Abstract
Introduction
Although the posterior cruciate ligament (PCL) is considered to contain not only proprioceptive but also nociceptive sensory fibers, there is a lack of information about nociceptive sensory innervation of the PCL. We hypothesized that the PCL has constant nociceptive sensory innervation, suggesting the possible source of osteoarthritic (OA) knee pain.
Materials and methods
Innervation of the PCL was examined by immunohistochemistry with particular reference to nociceptive nerve fibers in OA knees. Sensory nerve fibers were semi-quantitatively counted in the PCL of OA knees, comparing with non-OA knees. Protein gene product 9.5 (PGP9.5) as a general neuronal marker and calcitonin gene related peptide (CGRP) as a marker for nociceptive neuron were used.
Results
The PCLs had constant CGRP-immunoreactive (IR) nerve fibers in both OA and non-OA knees. The difference of the CGRP-IR nerve density between groups did not reach a statistical significance (p = 0.062). For PGP9.5-IR nerve fibers, however, the PCLs in OA knees were statistically less innervated than non-OA knees (p = 0.0009).
Conclusions
Our results showed that, in spite of a significant decrease in total innervation in OA knees, the PCLs have constant nociceptive sensory innervation. Although the relationship between the decrease in total innervations in the PCL and OA pathophysiology is still unclear, the PCL is the possible source of OA knee pain. Our results should be taken into account when examining the pain source of the OA knees and handling the PCL during total knee arthroplasty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The posterior cruciate ligament (PCL), the strongest ligament in the knee joint, is considered to have sensory function as well as stabilizing function. Historically, morphological studies using nonspecific staining, such as gold chloride, silver, and Masson’s trichrome methods, confirmed the presence of neural network within the PCL [1–3]. Recently, nerve-specific staining methods used in a few immunohistochemical studies [4, 5] revalidated this observation. However, most previous studies paid attention only to the low-threshold mechanoreceptors [1, 2, 5, 6], which play a significant role in the proprioceptive function of the knee joint. To date, there is a lack of information about nociceptive sensory innervation of the PCL. We hypothesized that the PCL has constant nociceptive sensory innervation, suggesting the possible source of OA knee pain. We semi-quantitatively counted the sensory nerve fibers of the PCL taken from OA knees (OA-PCL) and non-OA knees (N-PCL) with immunohistochemical methods. We used protein gene product 9.5 (PGP9.5) as a general neuronal marker and calcitonin gene related peptide (CGRP) as a marker for nociceptive neuron.
Materials and methods
All samples were obtained from patients during total knee arthroplasty (TKA) or anterior cruciate ligament (ACL) reconstruction. The OA-PCLs were from 10 patients with OA knees with a mean age of 77 years (70–85) and the N-PCLs were from 5 patients with ACL rupture with a mean age of 26 years (16–42). The severity of pain in patients with OA knees was evaluated using 100 mm visual analogue scale, preoperatively. The mean walking knee pain was 59 mm (range 36–100 mm). On the other hand, none of the patients with ACL rupture complained of knee pain. Two PCL samples for each patient, approximately 4 mm2, were taken with 3-mm forceps, one from its femoral insertion and the other from middle of the PCL. As a positive control for immunohistochemistry test, inflamed synovium was also obtained from patients with OA knees. Ethics committee approval from our institution was obtained prior to the study. Each patient was informed of the objectives and risks of the study and gave his or her consent. The study was conducted in compliance with the Declaration of Helsinki.
Immunohistochemistry
All samples were fixed in 4% paraformaldehyde for 24 h, placed in 30% sucrose for another 24 h, embedded in OCT compound (Sakura Finetek, Torrace, California), rapidly frozen, and kept at −80°C until sectioning. Fourteen micrometer frozen sections were then cut using a cryostat. Each section was observed with a microscope before staining to confirm the representative section.
The sections were soaked in methanol containing 0.3% H2O2 for 10 min to quench endogenous peroxidase, blocked in 3% normal goat serum for 30 min, and then incubated with rabbit anti-human PGP9.5 antibody (Ultraclone, 1:1,000) or rabbit anti-human CGRP antibody (Peninsula T-4239 1:1,000) overnight at room temperature. The next day, the sections were incubated with biotinylated goat anti-rabbit IgG diluted 1:200 in PBS containing 0.05% Triton X-100 and 1% normal goat serum for 30 min at room temperature. The sites of antibody binding were visualized using avidin–biotin peroxidase method (ABC kit, Santa Cruz Biotechnology, Santa Cruz, CA) diluted at 1:200 for 30 min at room temperature. Finally, the sections were treated with 3.3′-Diaminobenzidine for 3 min, counterstained with hematoxylin, and mounted on slides. Before, between, and after each incubation step, the sections were washed three times for 5 min in PBS. Negative controls were made by substituting primary antibody with non-immune rabbit serum.
