Abstract
Introduction
The intraoperative estimation of the anteversion of the femoral component of a total hip arthroplasty is generally made by the surgeon’s visual assessment of the stem position relative to the condylar plane of the femur.
Aim
The aim of this study was to evaluate the femoral component anteversion relative to lesser trochanter during cementless total hip arthroplasty. The intraoperative estimation of the anteversion of the femoral component of a total hip arthroplasty is generally made by the surgeon’s visual assessment of the stem position relative to the condylar plane of the femur. The aim of this study was to evaluate the femoral component anteversion relative to lesser trochanter during cementless total hip arthroplasty.
Method
The authors investigated the version of the lesser trochanter (LTV) relative to the posterior femoral condyles. Fifty-seven patients (59 hips) scheduled for primary cementless total hip arthroplasty underwent preoperative computed tomography and it was measured the LTV and collo-femoral version at the level of the proximal-most portion of the inferior neck, with respect to the lesser trochanter (native collo-trochanteric angle, NCTA). During surgery, the operative collo-trochanteric angle (OCTA) was measured.
Results
The mean LTV was 34.1 ± 3.0°, the mean NCTA was 49.1 ± 5.6°, and the mean OCTA was 48.8 ± 6.0°, which did not differ significantly from the NCTA (p = 0.495); the correlation coefficient was 0.872 (p < 0.0001). Based on the data, there was a constant relationship between the lesser trochanter and posterior femoral condyles and a good correlation between NCTA and OCTA.
Conclusion
The authors recommend first estimating the anteversion of the femoral component relative to lesser trochanter and then adjusting the position of the acetabular component to that anteversion of the femoral component to improve stability and reduce impingement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Proper positioning of both the acetabular and femoral components in total hip arthroplasty (THA) minimises impingement and its attendant complications [1, 2]. Several studies support the concept that, for the optimum range of motion in THA, the combined femoral and acetabular anteversion should be some constant value; previous studies have proposed a mean combined anteversion of approximately 37° (range 25–50°) to avoid impingement or dislocation [2–6].
Typically, the surgeon visually estimates the intraoperative anteversion of the femoral component by assessing the stem position relative to the condylar plane of the femur, unless computer navigation is used. However, because the available osseous landmarks are limited to the femoral neck and coronal femoral axis, as determined by palpating the epicondyles, even experienced surgeons may have difficulty estimating femoral anteversion during THA [5, 7]. In cementless THA, the available bone coverage provides the surgeon with a degree of flexibility in intraoperative estimation of acetabular anteversion, but the anteversion of a tight press-fit femoral component may be restricted by the anatomy of the femoral neck, the diaphyseal bow, and the anterior–posterior isthmus at the level of the lesser trochanter created by the calcar femorale. Therefore, the surgical intervention may not achieve the desired degree of anteversion.
This study estimated the version of the femoral component relative to the lesser trochanter and investigated the use of this parameter as a guide for implant position in THA. The authors asked two questions: is there a constant relationship between the lesser trochanter and the posterior femoral condyles and, if so, is it possible to estimate the version of a “best-fit” cementless femoral component with respect to the lesser trochanter?
Materials and methods
Patients
Institutional review board approval was obtained for this study, and all patients gave informed consent. Between June 2009 and February 2010, 57 osteoarthritis patients (59 hips) who were candidates for THA using a cementless femoral component were included in this study. The participants included patients with primary osteoarthritis of the hip or osteoarthritis secondary to mild dysplasia (less than 50% subluxation according to the classification of Crowe et al. [7]) and non-traumatic osteonecrosis of the femoral head. The authors excluded patients who had experienced osteoarthritis and arthroplasty elsewhere than the hip, those with moderate to severe dysplasia (more than 50% subluxation according to the classification of Crowe et al. [7]), and those who had undergone prior surgery on the affected hip. Bilateral cases were treated at least 2 weeks apart. The participants included 27 men (27 hips) and 30 women (32 hips). The reason for surgery was primary osteoarthritis in 35 hips, mild developmental dysplasia of the hip and severe degenerative joint disease in 21 hips, and osteonecrosis in 3 hips. Nineteen patients (21 hips) showed dysplastic femora. The remaining 38 patients (38 hips) did not show dysplastic femora and were classified as having normal anatomy. The mean age at the time of surgery was 62.1 ± 7.4 years (range, 47–77 years). The femoral components used were anatomic titanium plasma-sprayed, hydroxyapatite-coated replacements (F40 Press Fit; Biomet Europe) in all hips.
