Abstract
Successful treatment of tears to the avascular region of the meniscus remains a challenge. Current repair techniques, such as sutures and anchors, are effective in stabilizing the peripheral, vascularized regions of the meniscus, but are not adequate for promoting healing in the avascular region. The purpose of this study was to demonstrate the healing ability of a tissue-engineered repair technique using allogenic chondrocytes from three different sources for the avascular zone of the meniscus. Material and methods: Articular, auricular, and costal chondrocytes were harvested from 3-month-old Yorkshire swine. A 1-cm bucket-handle lesion was created in the avascular zone of each three swine. A cell-scaffold construct, composed of a single chondrocyte cell type and Vicryl mesh, was implanted into the lesion and secured with two vertical mattress sutures. Controls consisted of each three sutured unseeded mesh implants, suture only, and untreated lesions. The swine were allowed immediate post-operative full weight bearing. Menisci and controls were harvested after 12 weeks. Results: In all experimental samples, lesion closure was observed. Gross mechanical testing with two Adson forceps demonstrated bonding of the lesion. Histological analysis showed formation of new tissue in all three experimental samples. None of the control samples demonstrated closure and formation of new matrix. Conclusion: We present preliminary data that demonstrates the potential of a tissue-engineered, allogenic cellular repair to provide successful healing of lesions in the avascular zone in a large animal model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Successful treatment of tears to the avascular region of the meniscus remains a challenge. Although current repair techniques, such as sutures and anchors, are effective in stabilizing the peripheral vascularized regions of the meniscus, they are not adequate for promoting healing in the avascular region due to the lack of vascular supply [2]. Tissue engineering techniques could provide an alternative approach to achieve repair of lesions in the avascular meniscus by delivering cells directly to the injury site. The concept of a tissue-engineered cell-based repair technique for meniscal repair or replacement has been previously recognized [3, 9–11]. However, one of the primary obstacles to engineering meniscal tissue is a source of appropriate cells, as autologous cell procurement involves unwanted donor site morbidity.
Alternatively, allogeneic cells that possess a capacity for meniscal repair or regeneration would permit an unlimited supply of cells for further application; the most likely cell type is the allogenic chondrocyte. However, chondrocytes, like many other cell types, are believed to express major histocompatibility complex (MHC) antigens on the cell surface, which could lead to immunologic rejection by the recipient. For example, chondrocytes have been found to express MHC class II proteins on the surface in patients with rheumatoid arthritis and patients with articular surface defects [17]. It is not clear if these pathologic conditions have any relationship to lesions in the meniscus where the cartilage is largely isolated from the vascular system, and, therefore, less susceptible to cell-mediated rejection.
Previous experiments in our laboratory have shown the ability of autologous chondrocytes seeded onto a scaffold (the basis of tissue engineering) to bond artificially created meniscal tears in a large animal model [15]. However, only partial bonding occurred, probably due to the lack of a uniform distribution of cells on the scaffold. Subsequently, we have developed a seeding technique (dynamic oscillating seeding) that has been successful in providing uniform cell distribution onto a scaffold (C. Weinand et al., submitted for publication).
The purpose of this study was to build upon previous work by combining an improved seeding technique with a novel cell type, as we have demonstrated healing potential of autologous chondrocytes [15]. We hypothesized that allogenic chondrocytes from different anatomical sources could achieve repair of artificially created meniscal tears in the porcine animal model.
Materials and methods
The Institutional Animal Care and Use Committee of the Massachusetts General Hospital approved all animal procedures. A single surgeon (T.J.G.) under sterile conditions performed all surgical procedures.
Chondrocyte preparation
Under sterile operating conditions articular, auricular, and costal cartilage from three 3-month-old Yorkshire swine was harvested. The cartilage was excised, rinsed in phosphate-buffered saline (PBS) with 2% antibiotics, and minced into 1 mm3 pieces. The cartilage was digested at 37°C in a 5% CO2 incubator for 12–18 h in Ham’s F-12 media with Glutamax-1 (Gibco/BRL, Life Technologies, Grand Island, NY, USA) containing 0.1% of collagenase Type 2 (Worthington Biochemical, Freehold, NJ, USA) and 1% antibiotics. The solutions were then passed through a 100-μm filter to remove undigested particles. The cell suspension of isolated chondrocytes was centrifuged at 1,500 rpm for 10 min, washed three times with PBS containing 1% antibiotic/antimycotics (10,000 units penicillin, 10 mg streptomycin, and 25 μg amphotericin B; Sigma Chemical Co., St Louis, MO, USA) and cell viability assessed using trypan blue exclusion. Cell counting was done by a hemacytometer to the nearest 1×106 cells/ml. Only digests with a cell viability of >95% were used in this study.