Semi-quantitative analysis
Each section was observed with a Zeiss Axiophot microscope (Carl Zeiss, Oberkochen, Germany). The CGRP-and PGP9.5-immunoreactive (IR) nerve fibers were counted in randomly selected five sections for each sample at a magnification of 200×. Only nerve fibers longer than 30 um in length were counted. The area of each observed section was measured using Image J software (National Institutes of Health: http://rsb.info.nih.gov/nih-image/) and the nerve density was expressed as nerve fibers/mm2.
Statistical analysis
All values were expressed as median (interquartile range). For comparison of the nerve density within one group and between groups, Mann–Whitney U test was used (SPSS version 16.0, SPSS Inc, Chicago, USA). A p < 0.05 was considered statistically significant.
Results
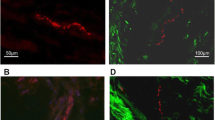
In general, both CGRP- and PGP9.5-IR nerve fibers were consistently observed in all samples with little nonspecific staining (Fig. 1). The harvested PCLs included a small amount of richly vascularized and innervated epiligament (approximately less than 1/10 of the total area). Nerve fibers were found predominantly in the epiligament in a perivascular distribution. In the deep substance of the ligament, less blood vessels and nerve fibers were found than in the epiligament.
The nerve density was higher at the femoral insertion site than the middle part of the PCL for CGRP- and PGP9.5-IR nerve fibers. The OA-PCLs showed various degenerative changes such as loose collagen fibers, mucomyxoid degeneration, and disorganized collagen architecture with some disruption.
The OA-PCLs tended to have less innervation than the N-PCLs. However, the difference of the CGRP-IR nerve density between the OA-PCLs and the N-PCLs did not reach a statistical significance [0.140 (0.120, 0.153) vs. 0.155 (0.140, 0.183) fibers/mm2; p = 0.062] (Fig. 2a). For PGP9.5-IR nerve fibers, the OA-PCLs were statistically less innervated than the N-PCLs [0.230 (0.208, 0.295) vs. 0.375 (0.348, 0.450) fibers/mm2; p = 0.0009] (Fig. 2b). The ratio of the density of CGRP-IR to PGP9.5-IR nerve fibers (% CGRP) was 59 (45, 71)% in the OA-PCLs and 45 (34, 48)% in the N-PCLs. The OA-PCLs had significantly higher % CGRP than the N-PCLs (p = 0.031).
Discussion
Proprioceptive function of the knee joint is reported to decline with aging [7–10] and certain joint diseases [8, 9, 11]. The decrease in number of mechanoreceptors within ligaments, reported in animals [12] and humans [2, 6, 13], is presumably one of the underlying mechanisms. In addition, total number of knee joint afferents decreases with aging [14] and OA changes [15, 16] in rodents. Taken together, the decrease in the number of mechanoreceptors at least partially accounts for the age-related loss of joint innervation and progression of OA changes. Our results were consistent with this notion. The number of PGP 9.5-IR fibers in the OA-PCL was significantly smaller than the N-PCL. Anti-PGP 9.5 labels all neurons, including sympathetic and motor neurons as well as sensory neurons [17]. Ligaments, like many other connective tissues, are innervated with both sensory and sympathetic nerves [18]. Therefore, our results showed that, comparing with the normal knees, there was a significant decrease in total innervation of the PCL including a subpopulation of proprioceptive and sympathetic nerves in the OA knees. Because peripheral nerves within the ligament play an essential role in homeostasis of ligaments and joints [19, 20], the decrease in total innervations can be detrimental to the structural integrity of ligaments and joints.
Although the issue of PCL retention versus sacrifice during TKA has been discussed largely over the past 20 years, there is, so far, no solid conclusion in this regard [21]. It is clear that the PCL plays an important role in the kinematics of the normal knee joint because of its biomechanical and proprioceptive functions. Therefore, it would be reasonable to retain the PCL during TKA if its functions were expected to be normal. However, all these functions of the PCL are doubtful in OA knees [4, 8]. As mentioned above, our results support previous reports regarding loss of the proprioceptive function of the PCL in OA knees. Furthermore, our results showed an interesting finding that the difference of the CGRP-IR nerve density between OA-PCL and N-PCL was not statistically significant. CGRP is a marker of somatic sensory neurons, typically responsible for nociceptive fibers [22]. Therefore, our results indicate that nociceptive inputs selectively remain in the PCL of OA knees, while the total innervation decreases. One possible reason for this finding is that OA and arthritic changes in knee cause an up-regulation of CGRP in rodent dorsal root ganglia [16, 23], These findings will give new insights to the PCL issue in TKA and should be taken into account.
There are several limitations in this study. First, the N-PCLs might not be representative of normal PCL because they were obtained from chronic ACL-deficient knees. Although the N-PCLs were obtained more than 3 months after the ACL injury, there could have been injury- or instability-related changes of innervation within the PCL. Second, the N-PCLs were obtained from younger patients than the OA-PCLs. Not only OA changes but also aging presumably affected the differences of innervation between two groups. Third, there were more nerve fibers in the epiligament than the ligament substance. Although the area of the epiligament in each section was relatively constant, the counting results could have been affected by the way of sampling the tissue.