Radiological techniques
Fifty-seven patients scheduled for primary THA underwent preoperative computed tomography (CT; Somatom Sensation Cardiac, Siemens, Erlangen, Germany). All CT procedures were performed with the patient supine and positioned symmetrically in the scanner. A neutral position of the pelvis was obtained by requesting the patient to lie prone with the anterior tips of the iliac crests and symphysis pubis resting evenly on the table. A support placed beneath the ankles was used to keep the patient’s feet parallel to each other. The scanned region extended from the level of the fourth lumbar vertebra proximally to the knee, including the distal femoral condyles. Each examination was performed using a 5-mm slice thickness and reconstruction was performed from the raw data using 2-mm slice thicknesses at 1-mm intervals. The images were imported into the Virtuoso software package (Siemens Medical Systems) in generic Digital Imaging and Communications in Medicine (DICOM) [8] format for measurements. All measurements were made using axial CT images.
Measuring the lesser trochanteric version
The lesser trochanteric version is the angle between the epicondylar axis of the knee joint and the transverse axis of the lesser trochanter (lesser trochanteric axis). Measurements of the posterior condylar axis were used to validate the epicondylar axis [9]. The lesser trochanter cannot be visualised as a whole in the transverse plane because of its posterior and cephalad orientation; therefore, the lesser trochanter axis was defined as the line passing through the centroid of the base of the lesser trochanter on the slice that showed the largest width of the lesser trochanter and the centroid at the tip of the lesser trochanter (Fig. 1).
Measuring the native collo-trochanteric angle
The collo-femoral version with respect to the lesser trochanter (native collo-trochanteric angle) was defined as the angle between the collo-femoral axis and the lesser trochanter axis. The slice at the level of the most proximal portion of the inferior neck that had no head portion was selected for measuring the collo-femoral axis. This level provides the most accurate estimate of the neck axis with the single slice method [10] and is consistent with the level of the collum osteotomy in THA. At this level, the collo-femoral axis was defined as the line passing through the midpoints at both the medial and lateral edges of the central portion of the neck (Fig. 2).
Surgical technique
After completing the preoperative CT measurements as a pilot study, all 59 patients underwent cementless primary THA performed by one surgeon (M.C.U.) using a posterior mini-incision technique [11] with minimal modification of the exposure of the lesser trochanter (incision length was 7–14 cm). After the collum osteotomy, the femur was prepared first, allowing the surgeon to estimate the anteversion of the femoral stem and then adjust the anteversion of the acetabular component accordingly to provide a combined anteversion of stem and cup in the safe zone of 25–50° [6].
The femur was prepared by flexing the knee and placing the tibia in a vertical position. The surgeon sighted the version of the femoral broaches and then the trial component in a parallel relation to the posterior wall of the medullary cavity at the level of the collum osteotomy. Just proximal to this level, the anterior and posterior walls of the medullary cavity are parallel, but closer to this level, only the posterior wall is still flat [10]. Because cementless THA uses an anatomic femoral component that fills the metaphysis and proximal diaphysis in the mediolateral plane, and this stem fills the metaphysis from medial to lateral, the position of the femoral component is dictated in part by the native femoral neck posterior wall, which is somewhat flat. Therefore, the femoral component axis was also parallel to the posterior wall of the medullary cavity at the level of the collum osteotomy. Subsequently, the operative collo-femoral version with respect to the lesser trochanter was measured (the operative collo-trochanteric angle): the angle between the operative axis of the lesser trochanter as it bisects the posterior wall of the medullary cavity at the level of the collum osteotomy (Fig. 3). The surgeon was blinded to the value of the native collo-trochanteric angle. Afterward, the anteversion of the femoral component was calculated as the operative collo-trochanteric angle minus 34° (the mean lesser trochanteric version). Then, the position of the acetabular component was adjusted to that of the calculated anteversion and the neck shaft angle of the femoral component to provide a mean combined anteversion of approximately 37° (range, 25–50°) [2–6]. A specific manoeuvre was used to assess the posterior stability with the trial components [12]. With the patient in the true lateral decubitus position, the femur was internally rotated without hip flexion until the degree of combined anteversion was individualised to the patient. Posterior dislocation was not observed in any patient.
