Abstract
Introduction
An adeno-associated virus (AAV) derived vector in gene transfer model that induces IGF-1 expression could repair articular cartilage.
Materials and methods
Male Wistar rats, 150 and 200 g, and 7 weeks old, were used. Effectiveness of constructed vectors was assayed inoculating them in rat knees of control and damaged animals either mechanically or by collagen-induced arthritis. Inoculation was intra-articular with 50 μL of recombinant AAV-Luciferase (1.25 × 108 particles). The rats were killed after 1, 2, 4 and 8 weeks. IGF-I activity was analyzed by injecting 50 μL of recombinant AAV (1.25 × 108 particles) in animals with damaged knees. Final analysis was performed after 8 weeks.
Results
The activity of AAV vectors in vitro shows the presence of mRNA coding to IGF-I in cells infected with AAV-IGF and not in control cells without viral vectors and an increase in secreted IGF-I protein in culture medium. In vivo, AAV derived vectors induced protein expression in cartilage 2 months after inoculation. In the animals killed after 1 and 2 weeks, no significant increase in the reaction of luciferase was observed (P > 0.05). In the group of animals with no injury an increase was observed at 4 weeks, which was more marked and significant after 8 weeks (P = 0.029). The same behavior occurred in the animals with induced arthritis and in the mechanical injury group. In the levels of expression after 8 weeks, no significant differences were found between the two groups of injured animals and the group of healthy animals infected with the virus. The joints of the animals that were subjected to injuries in the cartilage and inoculated with AAV-IGF-I presented a similar appearance to those animals inoculated with saline solution.
Conclusion
Autoimmune and mechanical lesions did not show improvement in the state of its cartilage after the treatment. The use of AAV vectors capable of inducing the expression of IGF-I in vitro is therefore not sufficient to protect the cartilage from the serious damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Articular cartilage is an avascular articular tissue which protects the ends of the bones, and allows less friction in the movement of the joints. The maintenance of a healthy and functional cartilage is essential for physical activity [3, 19]. One consequence of the lack of vascular supply to the cartilage is its limited capacity to self repair, which results in the gradual spreading of the injuries to the cartilage [4, 8, 15]. When the lesion includes the underlying bone, a response originated through the release of bone marrow content may occur, which generates a tissue that fills the injured area. This tissue will be similar to cartilage for 6–8 months, but the type I collagen will increase, generating a fibrocartilage tissue which is incapable of fulfilling the mechanical functions of the articular cartilage [5, 15, 24].
Nowadays there are no methods that completely restablish the function of damaged cartilage. Techniques like microfracture, allografts or even autollogous chondrocyte implantation do not achieve a complete cartilage healing, and most of them originate from fibrocartilage with bad mechanical conditions. Tissue engineering and gene therapy could provide a useful tool to produce a tissue capable of maintaining the mechanical and biological functions of the original cartilage. Some techniques introduce an implant at the site of the injury, consisting of a three-dimensional structure (such as collagen, alginate or chitosan) in which the cellular component is seeded. This generally consists of auotologous cells, which can be modified in the laboratory so that they express growth factors [2, 18, 23]. These strategies need two surgical procedures: the first one to obtain the cells to be modified and implanted in the second, which constitutes a disadvantage. Nevertheless, they have been widely studied using insulin like growth factor (IGF-I), which have been considered as the main anabolic factor for cartilage cells, and have proved to have many effects on these kind of cells. The greatest obstacles to this kind of treatment include disappearance of the cellular response to IGF-I inside the damaged cartilage, and the short average life of the protein. Despite these handicaps, some studies have obtained positive results [7, 13, 20, 25].
The aim of the present study is to construct a model of gene therapy using adeno-associated viruses and to study whether an IGF-I delivery system directly injected into the knee without any surgery is capable of preventing injuries caused by mechanical or auto-immune damage.
Materials and methodology
Experimental design
The study was divided into two parts. The first, in order to assay the induction of expression, recombinant adeno-associated viruses (AAV) capable of inducing luciferase expression (AAV-luciferase) were used. Animals were divided into four groups as follows (Table 1):
-
Group 1: Control
-
Group 2: Inoculation with AAV-luciferase
-
Group 3: Collagen induced arthritis (CIA) model inoculated with AAV-luciferase
-
Group 4: Mechanical injury model inoculated with AAV-luciferase.