Scaffold
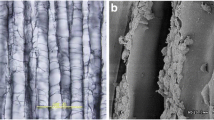
Woven Vicryl® mesh, PLGA (Ethicon Inc., Somerville, NJ, USA) was used as the scaffold. The scaffold was cut to dimensions of 2×0.5 cm2 in and had a thickness of 0.1–0.15 cm. The dimensions of the scaffold were chosen in order to have a large scaffold/cell carrier that could be modified intraoperatively into smaller dimensions. This scaffold has been employed in a previous study (C. Weinand et al., submitted for publication).
Dynamic oscillating seeding
One scaffold was placed into a 15-ml conical tube and 5 ml of chondrocyte solution at a concentration of 1 million cells/ml was added. The tube was tightly capped and placed horizontally onto a mobile platform in a bioreactor at an oscillation rate of 40 Hz at 37°C. Thus, the scaffold was free floating in the cell suspension and the fluid containing the cells was mixed by continuous oscillation. After co-culturing for 1 week, the scaffolds were removed from the media. Cell attachment, cellular phenotype, and cellular distribution for each chondrocyte type were evaluated histologically and quantitatively by DNA count. Extra scaffolds were prepared for histological examination to confirm successful seeding as evidenced by toluidine blue staining. In total, nine scaffolds, three with each chondrocyte type were evaluated for cell attachment.
Implantation of experimental constructs and controls [15]
Three recipient experimental swine were placed supine on an operating room table under general anesthesia. Cephazolin (1 g) and 600,000 IU bicillin were given intramuscularly preoperatively. An antero-medial incision was made over the left knee and the tibia was exposed under sterile conditions. A capsulotomy was performed with a #10 scalpel blade and the medial meniscus exposed. A 2-0 silk traction suture was placed in the superior rim of the meniscus. While applying traction, a 1-cm bucket-handle lesion was created at the anterior middle aspect of the medial meniscus in the white–white zone, anterior to the anterior cruciate ligament using a #11 scalpel blade. The bucket-handle lesion was opened with a mosquito hemostat and by pulling on the traction suture.
The seeded construct was cut to fit the lesion and implanted. The construct was fixed within the lesion with two, 2-0 Ethibond (Ethicon Inc., Somerville, NJ, USA) vertical mattress sutures. Eleven controls were employed, eight from a previous experiment in our lab, using the same techniques. They consisted of three unseeded control mesh, four suture only, or four no treatment [15]. The knee joint was washed gently with several irrigations of normal saline, and the incision was closed in layers. The wound was covered with a sterile Telfa (Kendall, Mansfield, MA, USA) dressing, Aerozoin adhesive spray (Graham-Field, Hauppauge, NY, USA), and Tegaderm (3M Health Care, Borken, Germany). A temporary Coflex (Andover, Covendish Scott Ltd., England) hip spica was applied for 1 day. All animals received analgesics (buprenex 0.03 mg/kg and fentanyl 50 μg/h) for 3–4 days to reduce pain and discomfort. After operation, all pigs were allowed full weight bearing and full motion of the knee joints. The menisci were harvested at 12 weeks and fixed in 10% buffered formalin.
Histological analysis
The Vicryl mesh scaffolds were stained with toluidine blue 1 day before implantation to validate the presence and distribution of cells on the scaffold. At the time of harvest, the entire menisci were embedded in 10% phosphate-buffered formalin, embedded in paraffin and cut in serial 4 μm sections in such a way, that the lesions with the seeded scaffolds could be evaluated. Hematoxylin and eosin staining was performed. Cellular integration of the newly formed tissue into the native meniscus tissue and cellular phenotype were analyzed. A pathologist blinded to the treatment groups evaluated the new tissue.
Results
Scaffold seeding
Histological analysis of all mesh scaffolds and the ones prior to implantation showed uniform distribution of the chondrocytes throughout the pores of the mesh and in several layers over the entire scaffold. Native chondrocyte morphology was preserved. The cells were surrounded by newly formed extracellular matrix, which was also present in the interstitial areas of the mesh. Interdigitation of the chondrocytes and the matrix throughout the meshes was found (Fig. 1).
Control implants
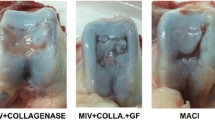
After harvest, one of the menisci with unseeded scaffold showed adherence at the lesion margin. However, the remaining controls did not demonstrate macroscopic healing or bonding to each other at the adjacent lesion margins (Fig. 2) [15]. Gross mechanical testing of the bonding using two Adson forceps confirmed the impression. The samples were transected and were examined. No new tissue was found to bridge the entire interface of the meniscus. Histological evaluation confirmed the gap between the native meniscus tissues after 12 weeks in vivo (Fig. 2). Evaluation of the histological processed samples by a pathologist blinded to the study revealed no immune cell infiltration or reaction to the implanted mesh in the meniscus host. These results suggested that there was no cellular rejection.