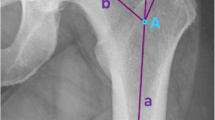
An image showing the measurement of the operative collo-trochanteric angle for the right hip of a 47-year-old woman. The operative axis of the lesser trochanter was defined as the line passing through the midpoint of the base (largest width) of the lesser trochanter and the caudal projection of the tip of the lesser trochanter. Kirchner wire (no. 1.5) was used to determine the operative axis of the lesser trochanter, and the operative collo-trochanteric angle was measured with a sterile goniometer. The axis of the goniometer was placed over the posterior wall of the medullary cavity, and the projection of the Kirchner wire over the goniometer was recorded as the operative collo-trochanteric angle. Note that the femoral trial component axis is parallel to the posterior wall of the medullary cavity at the level of the collum osteotomy
Statistical analysis
Statistical methods designed for independent observations were used. The Kolmogorov–Smirnov test was used to test for normal distributions before any statistical analysis was performed. One musculoskeletal magnetic resonance imaging (MRI) radiologist measured the lesser trochanteric version and native collo-trochanteric angle on all CT images. The mean, standard deviation, standard error, and 95% confidence interval were calculated. Univariate analysis of variance (ANOVA) was used to analyze the factors (sex and dysplastic/anatomically normal femur) potentially influencing the lesser trochanteric version.
The surgeon’s estimates of the collo-trochanteric angle (operative collo-trochanteric angle) were compared with the computed tomography values (native collo-trochanteric angle) using the scan as the true reference. Bias, precision, and Pearson’s correlation coefficient were calculated based on these operative and native values, and then the paired samples t-test was used to compare the means of the two variables.
Precision (randomised error) is the closeness of agreement between repeated measurements made under similar conditions and represents the reliability and reproducibility of a test. In this study, precision was measured between each independent measurement of the native and operative collo-trochanteric angles. Bias (system error) is a consistent difference between a set of measurements and an accepted reference or true value [13]. In this study, bias was defined as the numerical difference between the mean value of the operative and native collo-trochanteric angles. All analyses were performed using the SPSS statistical software package (ver. 17.0 for Windows, SPSS, Chicago, IL, USA).
Results
Operative versus native collo-trochanteric angles
The mean native collo-trochanteric angle (49.1 ± 5.6°; range, 37.3–60.5°) did not differ significantly from the mean operative collo-trochanteric angle (48.8 ± 6.0°; range, 35.0–63.0°; p = 0.495, paired samples t-test). The correlation coefficient was 0.872 (p < 0.0001) and the values for bias and precision were calculated at 2.84 and 0.99, respectively (Fig. 4). The correlation coefficient, which described the degree of association between the native and operative collo-trochanteric angles, had 100% statistical power with the sample size of 59 hips Table 1.
Lesser trochanteric version
The mean lesser trochanteric version was 34.1 ± 3.0° (range, 30.1–39.0°; 95% confidence interval 33.33–34.87°) with 100% of values differing from the mean by less than 5°; this value showed 80% power for predicting the mean within 1° of error with the sample size of 59 hips.
