Abstract
Introduction
Functional reconstruction of the shoulder joint following excision of a malignant proximal humeral tumour is a difficult proposition.
Method
Eleven patients with primary osteosarcoma or Ewing’s sarcoma underwent reconstruction with a composite of extra-corporeally irradiated autograft with the addition of a long stemmed hemiarthroplasty. At a mean follow-up of 5.8 years two patients had died from disseminated disease and one patient had undergone amputation for local recurrence. The eight patients with a surviving limb were examined clinically and radiographically.
Result
The mean Toronto Extremity Salvage Score was 74 and Musculo-Skeletal Tumour Society score 66. Rotation was well preserved but abduction (mean 32°) and flexion (40°) were poor. There was a high rate of secondary surgery, with five out of eleven patients requiring re-operation for complications of reconstruction surgery. Radiographic estimate of graft remaining at follow up was 71%. There were no infections, revisions or radiographic failures.
Conclusion
Whilst the reconstructions were durable in the medium term, the functional outcome was no better than with other reported reconstructive methods. The composite technique was especially useful in subtotal humeral resections, allowing preservation of the elbow joint even with very distal osteotomy. Bone stock is restored, which may be useful for future revision surgery in this young group of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reconstruction following excision of malignant primary tumours of the proximal humerus has been attempted with arthrodesis [1], local tissue transfer, for example clavicula pro humero [2, 3], massive allograft [4–7], endoprosthesis alone [8–10] and endoprosthesis/graft composites [11, 12]. Restoration of function to the shoulder joint following excision of the proximal humerus is still an unsolved problem. In most cases the shoulder reconstruction acts as a spacer to provide a fulcrum for the distal limb. The elbow usually obtains a functional range of motion with reduced strength and the hand functions near normally [1]. In addition to a poor range of motion, each method of shoulder reconstruction has its own spectrum of common complications that may further compromise function of the limb.
Extracorporeal irradiation (ECI) and reimplantation of bone was first described by Spira and Lubin in 1968 [13] and has been used in our unit since 1996 [14]. The irradiated autograft provides an anatomically perfect fit with preservation of muscle attachments and 100% kill of tumour cells within the excised specimen [15]. There is the possibility of a life long biologic reconstruction, or at least a restoration of bone stock for future reconstructions.
The aim of this study was to review the oncological, functional and radiographic outcomes of intra-articular excision and reimplantation of the proximal humerus following extra-corporeal irradiation of the diseased bone along with a long stem endoprosthesis (EPR) and to compare our outcomes with those of other published techniques.
Patients and methods
Eleven consecutive patients were identified who had undergone en bloc excision of a high grade malignant primary bone tumour of the proximal humerus followed by extra-corporeal irradiation and reimplantation, between 1996 and 2005. The mean age for the six females and five males was 21.5 years (range 6–52). The minimum follow up was 24 months and the mean 5 years 8 months (range 24–113 months). The underlying diagnosis was osteosarcoma in eight patients and Ewing’s sarcoma in three patients. Two patients presented with a pathological fracture and the other nine presented with pain and/or swelling around the proximal humerus of mean 6 months (range 1–24 months) duration. At presentation one patient had lung metastases. A further patient was noted to have abnormalities on their staging CT chest scan (nodules of less than 4 mm) which disappeared following induction (pre-operative) chemotherapy. All patients received induction chemotherapy and ten patients received post-operative chemotherapy. The exact regime differed for each patient. One patient also received post-operative radiotherapy (50 Gy) to the shoulder.
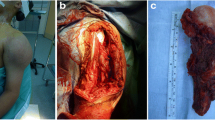
Following induction chemotherapy, surgery was performed through an extended deltopectoral approach to the proximal humerus. A wide intraarticular excision was preformed, including the biopsy tract, excising a variable amount of the deltoid muscle. The proximal humerus was divided 5 cm distal to the most distal extent of the tumour, excising a mean 17 cm (range 13–22 cm). In two patients the excision was at the level of the distal humeral metaphysis (Figs. 1, 2, 3). Using the Musculoskeletal Tumour Society classification, all resections were classified S345 [16] and by the classification system of Malawer et al. [17] all resections were classified IB.