Four animals of every group were killed at 1, 2, 4 and 8 weeks after the virus inoculation.
In the second part of the study (once optimized the time for expression induction by the vectors), recombinant adeno-associated viruses (AAV) capable of inducing IGF-I expression (AAV-IGF-I) were used to analyze the IGF-I effect in the joint disorders. The animals were divided as follows (Table 2):
-
Group 1: Control
-
Group 2: Control CIA model
-
Group 3. Control Mechanical injury model
-
Group 4: AAV-IGF-I inoculation.
-
Group 5: AAV-IGF-I inoculation and CIA model
-
Group 6: AAV-IGF-I inoculation and mechanical injury model.
Time of analysis was determined according to the results obtained in the first part (8 weeks).
Proceedings with animals
The animal experiments were carried out according to the policies and principles established by the Animal Welfare Act, and our national animal welfare guidelines. The study was approved by our Institutional Animal Care and Use Committee. Eighty-eight male Wistar rats were used, weighing between 150 and 200 g, and aged 7 weeks.
Recombinant AAV vectors were inoculated intra-articularly (1.25 × 108 particles) in both the knees suspended in 50 μL of saline solution. Control groups were inoculated in both the knees with 50 μL of the saline solution. The intra-articular injections were performed after sedation with 100 mg/kg ketamine (Imalgene™, Merial, Harlow, Essex, UK) administered intra-peritoneally. The joint damage model was carried out on the next day, after inoculation with the virus as described below.
Animals were killed by over-exposure to CO2. In order to evaluate the physical state of the animals, and to avoid possible variations in the knee biomechanics during the evolution of both the models, body weight parameter was monitored during all the experiments.
Collagen-induced arthritis model
The animals were inoculated with a collagen emulsion to produce arthritis according to Trentham et al. [27]. The model was induced as follows: 8 mg of chicken type II collagen (Sigma™, St Louis, MO, USA) was diluted in 2.5 mL of 0.5 M acetic acid. The resulting solution was added drop-wise to 2.5 mL of incomplete Freund adjuvant (BD™, Franklin Lakes, NJ, USA). They were mixed for 2–3 min using an Ultra Turrax homogenizer (IKA™, Staufen, Germany) until an emulsion was achieved. The inoculation of 0.5 mL emulsion was performed subcutaneously in the animal’s back, 1 day after inoculation with the virus. As a booster dose, 0.1 mL of the same collagen emulsion was injected 1 week after the first dose, under the same conditions.
Mechanical damage model
The mechanical lesion was produced in both the knees. A hypodermic needle was introduced through the anterior part of the knee, across the patellar tendon under fluoroscopy. A widespread osteochondral injury in the tibial plate was performed by scratching the tibial surface and reaching the subchondral bone. During the complete procedure, animals were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylacine (Rompun™, Bayer, Leverkusen, Germany) administered intraperitoneally.
Construction of adeno-associated viruses
The recombinant viruses were constructed according to the method used by Zaratiegui et al. [33]. Co-transfection of 293T cells was performed with two plasmids, one of which contained the genome of the virus except for the rep and cap genes and the cDNA, which codes for luciferase/IGF-I under the control of the cytomegalovirus promoter (CMV). The other had the two genes, rep and cap inserted. The cells used had the genes of the adjuvant adenovirus needed for replication. In the cytoplasm of the cells, the formation of the viral particles took place. The cells were lysed and their DNA and RNA were digested. Finally, the viruses were purified by chromatography in an agarose column, and their titer estimated by quantitative PCR.