ANOVA, with a post hoc Tukey honestly significant difference (HSD) test, revealed that neither gender nor the presence of a dysplastic/anatomically normal femur was significantly associated with discrepancies in the measurement of lesser trochanteric version. The mean lesser trochanteric version was 35.1 ± 3.0° (range, 30.1–39.0°) for women (n = 32 hips) and 32.9 ± 2.6° (range, 30.3–39.0°) for men (n = 27 hips). The mean lesser trochanteric version angle was 35.7 ± 3.0° (range, 31.1–39.0°) for dysplastic femora (n = 22 hips) and 33.2 ± 2.6° (range, 30.1–38.9°) for anatomically normal femora (n = 37 hips).
Descriptive statistics are given at Table 1.
Discussion
The authors analyzed 59 THAs preoperatively with CT and determined that there exists a constant relationship between the lesser trochanter and posterior femoral condyles. Even intraoperatively, it was possible to estimate the version of a ‘‘best-fit’’ cementless femoral component with respect to the lesser trochanter.
Many authors have studied the optimal position of the acetabular component, but little has been written regarding the final position achieved with a cementless femoral component, except to recommend approximately 15° of anteversion [3–5, 11]. In a previous study, the accuracy of surgeon-estimated femoral anteversion in 109 consecutive cementless THAs (99 patients) was evaluated prospectively with three-dimensional CT reconstruction of the femur; this analysis revealed that 47% of the femoral stems showed anteversion of less than 10° [14]. Another study of femoral anteversion compared surgeon estimates to values obtained via two-dimensional CT in 111 hips (111 patients) [5]. After measuring both cemented and cementless femoral components, the authors reported that only 30% (18 of 60 femoral components) achieved a range of 10–20° of anteversion, although 103 of the 111 surgeon estimates had ranged between 15° and 20°. Therefore, the surgeon’s intraoperative assessment of femoral anteversion may not be accurate. Furthermore, with cemented arthroplasty, the surgeon can easily control the anteversion of a smaller femoral component in the rasped canal. In contrast, surgeons performing cementless arthroplasty have little control over the femoral component, because its position is dictated by the native proximal femoral anatomy. In their prospective study, Bargar et al. [15] measured the native acetabular, native femoral, and prosthetic femoral anteversion of 46 patients who underwent THA using straight-stem cementless femoral components. They reported that the mean femoral component anteversion was 8.7° greater than the mean native femoral neck anteversion and there was no correlation between the native or prosthetic femoral anteversion and the native acetabular anteversion. Accordingly, the data from this previous study support the use of prosthetic femoral anteversion as a guide for optimising the position of the acetabulum.
These studies indicate that, especially for cementless applications, the final position is determined by the femoral component. Moreover, because the final position achieved may differ from the recommended target anteversion, an accurate estimate of the degree of femoral anteversion is required to permit the surgeon to adjust the acetabular anteversion to compensate, if necessary. Therefore, surgeons should avoid the tendency to routinely target the acetabular component anteversion to 15–20° [6, 16], and instead adjust this parameter to compensate for sub-optimal femoral anteversion.
For this reason, the authors investigated the use of the lesser trochanter for estimating femoral anteversion. The authors asked two questions: is there a constant relationship between the lesser trochanter and posterior femoral condyles, and if so, is it possible to estimate the version of a ‘‘best-fit’’ cementless femoral component with respect to the lesser trochanter? In answer to the first question, the results showed that the mean lesser trochanteric version was 34.1 ± 3.0° with 100% of values differing from the mean by less than 5°. Therefore, there is a constant relationship between the lesser trochanter and posterior femoral condyles. In this study, the precision and bias of the surgeon’s estimates of operative collo-trochanteric angle were 0.99 and 2.84, respectively; the surgeon achieved a consistent result within 3° precision and bias. The surgeon was relatively consistent, in that the difference between native and operative values did not exceed 3°. In answer to the second question, although this study focused on the work of one surgeon with 6 years of experience, we think that other surgeons will have at least the same degree of precision and bias, suggesting it is indeed possible to predict the ‘‘best-fit’’ cementless femoral component version that will obtained.