The major muscle insertions were tagged with marker sutres and detached from the proximal humerus, leaving a cuff of tendon on the resection specimen to reattach the muscles to. Specimens of tissue from the excision margins were sent to histopathology. The proximal humeral specimen was tightly wrapped in a damp sterile cotton sheet and then triple wrapped in sterile polythene bags before being transported to the radiotherapy centre. The method of irradiation (50 Gy) has been previously described [15]. When the specimen is returned the irradiated tumour is burred away and the specimen is cleared of muscle, leaving the tendonous muscle insertions. The humeral head was replaced with a long stem modular shoulder hemiarthroplasty (Bigliani/Flatow, Zimmer, Warsaw, USA). The proximal humeral autograft is prepared to accept an appropriate width of stem, in most cases the stem tip is left proud distally to engage in the remaining distal humerus (Figs. 4, 5). The stem is first cemented into the irradiated autograft and then separately press-fitted into the distal humerus. The host-graft junction is internally fixed with a short small fragment plate (Figs 3, 5). Once the graft-prosthesis composite is secured the muscles are sequentially reattached, starting posteriorly and proximally and working distally. In three patients, who required excision of the majority of their deltoid muscle, soft tissue cover was obtained with a vascularised, innervated latissimus dorsi flap.
All patients received intravenous prophylactic antibiotics peri-operatively and then oral antibiotics post-operatively for 6 weeks. The shoulder was immobilized in a sling for 4 weeks and at that stage active pendulum exercises were commenced. No lifting, overarm activities or contact sports were allowed until the distal host-graft junction had united.
The eight patients with a surviving limb were reviewed clinically and radiographically. The musculoskeletal tumour society score (MSTS) and Toronto extremity salvage score (TESS) were completed [18–20]. Clinical examination was carried out to assess the neurological status of the distal limb and range of motion of the shoulder, elbow and wrist (compared with the contralateral upper limb). Grip strength was assessed using a Jamar dynamometer and again compared to the contralateral side. A current AP and lateral radiograph of the humerus and shoulder joint were used for radiographic assessment. Graft resorption was measured using immediate pre-operative and current AP and lateral radiographs of the reconstruction. The radiographs were digitised and analysed using ImageJ software (ImageJ 1.38x, National Institute of Health, USA). The area occupied by the bone graft was measured and expressed as a percentage of the total area of the composite. The reduction in the relative area occupied by the bone graft on the immediate post-operative and current radiographs was calculated and this was taken to reflect the amount of resorption that had taken place. The measurements for bone resorption were repeated on two occasions 4 weeks apart and the mean result taken.
Results
The mean TESS was 74 (range 41–92) and MSTS 66 (range 46–86). Active abduction of the shoulder was a mean 32° (range 20–50°) and active flexion was 40° (range 20–70°). Passive abduction measured a mean 106° (range 30–170°) and passive flexion 96° (range 30–170°). Internal rotation was equal to the unaffected side and external rotation a mean 16° (range 5–45°). There was a fixed flexion deformity of the elbow of mean 3° (range 0–12°). The range of motion of the wrist was equal to the unaffected side. The mean grip strength was 87% of the normal side (range 66–100%). The outcome for each patient is given in Table 1.
Radiographic review showed no evidence of aseptic loosening or nonunion in any of the patients at latest follow up. Superior marked subluxation was seen in one patient. All of the other patients had a slight superior migration of the humeral head within the glenoid. One patient, who had extensive distal resorption of bone and subsequent strut grafting, had a painless and radiographically stable implant, but the tip of the stem was seen to lie outside the humeral shaft. At latest follow up there was an estimated 71% (range 59–91%) of graft bone still present.
Tumour complications
There were seven tumour complications in five patients. Since surgery two patients have died from lung metastases. One of the two deaths occurred in the patient with lung metastases at presentation. This patient also had axillary node disease prior to surgery and underwent lymph node dissection at the time of proximal humeral reconstruction followed by post-operative radiotherapy. The second death occurred in a patient with no clinically detectable pulmonary metastases at the time of surgery. This patient had a poor response to induction chemotherapy (estimated necrosis 15%). Three further patients developed pulmonary metastases, two had isolated metastases and were managed with local resection. A further patient is alive but has lung metastases which are not resectable and is receiving chemotherapy. The mean time to the development of pulmonary metastases after surgery was 26 months (range 11–60 months). There were two local recurrences, one occurred in one of the two patients who have since died. The other local recurrence occurred in a patient with a narrow margin and 25% necrosis following induction chemotherapy. She was managed with a shoulder disarticulation and this patient is now alive and disease free 5 years following amputation.
