Abstract
Background: Transcatheter arterial embolization (TAE) can cause gluteal skin and muscle necrosis. However, the ultimate and typical signs of gluteal necrosis resulting from TAE have not yet thoroughly been investigated.
Methods: From January 1995 to December 2003, 165 pelvic fractures were managed with TAE to control retroperitoneal bleeding at our level 1 trauma center. From these, 12 patients suffered gluteal muscle and skin necrosis. We reviewed the medical records of these 12 patients for age, gender, fracture type, embolic sites, computed tomography (CT) findings, serum creatine kinase level, site of skin necrosis, time from injury to skin necrosis, treatment, and outcome.
Results: All 12 patients underwent TAE of the bilateral internal iliac arteries with gelatin sponge slurries. One patient suffered from an infection of the gluteal muscle from an open fracture site. Five patients presented with signs of gluteal soft tissue injuries on admission. Of these, four had skin abrasions and three revealed fluid or air collection under the gluteal skin on CT. The remaining six patients showed no evidence of soft tissue injuries on admission, and the lesions appeared between 2 days and 7 days after their admission. In these six patients, low-density areas (LDAs) of gluteal muscle with a clear border on the CT were observed following the appearance of skin lesion. The skin necrosis was located in the center of either or both buttocks, and signs of ischemia were clearly demarcated from the adjacent normal tissue. Four of 12 patients died from sepsis, three of whom suffered from uncontrollable gluteal infections that had been pointed out as LDAs on the CT.
Conclusions: In every patient with gluteal necrosis associated with pelvic fracture following TAE, initial traumatic contusion cannot be ruled out as contributing to the development of the necrosis. However, for patients who undergo TAE of the bilateral internal iliac artery and who show clear-border LDAs on CT, skin necrosis centered on the buttock, and the delayed appearance of a skin lesion, careful attention must be given in the event of an arterial obstruction due to TAE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sacral region over bony prominences is one of the most frequent sites of pressure sores [8]. In contrast, skin and muscle necrosis of the gluteal region is relatively rare, since the soft tissue has such a rich blood supply [8, 11]. Direct injuries to this region can produce gluteal necrosis, and recently it has been reported that transcatheter arterial embolization (TAE) can also cause necrosis [1, 13]. To distinguish clearly the contribution of each factor is difficult in patients with pelvic fractures following TAE, and the definition of TAE-induced gluteal necrosis, and what type of necrosis is considered a complication of TAE, remains unclear [13].
We have had experience of 12 patients with gluteal necrosis with pelvic fractures. All the patients had undergone TAE for retroperitoneal bleeding associated with the pelvic fracture, but we could not conclude at that time that these necroses resulted solely from the TAE. Our purpose in this study was to evaluate the clinical findings of the patients with gluteal necroses and to identify the ultimate and typical clinical characteristics of these necroses as complications of TAE.
Patients and methods
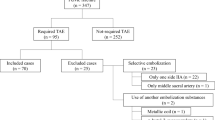
From January 1995 to December 2003, 165 pelvic fractures were managed with TAE of the bilateral internal iliac arteries for the control of retroperitoneal bleeding at our level 1 trauma center. Thirty-three patients died during the first 48 h of their hospitalization. Of the remaining 132 patients (79 men, 53 women), 12 (nine men, three women) were diagnosed as having gluteal muscle necrosis associated with gluteal skin necrosis. The medical records of these 12 patients were thoroughly evaluated and included details of age, gender, systolic blood pressure on arrival, injury severity score (ISS), revised trauma score (RTS), cause of injury, fracture type, embolic sites, computed tomography (CT) findings, serum creatine kinase (CK) level, site of skin necrosis, time from injury to skin necrosis, treatment, and outcome. The CT was performed at least on admission and after the appearance of skin necrosis or exacerbation of a skin lesion. The pelvic fracture types in this study were derived from the Tile classification in the pelvic ring and the Judet–Letournel classification in the acetabulum.
Results
The average age was 48.8 years (range 17–81 years), with an average systolic blood pressure on admission of 67.2 mmHg (range 0–90 mmHg), an ISS of 41.6 (range 14–59), and an RTS of 5.72 (range 0–7.86). The causes of injuries included pedestrians being hit by a motor vehicle (n=5), automobile accidents (n=2), motorcycle accidents (n=2), crush injuries (n=2), a fall from a height (n=1). All the patients underwent embolization of the bilateral internal iliac artery with gelatin sponge slurries, and other selective embolizations with gelatin sponge slurries, stainless steel coils, or microcoils (Table 1).