Clinical and histological evaluation of menisci in the large animal
The knees of the pigs were examined on a daily basis during the 12-week post-operative period (Table 1). The animals were able to ambulate well and no adverse reactions including infections, swelling of the joint, or redness were noted. Gross visual analysis of the harvested menisci after 12 weeks revealed new tissue filling the lesion and closure of the lesion in all three cases (Figs. 3–5; Table 2). This newly formed tissue had a fibrous scar-like appearance and was well integrated into the native meniscus cartilage. Gross mechanical testing with Adson forceps confirmed bonding of the borders of the lesion by the newly formed tissue (Table 2).
Implant seeded with articular chondrocytes after 12 weeks in vivo. Scar-like tissue is integrating into both sides of the lesion without lymphocytic infiltration. No complete healing is observed. At the margin of the lesion (arrows) a few histiocytes can be observed. Arrows pointing toward the meniscal center. Magnification 100×, staining H&E
Auricular chondrocyte-based implant showing new scar-like tissue integrating into the native meniscus without cellular rejection. Here as well some histiocytes are present at the borders of the lesion with some reactive vascular formation. This vascular reaction has been described after implantation of Vicryl. Arrows at the margins of the lesion, pointing toward the center. Magnification 100×, staining H&E
All menisci were subject to histological examination. The lesions of menisci in the experimental group were filled with newly formed fibrous scar-like tissue and cartilaginous cells. Extracellular matrix integrated into the meniscus tissue throughout the entire lesion (Figs. 3–5). The new tissue was hypercellular with some new cartilagenous tissue concentrated at the superior and inferior edges of the lesion. The healing seemed to originate from an ovoid collection of cells and extracellular matrix in the former gap of the lesion that was surrounded by darker staining fibroblast-like cells. This was a consistent finding, except for the articular sample, where new tissue was found close to the distal end of the lesion. The incomplete healing using articular chondrocytes was consistent with our finding from our previous study [15].
A pathologist blinded to the study examined all histological samples. The new tissue was more scar-like than cartilaginous. Some histiocytes and new vessel formation were found in the native meniscus tissue. No lymphocytic infiltration was found inside the newly formed tissue or in the native meniscus cartilage. Thus, cellular rejection of the allogeneic chondrocytes was not apparent.
Discussion
The meniscus is a critically important structure in the knee that provides stability, shock absorption, and proper transference of load-bearing forces. Therefore, preservation of this meniscus is of critical importance. Various repair techniques have been described, but they are often ineffective in the avascular zone of the meniscus, and spontaneous healing in this region is rare [16]. Current repair techniques to treat tears in the avascular zone of the meniscus, such as sutures or resorbable devices, fail to effect healing in more than 60% of the cases [13]. Without suitable means for stabilization and repair of the inner-third of the meniscus, partial or subtotal meniscectomy is often required. Such radical treatment can result in increased contact pressure as high as 50% to the articular surface of the knee and possibly result in early onset of osteoarthritis.
The field of tissue engineering offers an opportunity to develop techniques to repair torn menisci in order to preserve meniscal function and, thus, prevent degenerative joint disease. This study demonstrates complete healing of tears in the avascular zone of the meniscus using a tissue-engineered cell-based implant composed of allogeneic cells.
Histologically, all chondrocyte sources provided healing and integration into the native meniscus tissue. However, the auricular chondrocytes provided complete integration into the native meniscus tissue throughout the entire lesion. Auricular chondrocytes may provide the best healing capacity, due to the increased production of elastin over articular and costal chondrocytes [14]. Understandably, the elastin may grant better mechanical stability for the healing meniscal tissue. The articular chondrocytes provided only partial healing, which was observed as well in our previous results [15]. Interestingly, no inflammatory reaction was found histologically when using the allogeneic cells in the implants. Only few lymphocytes and histiocytes were found at the borders of the fibrous scar-like tissue. It has been suggested that articular chondrocytes may play an active role in immune response by presenting antigen to T cells. Indeed, a number of studies in animals have looked for MHC class II restricted responses against chondrocytes [6, 8, 18]. However, variations in chondrocyte phenotype between different species are evident as chondrocytes isolated from humans [1, 5, 12], pigs [7], and mice [4] do not appear constitutively to express MHC class II molecules and require activation with IFN-γ before they can present antigen to CD4+ T cells. In contrast to the results obtained with rabbits and rats, human chondrocytes, IFN-γ-treated or not, were very poor inducers of an allogeneic or autologous T-cell response and were able to present only to an antigen-specific T-cell line [1, 5]. Therefore, the use of allogeneic chondrocytes as cell-based therapy for an implant seemed to be possible. An inflammatory response was found only around the suture material. The use of allogeneic cells in this study was an attempt to overcome the potential for significant donor site morbidity. The problem of inadequate cell numbers inherent to the use of articular chondrocytes was overcome by applying dynamic oscillating seeding, thereby expanding our ability to provide easy to use cell-based implants for meniscus repair.