Reconstruction complications
Seven complications of the surgical reconstruction occurred in five patients. The most common complication was shoulder joint instability, which affected four patients. One patient with minor intermittent symptoms was managed non-operatively. Three patients with more severe symptoms underwent stabilisation with a mesh sleeve, which was successful in two patients and in the third patient two further operations were required to achieve a stable joint. There were two nonunions of the graft-host junction, both were successfully treated with the use of bone grafting and refixation of the graft-host junction. A single patient had a strut allograft reconstruction for resorption around the distal humerus. No patients suffered a fracture, deep infection, nerve injury or loosening of the prosthesis.
Discussion
This study contains a small but relatively homogenous group of patients, all with high grade primary bone tumours of the proximal humerus. According to the MSTS, six out of eight patients in this study had good or excellent outcomes (MSTS > 60%). The function of the limb from the elbow to the hand was near normal, with only a small detectable decrease in grip strength and no clinically detectable neurological deficit. The active range of motion of the shoulder was poor when compared to the contralateral side, with marked reduction in elevation of the limb but reasonable preservation of rotation.
One of the potential benefits of using autograft in addition to an endoprosthesis is the restoration of bone stock and we felt that an estimation of graft resorption was important. Estimation of the remaining graft on the current radiographs was difficult because of the presence of bone cement, loss of the demarcation between bone and cement and the use of additional bone graft to treat post-operative complications in some patients.
The five patients with pulmonary metastases and/or local recurrence were also the patients with a poor response to induction chemotherapy, with less than 25% necrosis. In addition the two patients with local recurrence both had a narrow margin of excision. In retrospect they may have been better served with amputation as a primary procedure, however it is unlikely that the decision to perform limb salvage has affected their prognosis in the long term. The first patient is disease free with a shoulder disarticulation and the second patient died from pulmonary metastases, which were evident at presentation. This patient had a poor prognosis from the beginning of treatment and was spared from having an amputation, which was not likely to have conferred any survival advantage over limb salvage.
Massive allografts alone in the reconstruction of the proximal humerus are problematic. There is a significant risk of graft resorption and fracture, infection, instability of the shoulder and delayed or nonunion [4, 6, 15, 21]. The survivorship is reported at approximately 70% at 5 years [5]. We have used ECI autograft alone in two patients, both of which resulted in massive resorption of proximal bone, resulting in a switch to a composite technique described here. Endoprosthetic replacement on its own allows early supervised movement of the limb and eliminates the risk of nonunion and graft resorption as well as markedly reducing the fracture risk. The survivorship is superior to the use of allograft alone and the functional results are equivalent [10]. Using an EPR, there is no prospect of a biological reconstruction and since many malignant tumours occur in a young population future revision surgery is likely. Reattachment of muscles to the prosthesis is often unsatisfactory, reducing the chance of obtaining a good range of motion of the shoulder.
The use of a composite of bone graft and endoprosthesis may mitigate some of the problems of the use of allograft or EPR alone. The graft component provides an anatomic fit with restoration of bone stock and tendon insertions for the reattachment of muscles. The prosthesis component reduces the risk of fracture and of resorption of the vulnerable proximal bone. We chose to use ECI bone as the graft material. The dose of radiation used in ECI is 50 Gy, about 500 times less than the dose used in the preparation of irradiated allograft bone. The effect of radiation on the strength of bone is dose dependent [22, 23] and doses of up to 5 kGy have been shown to allow bone to retain most of its biological potential [24]. ECI grafts have the capacity to heal at the graft junction and to regenerate bone in the graft and attached tendons [25]. Therefore, the quality of ECI bone is likely to be superior to allograft, which should also decrease the time to incorporation and reduce the rate of graft-host nonunion. The use of ECI bone does not require the maintenance of a bone bank for massive allografts, and the fit of the graft is anatomic.
