of the femoral head”. Med Hypotheses 75:132
Ng VY, Granger JF, Ellis TJ (2010) Calcium phosphate cement to prevent collapse in avascular necrosis of the femoral head. Med Hypotheses 74:725–726
Mont MA, Einhorn TA, Sponseller PD, Hungerford DS (1998) The trapdoor procedure using autogenous cortical and cancellous bone grafts for osteonecrosis of the femoral head. J Bone Joint Surg Br 80:56
Bakx PA, van Biezen FC, van Linge B (1991) Failure of tibial bone grafting for femoral head necrosis. Acta Orthop Scand 62:230
Buckley PD, Gearen PF, Petty RW (1991) Structural bone-grafting for early atraumatic avascular necrosis of the femoral head. J Bone Joint Surg Am 73:1357
Seyler TM, Marker DR, Ulrich SD, Fatscher T, Mont MA (2008) Nonvascularized bone grafting defers joint arthroplasty in hip osteonecrosis. Clin Orthop Relat Res 466:1125–1132
Beris AE, Lykissas MG, Payatakes A, Kontogeorgakos VA, Mavrodontidis A, Korompilias AV (2009) Free vascularized fibular graft for treatment of pathological femoral neck fracture and osteonecrosis of the femoral head: a case report with a long-term follow-up. Microsurgery 29:240–243
Korompilias AV, Lykissas MG, Beris AE, Urbaniak JR, Soucacos PN (2009) Vascularised fibular graft in the management of femoral head osteonecrosis: twenty years later. J Bone Joint Surg Br 91:287–293
Shuler MS, Rooks MD, Roberson JR (2007) Porous tantalum implant in early osteonecrosis of the hip: preliminary report on operative, survival, and outcomes results. J Arthroplasty 22:26
Veillette CJ, Mehdian H, Schemitsch EH, McKee MD (2006) Survivorship analysis and radiographic outcome following tantalum rod insertion for osteonecrosis of the femoral head. J Bone Joint Surg Am 88(Suppl 3):48
Tanzer M, Bobyn JD, Krygier JJ, Karabasz D (2008) Histopathologic retrieval analysis of clinically failed porous tantalum osteonecrosis implants. J Bone Joint Surg Am 90:1282
Aaron RK, Lennox D, Bunce GE, Ebert T (1989) The conservative treatment of osteonecrosis of the femoral head. A comparison of core decompression and pulsing electromagnetic fields. Clin Orthop Relat Res 249:209–218
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Helbig, L., Simank, H.G., Kroeber, M. et al. Core decompression combined with implantation of a demineralised bone matrix for non-traumatic osteonecrosis of the femoral head. Arch Orthop Trauma Surg 132, 1095–1103 (2012). https://doi.org/10.1007/s00402-012-1526-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-012-1526-3