In vitro assay
Adeno-associated virus vectors were first assayed in vitro for its capability to induce expression of IGF-I in cultured chondrocytes. All cell culture reagents were purchased from Gibco-BRL™ (Gaithersburg, MD, USA). Cells were extracted from rat cartilage using collagenase/dispase digestion and grown in monolayer up to passage 2 in DMEM medium supplemented with 10% FBS, penicillin/streptomicyn, l-Cys and Hepes. They were then detached using a tripsin/EDTA solution and counted with trypan blue dye. A total of 5 × 105 cells were seeded on 75 cm2-flasks with medium consisting of DMEM supplemented with 0.1% FBS, penicillin/streptomicyn, l-Cys and Hepes. Two hours after seeding, viral vectors were added (m.o.i 50.000) and resuspended in 1 mL culture medium. Control flask was supplemented with 1 mL medium without viral particles.
AAV vectors were allowed for the infection and induction of protein expression during 72 h, and then cells were collected by tripsinization and centrifugation (5 min at 1,500g). Conditioned media were also collected and stored at −20°C. After resuspension of cell pellet in Trizol™ reagent, RNA was extracted according to manufacturer’s guidelines, and final RNA pellet was resuspended in 20 μL RNAse free water. Conditioned media were assayed for the presence of IGF-I by ELISA assay, and RNA was subjected to RT-PCR experiments.
Protein determination
Prior to ELISA assay, conditioned media were subjected to Bradford assay to determine the protein content. Bradfor reagent (Bio-Rad™, Hercules, CA, USA) was used in 96 well plates in a total volume of 200 μL per well using Bovine Serum Albumin as a standard. Absorbance was measured at 595 and 450 nm using a plate reader (Organon Teknica™, Boxtel, Netherlands), and the ratio 595/450 nm was used to calculate protein concentration.
ELISA
A volume of 100 μL of every extract was added per well (four repeats) in 96 well plates, using 100 μL per well of fresh medium and PBS as controls. After 2 h of incubation at room temperature, plates were blocked with 100 μL of TBS/0.5% Tween 20 and 1% BSA for 2 h at RT. Specific antibody to IGF-I (Upstate-Millipore™, Billerica, MA, USA) was diluted 1:1,000 in blocking buffer and 100 μL was added to every well. After 2 h of incubation at RT, three washes were performed using blocking buffer and then secondary antibody HRP conjugated (Amersham-Biosciences™, Piscataway, NJ, USA) was added at a 1:500 dilution and allowed for its binding for 30 min. After washing again as described, colour was developed adding citrate buffer supplemented with 1.1 mg/10 mL 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) and H2O2 (45 μg/10 mL) and waiting until the appearance of color. Absorbance was measured at 405 nm with a plate reader (Organon Teknika™).
RT-PCR
After RNA concentration determination by spectrophotometry (Genequant II, Amersham Biosciences™), RT-PCR assays were performed (Superscript Platinum RTPCR System™ Invitrogen™). For each reaction, 10 ng of RNA and 20 nM of each of the two primers (Proligo™, Boulder, CO, USA) were used. Finally, a thermal cycler was programmed (Omnigene™ Hybaid™, Franklin, MA, USA) to achieve the following reaction: 50°C, 30 min; 94°C, 2 min, 1 cycle; 94°C; 1 min; Tm 1 min; 72°C, 1 min, 30 cycles and 72°C, 10 min, 1 cycle. Primers used were tctacaacgagctgcgtgtg (forward) and cgtgaggatcttcatgaggt (reverse) for β-actin gene and atgtcgtcttcacatctcttctacc (forward) and gatggaacgagctgactttgtag (reverse) for IGF-I gene.
The results were visualized in a 2% agarose gel and photographed and analyzed using Gel Doc 2000™ system (Bio-Rad™). Every experiment was repeated three times, to asses its reproducibility and a representative gel is presented.
Obtaining and processing the tissue
After killing the animals, the two femora–tibial joints were removed. The right knees were stored at −20°C for total protein extraction, while the left knees were directed for histological processing. The specimens for the morphological study were maintained in 4% formaldehyde, for 24 h. Once fixed, were decalcified in a solution of 7.5% poly-vinyl-pyrrholidone (PVP), and 10% EDTA, in Tris 0.1 M and pH 6.95 for at least 3 weeks, at room temperature. Subsequently, they were embedded in paraffin. Finally, 4 μm thick sections were obtained using a conventional microtome (Microm™, Neuss, Germany) and stained with Masson’s trichrome and Safranine-O. Safranine stained samples were graded for the histological state of cartilage using Mankin score [14] (Table 3).