In conclusion, the PCL has constant nociceptive sensory innervation even in OA knees. Although the relationship between the decrease in total innervations in the PCL and OA pathophysiology is still unclear, the PCL is the possible source of OA knee pain. Our results should be taken into account when examining the pain source of the OA knees and handling the PCL during TKA.
References
Katonis PG, Assimakopoulos AP, Agapitos MV, Exarchou EI (1991) Mechanoreceptors in the posterior cruciate ligament. histologic study on cadaver knees. Acta Orthop Scand 62:276–278
Franchi A, Zaccherotti G, Aglietti P (1995) Neural system of the human posterior cruciate ligament in osteoarthritis. J Arthroplast 10:679–682
Biedert RM, Stauffer E, Friederich NF (1992) Occurrence of free nerve endings in the soft tissue of the knee joint. a histologic investigation. Am J Sports Med 20:430–433
Nelissen RG, Hogendoorn PC (2001) Retain or sacrifice the posterior cruciate ligament in total knee arthroplasty? a histopathological study of the cruciate ligament in osteoarthritic and rheumatoid disease. J Clin Pathol 54:381–384
Del Valle ME, Harwin SF, Maestro A, Murcia A, Vega JA (1998) Immunohistochemical analysis of mechanoreceptors in the human posterior cruciate ligament: a demonstration of its proprioceptive role and clinical relevance. J Arthroplast 13:916–922
Schultz RA, Miller DC, Kerr CS, Micheli L (1984) Mechanoreceptors in human cruciate ligaments. a histological study. J Bone Jt Surg Am 66:1072–1076
Kaplan FS, Nixon JE, Reitz M, Rindfleish L, Tucker J (1985) Age-related changes in proprioception and sensation of joint position. Acta Orthop Scand 56:72–74
Barrett DS, Cobb AG, Bentley G (1991) Joint proprioception in normal, osteoarthritic and replaced knees. J Bone Jt Surg Br 73:53–56
Pai YC, Rymer WZ, Chang RW, Sharma L (1997) Effect of age and osteoarthritis on knee proprioception. Arthritis Rheum 40:2260–2265
Skinner HB, Barrack RL, Cook SD (1984) Age-related decline in proprioception. Clin Orthop Relat Res 184:208–211
Barrack RL, Skinner HB, Buckley SL (1989) Proprioception in the anterior cruciate deficient knee. Am J Sports Med 17:1–6
Aydog ST, Korkusuz P, Doral MN, Tetik O, Demirel HA (2006) Decrease in the numbers of mechanoreceptors in rabbit ACL: the effects of ageing. Knee Surg Sports Traumatol Arthrosc 14:325–329
Morisawa Y (1998) Morphological study of mechanoreceptors on the coracoacromial ligament. J Orthop Sci 3:102–110
Salo PT, Tatton WG (1993) Age-related loss of knee joint afferents in mice. J Neurosci Res 35:664–677
Salo PT, Seeratten RA, Erwin WM, Bray RC (2002) Evidence for a neuropathic contribution to the development of spontaneous knee osteoarthrosis in a mouse model. Acta Orthop Scand 73:77–84
Ferreira-Gomes J, Adaes S, Sarkander J, Castro-Lopes JM (2010) Phenotypic alterations of neurons that innervate osteoarthritic joints in rats. Arthritis Rheum 62:3677–3685
Schofield JN, Day IN, Thompson RJ, Edwards YH (1995) PGP 9.5, A ubiquitin C-terminal hydrolase: pattern of mRNA and protein expression during neuronal development in the mouse. Brain Res Dev Brain Res 85:229–238
Ackermann PW, Li J, Finn A, Ahmed M, Kreicbergs A (2001) Autonomic innervations of tendons, ligaments and joint capsules. a morphologic and quantitative study in the rat. J Orthop Res 19:372–378
Grorud KW, Jensen KT, Provenzano PP, Vanderby R (2007) Adjuvant neuropeptides can improve neuropathic ligament healing in a rat model. J Orthop Res 25:703–712
Halstead J, Bergin D, Keenan AM, Madden J, McGonagle D (2010) Ligament and bone pathologic abnormalities more frequent in neuropathic joint disease in comparison with degenerative arthritis of the foot and ankle. Arthritis Rheum 62:2353–2358
Jacobs WC, Clement DJ, Wymenga AB (2005) Retention versus sacrifice of the posterior cruciate ligament in total knee replacement for treatment of osteoarthritis and rheumatoid arthritis. Cochrane Database Syst Rev 19:CD004803
Ishida-Yamamoto A, Tohyama M (1989) Calcitonin gene-related peptide in the nervous tissue. Prog Neurobiol 33:335–386
Ikeuchi M, Kolker S, Sluka KA (2009) Acid-sensing ion channel 3 expression in mouse knee joint afferents and effects of carrageenan-induced arthritis. J Pain 10:336–342
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Ikeuchi and Q. Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ikeuchi, M., Wang, Q., Izumi, M. et al. Nociceptive sensory innervation of the posterior cruciate ligament in osteoarthritic knees. Arch Orthop Trauma Surg 132, 891–895 (2012). https://doi.org/10.1007/s00402-012-1478-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-012-1478-7