There are limitations to this study. The first is that these findings are limited to the specific femoral component used in this study. Although its design is not dissimilar to many other anatomic implants currently available, these findings may not be generalisable to all other total hip designs. Second, although the statistical power for predicting the lesser trochanteric version mean with 1° error with the sample size of 59 hips was 80%, the statistical power dropped below 80% when these hips were grouped according to gender or the presence of a dysplastic/anatomically normal femur. However, if the authors accept 2° error for predicting the lesser trochanteric version mean, all subgroups will have a statistical power exceeding 90%. Third, the authors did not obtain repeatability or intra-observer variability data for the CT measurements. Finally and most importantly, the clinical relevance of this study is dependent on the assumption that the various papers proposing the use of combined femoral and acetabular anteversion for the optimal position of the implants are correct.
Although, the morphological features of the proximal femur have been used in preoperative planning before THA [17], the authors are not aware of any study examining the position of the lesser trochanter relative to the posterior femoral condylar plane (i.e., lesser trochanteric version). It is well known that the dysplastic femur often has a narrow medullary canal and increased anteversion [7, 18–20]. However, although several studies examining patients with primary osteoarthritis have supported the hypothesis that a persistent increase in femoral anteversion predisposes the patient to osteoarthritis of the hip [21–24], several other studies have reported contradictory findings [25–27]. The size and shape of the human femur varies with the gender, age, stature, and ethnic background [28]. The authors choose the lesser trochanter because it was hypothesised that the position (version) of the lesser trochanter will be constant relative to the changes in the magnitude and direction of the forces affecting the shape and anteversion of the femur due to the attached iliopsoas muscle. These findings supported the validity of this hypothesis.
The typical technique used by many surgeons is to place the acetabular component in line with the native acetabular anteversion and then place a best-fitting implant in the femoral canal. Because suboptimal combined anteversion increases the likelihood of dislocation [29], the authors recommend first preparing the femur parallel to the posterior wall of the medullary cavity at the level of the collum osteotomy and then adjusting the position of the acetabular component to that of the calculated anteversion of the femoral component [measured operative collo-trochanteric angle minus 34° (mean lesser trochanteric version)] to improve stability and reduce impingement.
References
Trousdale RT, Cabanela ME, Berry DJ (1995) Anterior iliopsoas impingement after total hip arthroplasty. J Arthroplasty 10(4):546
Yoshimine F (2006) The safe-zones for combined cup and neck anteversions that fulfill the essential range of motion and their optimum combination in total hip replacements. J Biomech 39(7):1315
Dorr LD, Malik A, Wan Z, Long WT, Harris M (2007) Precision and bias of imageless computer navigation and surgeon estimates for acetabular component position. Clin Orthop Relat Res 465:92
Pierchon F, Pasquier G, Cotten A, Fontaine C, Clarisse J, Duquennoy A (1994) Causes of dislocation of total hip arthroplasty CT study of component alignment. J Bone Joint Surg [Br] 76-B(1):45
Wines AP, McNicol D (2006) Computed tomography measurement of the accuracy of component version in total hip arthroplasty. J Arthroplasty 21(5):696–701
Dorr LD, Malik A, Dastane M, Wan Z (2009) Combined anteversion technique for total hip arthroplasty. Clin Orthop Relat Res 467(1):119