To extract the protein, the joints were frozen in CO2 and triturated. They were subsequently homogenized using an Ultra-Turrax homogenizer (IKA®), and 1.5 mL RLB buffer (Reporter Lysis Buffer) was added corresponding to the Luciferase Assay System (Promega®, Madison, WI, USA). The homogenate was centrifuged for 10 min at 15,000g to obtain a supernatant which constituted the protein extract from each knee. A volume of 100 μL of this extract was added to 100 μL of the substrate for the luciferase. To read the results at 562 nm, a luminometer (Berthold Systems®, Bad Wildbad, Germany) was used. The total protein concentration for each sample was determined using Bradford’s method (Bio-Rad™), and the result was used to normalize the data corresponding to the luciferase activity.
Immunhistochemistry
Immunostaining was performed using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA USA). Briefly, after trypsin treatment and following deparaffinization, endogenus peroxidase was blocked by placing the sections in 3% hydrogen peroxide solution for 30 min. They were then incubated in the following reagents with appropriate tris-buffered-saline (TBS 0.55 M, pH 7.36) washes: normal pig serum for 30 min, primary antibody (anti luciferase diluted 1:100, SIGMA™) for 1 h, biotinylated antirabbit antibody for 30 min, and avidin–biotin complex for 30 min. The reaction was visualized with chromogen substrate solution (0.3 mg/mL diaminobenzidine and hydrogen peroxide), and sections were then counterstained with Harris’ hematoxilin, dehydrated, and mounted. Normal serum was used as negative control.
Statistical analysis
The data obtained in the luminometer to detect the presence of luciferase and those obtained with the Mankin score were compared using the non-parametric Kruskal–Wallis and Mann–Whitney U tests. The former was used to determine the differences between all the different treatments, and the latter was used for the differences between each treatment. Significance was set at P = 0.05 for all tests. For the analysis, the program SPSS 9.0 for Windows™ was used.
Results
AAV induction in vitro
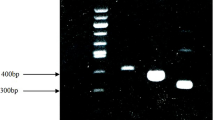
Chondrocytes in monolayer culture were used to asses the activity of AAV vectors in vitro. After infection, RNA from cells was subjected to RT-PCR. Results presented in Fig. 1 show the presence of mRNA coding to IGF-I in cells infected with AAV-IGF and not in control cells, without the viral vectors or with AAV-Luciferase. The mRNA presence agrees with the results obtained in the ELISA assays. The figure also shows an increase in specific binding of the antibody to IGF-I, as indicated by the absorbance measure, demonstrating a higher level of IGF-I protein in conditioned media from cells infected with AAV-IGF-I vectors, and thus an efficient induction of expression.
Induction of IGF-I expression in cultured chondrocytes using AAV vectors. a ELISA assay of conditioned culture media. b RT-PCR analysis. Both figures show an increase in IGF-I protein (a) and mRNA (b) content in AAV-IGF-I infected cells. Lanes on gel are 1: Φ × 174/HaeIII, DNA marker; 2: control cells; 3: AAV-IGF-I infected cells. Φ × 174/HaeIII DNA bands sizes are 1,353 bp, 1,078 bp, 872 bp, 603 bp and 310 bp. AAV-Luciferase: adeno-associated virus vector with luciferase DNA, AAV-IGF-I: adeno-associated virus vector with IGF-I DNA
Models of joint damage
To ensure that the state of the animals did not have any influence on the evolution of the damage models, the physical conditions of the animals were evaluated by monitoring body weight, as shown in Fig. 2. Animals in every group showed similar patterns of weight gain, independent of the treatment applied. Thus, condition of the cartilage was only affected by mechanical injuries or by injection of type II collagen. After 2 months, the aspect of the joints in animals inoculated with saline solution (control group) showed no type of alteration. Tibial cartilage had no injury, and was healthy with normal thickness and homogenous surface (Fig. 3).