One patient had suffered from an infection of the pelvis from an open fracture site (perineum), which advanced to gluteal necrosis. Five patients showed signs of gluteal soft tissue injuries on admission. Of these, four showed skin abrasions, three had fluid or air collection detected under the skin on the CT, and one patient presented with a degloving injury from the lateral thigh to the sacral region at the operation (Table 1).
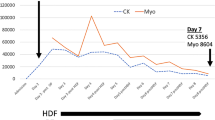
Pelvic CT several days after admission revealed a low-density area (LDA) of the gluteal muscle with a clear border in seven patients (Fig. 1). The LDA included at least the gluteus maximus. Those seven patients also presented with a high CK level (>10,000 IU) for more than 7 days; the most severe portion of gluteal skin necrosis was located in the center of either or both buttocks, and signs of skin and musculature ischemia were clearly demarcated from the adjacent normal tissues (Fig. 2). Of these, only one patient showed skin abrasions, and the other six patients had no evidence of gluteal soft tissue injury on admission. For these six patients the average time from injury to the appearance of gluteal skin change that progressed rapidly to necrosis was 4.0 days (range 2–7 days) (Table 1).
In nine patients, the gluteal muscle and skin necrosis was treated by several debridement. To control muscle infection we treated the other two patients with antibiotics, administered by intra-arterial infusion through the internal iliac artery. The remaining one patient was treated conservatively because the area of skin necrosis was small. Of 12 patients, four patients died from sepsis. The average time of death from injury was 25 days (range 22–28 days) (Table 1). In three of those patients, the focus of the sepsis was considered to be a gluteal abscess that had been pointed out as an LDA on the CT. The focus in the other case was a pelvic infection from the open fracture site.
Discussion
Pelvic fracture with hemodynamic instability is life threatening, and TAE provides a better alternative to surgery in managing retroperitoneal bleeding [14]. In most cases selective arterial embolization is advisable, but in some cases with severe hemodynamic instability and multiple bleeding points scatter embolization of the bilateral internal iliac arteries provides effective control of retroperitoneal bleeding [3, 14]. Velmahos et al. [14] reported that no severe morbidity was noted in relation to embolization of the bilateral internal iliac arteries in the 30 patients in their study.
Although bilateral internal iliac artery occlusion has generally been thought not to give rise to any complications [2, 5, 12], some disadvantages of TAE of the internal iliac artery have been reported. Avascular necrosis of the femoral head, bladder wall necrosis, and neurological complications were major complications [6, 13]. In 1987, Adamietz and Gleumes [1] reported gluteal skin and muscle necrosis as complications of TAE. In the English literature Takahira et al. [13] first showed five patients with gluteal necrosis following TAE. However, there are certain factors influencing the development of these complications [13].
Pelvic fractures sometimes accompany gluteal soft tissue injuries, although the significance of the injuries may not initially be apparent [7, 10]. This initial traumatic contusion can result in necrosis of the gluteal muscle and overlying skin [13]. For example, it is widely recognized that closed degloving injuries with an intact skin surface (Morel–Lavalee lesion) are often overlooked and may develop into central skin necroses or gluteal infections [7, 10]. Therefore, in the patients with gluteal necrosis following both pelvic fracture and TAE for the pelvis, the distinction of each factor is difficult.
Even in patients without pelvic trauma, gluteal necroses have been reported. Duff et al. [6] described an intraoperative embolization that caused gluteus maximus necrosis identified on the CT scan. Connolly et al. [5] reviewed 30 reported cases of gluteal necrosis after surgery of the infrarenal aorta. It was revealed that occlusion of the associated bilateral internal iliac arteries was noted in most of them. Several authors [2, 8] reported that the necrosis following ligation of the bilateral internal iliac arteries demonstrated the clear border between normal and necrotic tissue. Gradual skin discoloration was seen a few days after the operation [2, 6]. The most severe portion of the necrosis was located in the center of the buttock [2, 8, 11].
In our series, all 12 patients underwent TAE of the bilateral internal iliac arteries. Especially, the seven patients in whom an LDA with a clear border was pointed out, most of whom did not show soft tissue injuries on admission, showed almost the same characteristics as those with occlusion of the bilateral internal iliac arteries. The characteristics were a high CK level (>10,000 IU) for more than 7 days, gluteal skin necrosis located in the center of the buttock, and the delayed appearance of skin necrosis within 7 days. Thus, it is possible that the seven of the 132 patients (5.3%) were thought to show typical and ultimate gluteal necrosis resulting from TAE.