Instability is a common problem following proximal humeral reconstruction and is not solved by the use of a composite technique. Surgery for instability is reasonably successful in the control of symptomatic instability but does result in a poorer functional outcome (both patients with a MSTS < 60% were in this group) and reduced range of motion. For this reason we do not routinely use artificial capsular sleeves during initial reconstruction, accepting that some patients will need stabilisation procedures in the future.
Following reconstruction of the proximal humerus after tumour excision, the function of the shoulder is related to the size of the resected specimen [10] and the preservation of the abductor mechanism. These margins are pre-determined by the size and location of the presenting tumour. The MSTS and TESS scores of the patients in this series were no better than the use of an EPR alone. This was a surprise as we felt that the use of a perfectly fitting autograft with retained tendon for muscle reattachment, would improve muscle and shoulder function. The passive range of motion was substantially better than the active range, indicating that the restriction to movement is not due to scarring or stiffness of the joint. Our method of muscle reattachment, using large non-absorbable sutres may not be optimal, with resorption of bone weakening the tendinous insertion of the muscle. Muscles may be defunctioned by damage to their nerve or blood supply, partial resection, prolonged inactivity or erosion by the prosthetic head. The joint biomechanics are disrupted by the use of a hemiarthroplasty which may not restore the muscles to their correct tension or orientation. Reduced range of motion of the shoulder joint continues to be a problem with the use of graft/EPR composites and we suspect that the problem is not solved by simply improving the fixation of the proximal humeral musculature to the reconstruction. A reconstruction that is stable during motion and which optimizes the biomechanics of the shoulder joint is also necessary for the shoulder to function as normally as possible. The use of a reverse shoulder prosthesis with ECI graft in the reconstruction of the shoulder joint has been reported in proximal humeral resections, with preservation of the abductor mechanism [26]. The functional results are impressive but given the young age of our patients and the uncertain longevity of this type of prosthesis we have doubts as to whether reconstruction with a reverse prosthesis will be a durable solution. We feel that the use of a composite technique offers the best prospects for maximizing shoulder function following tumour resection from the proximal humerus. However, in its current form this technique has not solved the problem of poor shoulder function.
In keeping with the published literature on the use of massive bone grafts there was a high incidence of secondary surgery [4]. The main disadvantage of the use of an ECI/EPR composite, when compared with the use of EPR alone, is the higher rate of secondary surgery [10]. The advantages are restoration of distal bone stock and the possible, unproven, improvement in survival of the reconstruction. No prosthesis has required revision for any reason and it is therefore not possible to report the ease of revision or the usefulness of the autograft in future reconstructions. We would predict that subsequent revision surgery would be less extensive when compared to EPR alone because of the increased distal bone stock. The use of an ECI/EPR composite is most useful in subtotal humeral resections. It allows preservation of the elbow joint even with very distal resections (Figs. 1, 2, 3). In addition, once the osteotomy has healed, the strength of the reconstruction will be greatly enhanced with a greater length of bone to support the prosthesis. We had no revisions of reconstruction even with resections at the level of the distal humeral metaphysis. Further follow up is required to see if the reconstructions will be durable in the long term.