Histological analysis of damaged models using Massons Trichrome staining, a saline solution (×20), b collagen induced arthritis (CIA) (×20), c mechanical injury (×20), d IGF-I (×20), e CIA + IGF-I (×20), f mechanical injury + IGF-I (×20), g quantification of damage following Mankin score. Zones with an intense disorganization of cartilage structure are indicated by asterisks. T Tibia, C tibial Cartilage, M meniscus
In the joints of rats inoculated with the recombinant adeno-associated virus AAV-Luciferase, the morphology of the tibial cartilage was similar to that in the control group. In animals that suffered the CIA model, the articular cartilage was thinner than in the control group. In some regions, the cartilage disappeared leaving the subchondral bone of the tibia exposed. The surface of the tissue was rough and uneven (Fig. 3). Similar articular cartilage morphology was found in the animals that suffered a mechanical injury (Fig. 3).
Mankin score confirms the results presenting a significant increase in the grading of animals injured either mechanically or with collagen induced arthritis. This increase is not observed in control animals inoculated with saline solution or in control animals inoculated only with the virus (Fig. 3).
AAV induction in vivo
To asses the activity of AAV vectors in vivo, AAV-Luciferase vectors were used in control and damaged rats (Fig. 4), and total knee protein extracts assayed for the reaction of luciferase. In the animals killed after 1 and 2 weeks, no significant increase in the reaction was observed (P > 0.05). In animals with no injury, after 4 weeks of treatment, an increase in the parameter obtained using the luminometer was observed (P > 0,05). The increase was more marked and significant after 8 weeks (P = 0.029). The same behavior occurred in the animals with CIA, with a non-significant increase at 4 weeks and a significant increase at 8 weeks of treatment (P = 0.029). In the animals that suffered mechanical injury, the level of luciferase reaction did not increase after 4 weeks of treatment but delayed until 8 weeks (P = 0.029).
After 8 weeks, no significant differences were found between the two groups of injured animals and the group of healthy animals infected with the virus.
The joints corresponding to the animals killed at 8 weeks were analyzed by immunohistochemistry using a specific antibody for luciferase. The results show the expression induced by the adeno-associated virus. The cells in articular cartilage of AAV-Luciferase inoculated animals were stained specifically, compared with cells in control animals, that showed no staining (Fig. 3). Thus, data presented here prove that AAV derived vectors are efficient for the induction of protein expression in articular cartilage 8 weeks after a direct intra-articular administration in vivo.
Effect of IGF-I gene therapy on joint damage
The joints of the animals that were subjected to injuries in the cartilage and inoculated with AAV-IGF-I presented a similar appearance to those animals inoculated with saline solution (Fig. 4). The intra-articular inoculation of AAV-IGF-I did not modify the appearance of the joint after induction of collagen-induced arthritis, or after mechanical injury after 8 weeks. The injured animals inoculated with adeno-associated viruses presented similar characteristics, with de-structuring of the tibial cartilage, and disappearance of the tissue in certain parts, leaving the subchondral bone exposed.
Results obtained with the Mankin score (Fig. 4) showed no decrease in animals injured and previously treated with AAV-IGF-I. Both lesions, autoimmune and mechanical, did not show an improvement in the state of its cartilage, suggesting inability of the proposed strategy for protecting or inducing the repair of articular cartilage. The use of AAV vectors capable of inducing the expression of IGF-I injected intrarticularly in vivo is therefore not sufficient to protect the cartilage from the serious damage provoked by the models used in this study.
Discussion
IGF-I promotes the proliferation and synthesis of extracellular chondrocyte matrix, which has promoted several studies that support the use of IGF-I in the treatment of joint disorders [16, 28–30]. In canine models of arthritis, the intra-articular administration of 2 μg of IGF-I, three times a week for three weeks, produced an improvement in the state of the cartilage [25]. The treatment of chondral defects in rabbits and pigs by administering 50 ng/mL of IGF-I in fibrin patches improved the state of cells of the repair tissue [10], whereas in rats treated with recombinant adenoviruses for IGF-I, a better repair was achieved in the short term [9]. Nixon et al [20], in a horse model, improved the repair of large chondral defects by applying 25 μg of IGF-I on fibrin patches. The integration of implants of chondrocytes in alginate was boosted when the cells were modified to express IGF-I [13]. Nonetheless, there are no published studies which demonstrate that articular cartilage is protected by administrating IGF-I via gene therapy in vivo. The study by Mi et al. [17], achieved stimulation of the chondrocyte metabolism in rabbits in vivo using a strategy of IGF-I administration via recombinant adenoviruses, but this did not improve the state of the cartilage in arthritic joints, even though the IGF-I did produce an anabolic effect.