In this report, we describe a biologic repair of an avascular meniscal tear based on the use of allogeneic chondrocytes. It utilizes current techniques augmented with cells and thereby expands indications for meniscal repair. By use of allogeneic cells, the door might be open to an unlimited source of cell supply for further application. Future studies will concentrate on the use of allogeneic auricular chondrocytes in larger number of animals to confirm our results.
References
Alsalameh S, Jahn B, Krause A, Kalden JR, Burmester GR (1991) Antigenicity and accessory cell function of human articular chondrocytes. J Rheumatol 18:414–421
Arnoczky SP, Warren RF (1982) Microvasculature of the human meniscus. Am J Sports Med 10:90–95
Arnoczky SP (1999) Building a meniscus: biological considerations. Clin Orthop 367S:S244–S253
Brennan FR, Mikecz K, Buzas EI, Glant TT (1995) Interferon-gamma but not granulocyte-macrophage colony-stimulating factor augments proteoglycan presentation by synovial cells and chondrocytes to an autopathogenic T-cell hybridoma. Immunol Lett 45:87–91
Bujia J, Alsalameh S, Sittinger M, Hammer C, Wilmes E, Burmester G (1994) Antigen-presenting cell-function of class-II positive human nasal chondrocytes. Acta Otolaryngol 114:75–79
Busche K, Schlesier M, Runge M, Binder A, Kirchhoff H (1990) T-cell lines responding to Mycoplasma arthritidis and chondrocytes in the Mycoplasma arthritidis infection of rats. Immunobiology 181:398–405
Davies ME, Horner A, Franz B (1994) Intercellular-adhesion molecule-1 (ICAM-1) and MHC class-II on chondrocytes in arthritic joints from pigs experimentally infected with Erysipelothrix rhusiopathiae. FEMS Immunol Med Microbiol 9:265–272
Gertzbein S, Lance E (1976) The stimulation of lymphocytes by chondrocytes in mixed cultures. Clin Exp Immunol 24:102–109
Guilak F, Mow VC (2000) The mechanical environment of the chondrocyte: a biphasic finite element model of cell–matrix interactions in articular cartilage. J Biomech 33:1663
Iberra C, Janetta C, Vacanti CA, et al (1997) Tissue engineered meniscus: a potential new alternative to allogeneic meniscus transplantation. Transplant Proc 29:986–988
Iberra C, Koski JA, Warren RF (2000) Tissue engineering meniscus: cells and matrix. Orthop Clin North Am 31:411–418
Jobanputra P, Corrigall V, Kingsley G, Panayi G (1992) Cellular responses to human chondrocytes. Absence of allogeneic responses in the presence of HLA-DR and ICAM-1. Clin Exp Immunol 90:336–344
O’Shea JJ, Shelbourne KD Repair of locked bucket handle meniscus tears in anterior cruciate ligament-deficient knees. Paper No. 007, Dallas Convention Center, AAOS Meeting 2002
Lee KA, Pierce RA, Mecham RP, Parks WC (1994) Increased mesenchymal cell densities accompanies induction of tropoelastin expression in developing elastic tissue. Dev Dyn 200:53–67
Peretti GM, Gill TJ, Xu JW, Randolph MA, Morse KR, Zaleske DJ (2003) Cell based therapy for meniscal repair—a large animal study. Am J Sports Med 32:1–13
Shakespeare DT, Rigby HS (1983) The bucket-handle tear of the meniscus. A clinical and arthrographic study. J Bone Joint Surg 65B:383–387
Summers KL, O’Donnell JL, Hoy MS, Peart M, Dekker J, Rothwell A, Hart DN (1995) Monocyte-macrophage antigen expression on chondrocytes. J Rheumatol 22:1326–1334
Tiku M, Liu S, Weaver C, Teodorescu M, Skosey J (1985) Class II histocompatibility antigen-mediated immunologic function of normal articular chondrocytes. J Immunol 135:2923–2928
Acknowledgments
This work was funded by the AO/ASIF, Switzerland. We would like to thank Dr. Stuart L. Houser, Transplant Biology Research Laboratory, Director David Sachs, Charlestown, MA, USA for the histological and pathological evaluation. All procedures and experiments were performed in accordance with the “Principles of Laboratory Animal Care” (NIH Publication No. 86–23, revised 1985) and the guidelines of the Massachusetts General Hospital for experiments in animals and animal care.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weinand, C., Peretti, G.M., Adams, S.B. et al. Healing potential of transplanted allogeneic chondrocytes of three different sources in lesions of the avascular zone of the meniscus: a pilot study. Arch Orthop Trauma Surg 126, 599–605 (2006). https://doi.org/10.1007/s00402-005-0100-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-005-0100-7