References
Damron TA, Rock MG, O’Connor MI, Johnson M, An KN, Pritchard DJ, Sim FH (1996) Functional laboratory assessment after oncologic shoulder joint resections. Clin Orth Rel Res 348:124–134
Rodl RW, Gosheger G, Gerbert C, Lindner N, Ozaki T, Winkelmann W (2002) Reconstruction of the proximal humerus after wide resection of tumours. J Bone Joint Surg Br 84B:1001–1008
Tsukushi S, Nishida Y, Takahashi M, Ishiguro N (2006) Clavicula pro humero reconstruction after wide resection of the proximal humerus. Clin Orth Rel Res 447:132–137
Gebhardt MC, Roth YF, Mankin HJ (1990) Osteoarticular allografts for reconstruction in the proximal part of the humerus after excision of a musculoskeletal tumour. J Bone Joint Surg Am 72A:334–345
Getty PJ, Peabody TD (1999) Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am 81A:1138–1146
Rodl RW, Ozaki T, Hoffmann C, Bottner F, Lindner N, Winkelmann W (2000) Osteoarticular allograft in surgery for high grade malignant tumours of bone. J Bone Joint Surg Br 82B:1006–1010
Mankin HJ, Gebhardt MC, Jennings C, Springfield DS, Tomford WW (1996) Long-term results of allograft replacement in the management of bone tumors. Clin Orth Rel Res 324:86–97
Bos G, Sim F, Pritchard D, Shives T, Rock M, Askew L, Chao E (1987) Prosthetic replacement of the proximal humerus. Clin Orth Rel Res 224:178–191
Malawer MM, Chou LB (1995) Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high-grade bone sarcomas. J Bone Joint Surg Am 77A:1154–1165
Kumar D, Grimer RJ, Abudu A, Carter SR, Tillman RM (2003) Endoprosthetic replacement of the proximal humerus. Long term results. J Bone Joint Surg Br 85B:717–722
Black AW, Szabo RM, Titelman RM (2007) Treatment of malignant tumors of the proximal humerus with allograft-prosthesis composite reconstruction. J Shoulder Elbow Surg 16:525–533
Jensen KL, Johnston JO (1995) Proximal humeral reconstruction after excision of a primary sarcoma. Clin Orth Rel Res 311:164–175
Spira E, Lubin E (1968) Extracorporeal irradiation of bone tumours: a preliminary report. Isreal J Med Sci 4:1015–1019
Davidson AW, Hong A, McCarthy SW, Stalley PD (2005) En-bloc resection, extracorporeal irradiation, and re-implantation in limb salvage for bony malignancies. J Bone Joint Surg Br 87B:851–857
Hong A, Stevens G, Stalley P, Pendelbury S, Ahern V, Ralston A, Estoesta E, Barrett I (2001) Extracorporeal irradiation for malignant bone tumours. Int J Radiat Oncol Biol Phys 50:441–447
Enneking W, Dunham W, Gebhardt M, Malawar M, Pritchard D (1990) A system for the classification of skeletal resections. Chir org mov 75(Suppl 1):217–240
Malawer MM, Meller I, Dunham WK (1991) A new surgical classification for shoulder-girdle resections. Analysis of 38 patients. Clin Orthop Rel Res 267:33–44
Davis AM, Bell RS, Badley EN, Yoshida K, Williams JL (1999) Evaluating functional outcome in patients with lower extremity sarcoma. Clin Orthop Relat Res. 358:90–100
Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS (1996) Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res 5:508–516
Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ (1993) A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 286:241–246
O’Connor MI, Sim FH, Chao EYS (1996) Limb salvage for neoplasms of the shoulder girdle. Intermediate reconstructive and functional results. J Bone Joint Surg Am 78A:1872–1888
Hamer AJ, Strachan JR, Black MM, Ibbotson CJ, Stockley I, Elson RA (1996) Biochemical properties of cortical allograft bone using a new method of bone strength measurement: a comparison of fresh, fresh-frozen and irradiated bone. J Bone Joint Surg Br 78B:363–368
Currey JD, Foreman J, Laketic I, Mitchell J, Pegg DE, Reilly GC (1997) Effects of ionizing radiation on the mechanical properties of human bone. J Orthop Res 15:111–117
Voggenreiter G, Ascherl R, Blumel G, Schmit-Neuerburg KP (1996) Extracorporeal irradiation and incorporation of bone grafts: autogenic cortical grafts studied in rats. Acta Orthopaedica Scand 67:585–588
Hatano H, Ogose A, Hotta T, Endo N, Umezu H, Morita T (2005) Extracorporeal irradiated autogenous osteochondral graft: a histological study. J Bone Joint Surg Br 87-B:1006–1011
De Wilde LF, Plasschaert FS, Audenaert EA, Verdonk RC (2005) Functional recovery after a reverse prosthesis for reconstruction of the proximal humerus in tumor surgery. Clin Orth Rel Res 490:156–162
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moran, M., Stalley, P.D. Reconstruction of the proximal humerus with a composite of extracorporeally irradiated bone and endoprosthesis following excision of high grade primary bone sarcomas. Arch Orthop Trauma Surg 129, 1339–1345 (2009). https://doi.org/10.1007/s00402-008-0752-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-008-0752-1