AAV is an advantageous technique for the induction of protein expression in vivo. Using these vectors, in vivo and in vitro induction of expression by chondrocytes in different conditions has been achieved [12, 31, 32]. Several publications also report the successful introduction of genes with therapeutic capacities, such as IL-4, IL-10, and the TNF-α receptor [1, 6, 32, 34]. Zhang et al. [34], used an intra-articular injection in transgenic animals which over-express the same TNF-α factor. This same administration strategy was used in the arthritis model by Pan et al. [22] mediated by lipopolysaccharides in the rat and in that by Watanabe et al. [32] in mice with collagen-induced arthritis.
We performed and compared two models of joint damage. One comprised collagen-induced arthritis, while the other was based on a mechanical injury. Both models caused cartilage destruction, which disappeared in parts, leaving the subchondral bone of the tibia exposed. In spite of the damage produced in the knee, we have achieved induction of luciferase expression by cartilage cells. In animals that were subjected to the CIA model, the expression initiated after 4 weeks of inoculation was the same as in the control group without any damage. Nevertheless in mechanically injured knees, the detection of expression delayed until the 8th week, but the level of expression was not significantly different than other groups at that time. Perhaps mechanically produced damage, performed the day after the injection of the virus, provoked a loss of infected cells, which would cause a decrease in the viral particles that effectively infected cells. This fact would delay the time needed to achieve a sufficient and detectable level of luciferase, without delaying the beginning of the protein expression induction. On the other hand, cytokines released by dying cells in damaged cartilage could affect the metabolism and behavior of infected cells, which could also be responsible for the molecular mechanisms that delayed the induction of expression.
Based on those results, and due to the lack of studies that focus on the direct effect of IGF-I on joint disorders, the main goal of our work was to deliver IGF-I in damaged knees by inoculating AAV-IGF-I. According to recent references, the effect on the joint damage in most studies of gene therapy with IGF-I is based on boosting the effects of cell implants, without demonstrating whether IGF-I itself performs significant activities for preventing or repairing damage.
Although we achieved luciferase expression throughout the time of the study, administration of AAV-IGF-I was not enough to prevent damage. As shown in Fig. 4, the aspect of cartilage from animals inoculated with AAV-IGF-I that suffered any damage was similar to that of damaged animals inoculated with saline solution. Related to this, there are some facts that could explain this lack of effect. The greatest disadvantages of joint therapy using IGF-I are the short average life of the protein in biological systems, and the low response in cells from damaged or old cartilage [13]. The former problem was addressed in earlier studies by repeated administration or the use of fibrin patches capable of releasing the protein gradually [20]. Gene therapy should ensure that the cells themselves express IGF-I over a long period of time, eventually solving this problem. This might be the case of studies such as that by Mi et al. [17], or our own, in which the prolonged administration of IGF-I should not be necessary. Nevertheless it would seem that the cellular response to the growth factor was not enough to halt the damage caused, or that expression of IGF-I induced by these vectors did not provide a sufficient protein concentration to achieve any significant effect. Implantation of genetically modified cells could solve the second of the problems, as these would provide cells capable of responding to IGF-I. Madry et al. [13], uses these techniques to achieve the repair of chondral lesions. However, the implant used as a control also had a reparative effect on the injury, which does not provide any data in our understanding of the effect of IGF-I on the damaged cartilage or its potential for stimulating repair in the tissue by itself.