It is interesting that the most severe portion of gluteal skin necrosis was located not in the sacrum but in the center of either or both buttocks in the seven patients with LDAs revealed on the CT scan. This part of the skin is supplied by perforating arteries from the gluteus maximus muscle. Thus, the extent of the skin necrosis reflected the necrosis of the gluteus maximus muscle, which was naturally supplied with blood through the superior and inferior gluteal arteries [8]. The reason for the decrease in the blood supply through these arteries remains unknown. The gelatin sponge released at the base of the internal iliac artery may tend to flow into these arteries selectively. It may also associate with the occlusion in the anastomosis from the deep femoral artery or the medial and lateral femoral circumflex arteries due to trauma of a proximal part of the thigh, TAE, or pre-existing atherosclerosis [4, 8, 9, 11].
The necroses of the other five patients have not been excluded as complications of TAE. The short duration of the high CK level only indicates that the volume of damaged muscle was small. Direct trauma that causes soft tissue injuries may also cause an interruption in the collateral circulation, which, in turn, exacerbates the necrosis induced by TAE. We observed that the contribution of each of these factors was always gradual, gradient, and complex. If the ischemia from TAE is severe enough, the above-mentioned characteristics can easily be identified. However, further studies are warranted to clarify the increased risk factors of gluteal necrosis resulting from TAE used to manage pelvic fractures.
In conclusion, the morbid condition of every patient suffering gluteal necrosis after pelvic fracture following TAE could be the result of the combined effect of both the initial traumatic contusion and the TAE. For the patients presenting with LDAs with a clear border on the CT scan, skin necrosis located in the center of either or both buttocks, and the delayed appearance of skin necrosis, arterial obstruction from TAE is the major cause of the necrosis.
References
Adamietz IA, Gleumes L (1987) Extensive gluteal necrosis following bilateral embolization of the internal iliac artery (a case report). Geburtshilfe Frauenheilkd 47:63–64
Andriole GL, Sugarbaker PH (1985) Perineal and bladder necrosis following bilateral internal iliac artery ligation. Report of a case. Dis Colon Rectum 28:183–184
Ben-Menachem Y, Coldwell DM, Young JW, Burgess AR (1991) Hemorrhage associated with pelvic fractures: causes, diagnosis, and emergent management. Am J Roentgenol 157:1005–1014
Cikrit DF, O’Donnell DM, Dalsing MC, Sawchuk AP, Lalka SG (1991) Clinical implications of combined hypogastric and profunda femoral artery occlusion. Am J Surg 162:137–141
Connolly JE, Ingegno M, Wilson SE (1996) Preservation of the pelvic circulation during infrarenal aortic surgery. Cardiovasc Surg 4:65–70
Duff C, Simmen HP, Brunner U, Bauer E, Turina M (1990) Gluteal necrosis after acute ischemia of the internal iliac arteries. Vasa 19:252–256
Hak DJ, Olson SA, Matta JM (1997) Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel–Lavallee lesion. J Trauma 42:1046–1051
Ho PR, Yim K, Hui K, Lineaweaver WC (1996) Gluteal infarction as a complication of aortofemoral bypass grafting. Ann Plast Surg 37:645–649
Iliopoulos JI, Howanitz PE, Pierce GE, Kueshkerian SM, Thomas JH, Hermreck AS (1987) The critical hypogastric circulation. Am J Surg 154:671–675
Kottmeier SA, Wilson SC, Born CT, Hanks GA, Iannacone WM, DeLong WG (1996) Surgical management of soft tissue lesions associated with pelvic ring injury. Clin Orthop 329:46–53
Lose G, Jorgensen L, Lorentzen JE (1985) Regional ischemia due to compromised collateral circulation after arterial reconstruction. Acta Chir Scand 151:301–303
Sadahiro S, Ishida H, Suzuki T, Ishikawa K, Tajima T, Makuuchi H (1999) Vesicular blood flow after ligation of the internal iliac arteries in low anterior resection or abdominoperineal resection. Dis Colon Rectum 42:1475–1479
Takahira N, Shindo M, Tanaka K, Nishimaki H, Ohwada T, Itoman M (2001) Gluteal muscle necrosis following transcatheter angiographic embolisation for retroperitoneal haemorrhage associated with pelvic fracture. Injury 32:27–32
Velmahos GC, Chahwan S, Hanks SE, Murray JA, Berne TV, Asensio J, Demetriades D (2000) Angiographic embolization of bilateral internal iliac arteries to control life-threatening hemorrhage after blunt trauma to the pelvis. Am Surg 66:858–862
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, T., Shindo, M., Kataoka, Y. et al. Clinical characteristics of pelvic fracture patients with gluteal necrosis resulting from transcatheter arterial embolization. Arch Orthop Trauma Surg 125, 448–452 (2005). https://doi.org/10.1007/s00402-005-0827-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-005-0827-1