Crowe JF, Mani VJ, Ranawat CS (1979) Total hip replacement in congenital dislocation and dysplasia of the hip. J Bone Joint Surg Am 61-A(1):15
Jamali AA, Deuel C, Perreira A, Salgado CJ, Hunter JC, Strong EB (2007) Linear and angular measurements of computer-generated models: are they accurate, valid, and reliable? Comput Aided Surg 12(5):278
Murphy SB, Simon SR, Kijewski PK, Wilkinson RH, Griscom NT (1987) Femoral anteversion. J Bone Joint Surg Am 69-A(8):1169
Sugano N, Noble PC, Kamaric E (1998) A comparison of alternative methods of measuring femoral anteversion. J Comput Assist Tomogr 22(4):610
Inaba Y, Dorr LD, Wan Z, Sirianni L, Boutary M (2005) Operative and patient care techniques for posterior mini-incision total hip arthroplasty. Clin Orthop Relat Res 441:104
Lucas DH, Scott RB (1994) The Ranawat Sign: a specific maneuver to assess component positioning in total hip arthroplasty. J Orthop Techn 2(2):59
Kalteis T, Handel M, Bathis H, Perlick L, Tingart M, Grifka J (2006) Imageless navigation for insertion of the acetabular component in total hip arthroplasty: is it as accurate as CT-based navigation? J Bone Joint Surg [Br] 88(7):163
Dorr LD, Wan Z, Malik A, Zhu J, Dastane M, Deshmane P (2009) A comparison of surgeon estimation and computed tomographic measurement of femoral component anteversion in cementless total hip arthroplasty. J Bone Joint Surg Am 91-A(11):2598
Bargar WL, Jamali AA, Nejad AH (2010) Femoral anteversion in THA and its lack of correlation with native acetabular anteversion. Clin Orthop Relat Res 468(2):527
Barrack RL (2003) Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg 11(2):89
Unnanuntana A, Toogood P, Hart D, Cooperman D, Grant RE (2010) The evaluation of two references for restoring proximal femoral anatomy during total hip arthroplasty. Clin Anat 23(3):312
Charnley J, Feagin JA (1973) Low-friction arthroplasty in congenital subluxation of the hip. Clin Orthop 91:98
Dunn HK, Hess WE (1976) Total hip reconstruction in chronically dislocated hips. J Bone Joint Surg Am 58-A(6):838
Woolson ST, Harris WH (1983) Complex total hip replacement for dysplastic or hypoplastic hips using miniature or microminiature components. J Bone Joint Surg Am 65-A(8):1099
Giunti A, Moroni A, Olmi R, Rimondi E, Soldati D, Vicenzi G (1985) The importance of the angle of anteversion in the development of arthritis of the hip. Ital J Orthop Traumatol 11(1):23
Reikerås O, Høiseth A (1982) Femoral neck angles in osteoarthritis of the hip. Acta Orthop Scand 53(5):781
Reikerås O, Bjerkreim I, Kolbenstvedt A (1983) Anteversion of the acetabulum and femoral neck in normals and in patients with osteoarthritis of the hip. Acta Orthop Scand 54(1):18
Terjesen T, Benum P, Anda S, Svenningsen S (1982) Increased femoral anteversion and osteoarthritis of the hip joint. Acta Orthop Scand 53(4):571
Hubbard DD, Staheli LT, Chew DE, Mosca VS (1988) Medial femoral torsion and osteoarthritis. J Pediatr Orthop 8(5):540
Kitaoka HB, Weiner DS, Cook AJ, Hoyt WA Jr, Askew MJ (1989) Relationship between femoral anteversion and osteoarthritis of the hip. J Pediatr Orthop 9(4):396
Swanson AB, Greene PW Jr, Allis HD (1963) Rotational deformities of the lower extremity in children, their clinical significance. Clin Orthop Relat Res 27:157
Noble PC, Box GG, Kamaric E et al (1995) The effect of aging on the shape of the proximal femur. Clin Orthop 3163:1
Komeno M, Hasegawa M, Sudo A, Uchida A (2006) Computed tomographic evaluation of component position on dislocation after total hip arthroplasty. Orthopedics 29(12):1104
Acknowledgment
Thanks to Dr Nabuhiko Sugano for radiological technique.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Unlu, M.C., Kesmezacar, H., Kantarci, F. et al. Intraoperative estimation of femoral anteversion in cementless total hip arthroplasty using the lesser trochanter. Arch Orthop Trauma Surg 131, 1317–1323 (2011). https://doi.org/10.1007/s00402-011-1282-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-011-1282-9