Both IL-1 and TNF-α, and IGF-I itself, are capable of stimulating the appearance of proteins that bind IGF or IGFBPs. According to Olney et al. [21], IL-1 and TNF-α encourage the synthesis of IGFBP-3 but not IGFBP-4. However, Sunic et al. [26], describe the stimulation of IGFBP4 by IL-1, and IGF-I itself is capable of inducing the production of IGFBP-4 in a mechanism, which prevents its in vivo action even in tissues that have not undergone inflammatory damage. Thus the presence of all these proteins in the extracellular matrix of cartilage from damaged knees has been proposed as being responsible for the waning of the response to IGF-I in old or damaged tissue, and might partly explain the lack of response obtained in the present study.
Thus, IGF-I gene therapy approach used in our study is not sufficient for the repair of in vivo systems. Other attempts of repair have been published trying to eliminate the catabolic activity, which takes place in the extracellular matrix of damaged tissue [11]. However, although these are capable of slowing down cartilage degradation, they are not capable of promoting the synthesis of new tissue, which is also needed for the joint re-establishment. In short, the administration of AAV capable of inducing IGF-I production, in spite of IGF-I capacity to exert some effect on the metabolism [17], is insufficient to slow down the damaged caused by the two models used in our study.
References
Apparailly F, Millet V, Noel D, Jorgensen C (2002) Tetracycline-inducible interleukin-10 gene transfer mediated by an adeno-associated virus: application to experimental arthritis. Hum Gene Ther 13:1179–1188
Brittberg M, Peterson L, Sjörgen-Larsson E, Tallheden T, Lindahl A (2003) Articular cartilage engineering with autologous chondrocyte transplantation. J Bone Joint Surg (Am) 85-A(suppl 3):109–115
Buckwalter JA, Mankin HJ (1998) Articular cartilage: tissue design and chondrocytematrix interactions. AAOS Instr Course Lect 47:477–486
Cheung HS, Cottrell WH, Stephenson K, Nimni ME (1978) In vitro collagen biosynthesis in healing and normal rabbit articular cartilage. J Bone Joint Surg (Am) 60-A:1076–1081
Coletti JM Jr, Akeson WH, Woo SL (1972) A comparison of the physical behavior of normal articular cartilage and the arthroplasty surface. J Bone Joint Surg (Am) 54-A:147–146
Cottard V, Mulleman D, Bouille P, Mezzina M, Boissier MC, Bessis N (2000) Adeno-associated virus mediated delivery of IL-4 prevents collagen-induced arthritis. Gene Ther 7:1930–1939
Fortier LA, Mohammed HO, Lust G, Nixon AJ (2002) Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg (Br) 84-B:276–288
Fuller JA, Ghadially FN (1972) Ultrastructural observations on surgically produced partial-thickness defects in articular cartilage. Clin Orthop Relat Res 86:193–205
Gelse K, von der Mark K, Aigner T, Park J, Schneider H (2003) Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis Rheum 48:430–441
Hunziker EB, Rosenberg LC (1976) Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg (Am) 78-A:721–733
Lechman ER, Jaffurs D, Ghivizzani SC, Gambotto A, Kovesdi I, Mi Z et al (1999) Direct adenoviral gene transfer of viral IL-10 to rabbit knees with experimental arthritis ameliorates disease in both injected and contralateral control knees. J Immunol 163:2202–2208
Madry H, Cucchiarini M, Terwilliger EF, Trippel SB (2003) Recombinant adenoassociated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum Gene Ther 14:393–402
Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, Menger MD et al (2005) Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I). Gene Ther 12:1171–1179
Mankin HJ, Dorfman H, Lippiello L, Zarins A (1971) Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg (Am) 53-A:523–537
Mankin HJ (1982) The response of articular cartilage to mechanical injury. J Bone Joint Surg (Am) 64-A:460–466
McQuilaan DJ, Handley CJ, Campbell MA, Bolis S, Milway VE, Herington AC (1986) Stimulation of proteoglycan synthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J 240:424–430
Mi Z, Ghivizzani SC, Lechman ER, Jaffurs D, Glorioso JC, Evans CH et al (2000) Adenovirus-mediated gene transfer of insulin-like growth factor 1 stimulates proteoglycan synthesis in rabbit joints. Arthritis Rheum 43:2563–2570
Minas T, Chiu R (2000) Autologous chondrocyte implantation. Am J Knee Surg 13:41–50
Mow V, Rosenwasser M (1988) Articular cartilage: biomechanics. In: Woo SL, Buckwalter JA (eds) Injury and repair of the musculoeskeletal soft tissues. American Academy of Orthopaedic Surgeons, Park Ridge, pp 427–463
Nixon AJ, Fortier LA, Williams J, Mohammed H (1999) Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J Orthop Res 17:475–487
Olney RC, Wilson DM, Mohtai M, Fielder PJ, Smith RL (1995) Interleukin-1 and tumor necrosis factor-alpha increase insulin-like growth factor-binding protein 3 (IGFBP-3) production and IGFBP-3 protease activity in human articular chondrocytes. J Endocrinol 146:279–286
Pan RY, Xiao X, Chen SL, Li J, Lin LC, Wang HJ et al (1999) Disease-inducible transgene expression from a recombinant adeno-associated virus vector in a rat arthritis model. J Virol 73:3410–3417
Peterson L, Brittberg M, Kiviranta I, Akelund EL, Lindahl A (2002) Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med 30:2–12
Radin EL, Ehrlich MG, Chernack R, Abernethy P, Paul IL, Rose RM (1978) Effect of repetitive impulsive loading on the knee joints of rabbits. Clin Orthop Relat Res 131:288–289
Rogachefsky RA, Dean DD, Howell DS, Altman RD (1993) Treatment of canine osteoarthritis with insulin like growth factor-1 (IGF-I) and sodium pentosan polysulphate. Osteoarthr Cartilage 1:105–114
Sunic D, McNeil JD, Rayner TE, Andress DL, Belford DA (1998) Regulation of insulin-like growth factor-binding protein-5 by insulin-like growth factor I nad interleukin-1 alpha in ovine articular chondrocytes. Endocrinology 139:2356–2362
Trentham DE, Townes AS, Kang AH (1977) Autoimmunity to type II collagen: an experimental model of arthritis. J Exp Med 146:857–868
Trippel SB, Corvol MT, Dumontier MF, Rappaport R, Hung HH, Mankin HJ (1989) Effect of somatomedin-C/insulin-like growth factor I and growth hormone on cultured growth plate and articular and articular chondrocytes. Pediatr Res 25:76–82
Trippel SB, Van Wyk JJ, Foster MB, Svodoba ME (1983) Characterization of a specific somatomedin-c receptor on isolated bovine growth plate chondrocytes. Endocrinology 112:2128–2136
Trippel SB (1996) Growth factor actions on articular cartilage. J Rheumatol (suppl) 43:129–132
Ulrich-Vinther M, Duch MR, Soballe K, O’Keeffe RJ, Schwarz EM, Pedersen FS (2004) In vivo gene delivery to articular chondrocytes mediated by an adeno-associated virus vector. J Orthop Res 22:726–734
Watanabe S, Imagawa T, Boivin GP, Gao G, Wilson JM, Hirsch R (2000) Adeno-associated virus mediates long-term gene transfer and delivery of chondroprotective IL-4 to murine synovium. Mol Ther 2:147–152
Zaratiegui M, Castilla I, Garcia M, Quiroga J, Prieto J, Novo J (2002) IGF1 gene transfer into skeletal muscle using recombinant adeno-associated virus in a rat model of liver cirrhosis. J Physiol Biochem 58:169–176
Zhang HG, Xie J, Yang P, Wang Y, Xu L, Liu D et al (2000) Adeno-associated virus production of soluble tumor necrosis factor receptor neutralizes tumor necrosis factor alpha and reduces arthritis. Hum Gene Ther 11:2431–2434
Acknowledgments
This work was supported by the MAPFRE Foundation and PIUNA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Izal, I., Acosta, C.A., Ripalda, P. et al. IGF-1 gene therapy to protect articular cartilage in a rat model of joint damage. Arch Orthop Trauma Surg 128, 239–247 (2008). https://doi.org/10.1007/s00402-007-0407-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-007-0407-7