Abstract
Introduction: Tears in the peripheral part of the menisci have a better healing potential than tears in the central part, because the central two-thirds of the menisci are avascular. We hypothesized that healing of meniscus tears in the avascular zone can be promoted by the local application of the angiogenic factor vascular endothelial growth factor (VEGF). Materials and methods: A tear was created in the avascular zone of the medial meniscus in 18 merino sheep. The tear was then repaired with an uncoated suture (group 1), a suture coated with PDLLA (group 2), and by a suture coated with PDLLA/VEGF (group 3). Results: After 6 weeks, we observed increased immunostaining for factor VIII in the VEGF-treated group 3. However, in this treatment group no meniscus healed completely. In the uncoated suture group and in the PDLLA-coated-suture group, partial healing was observed in three animals and complete healing in three animals, respectively. Conclusion: In this experiment the local application of VEGF via PDLLA-coated sutures did not promote meniscus healing. Growth factors might not always be a promising tool for tissue repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The menisci are mobile joint surfaces and they cover approximately 70% of the tibial plateau [16]. They play an important role in the load transmission, shock absorption, and passive stabilization of the knee [9, 19, 32, 33, 35]. Several clinical long-term studies have shown that total or partial meniscectomy may lead to cartilage degeneration and osteoarthrosis [15, 52]. However, meniscectomy remains one of the most common orthopaedic surgeries today [14].
Repair should be considered depending on the type and location of the meniscal tear [26]. Meniscal healing principally depends on the vascularity of the zone that has been injured. Tears in the peripheral part of the menisci have a better healing potential than tears in the central part, because the central two-thirds of the menisci are avascular [2–4, 34, 35, 44]. Excellent clinical results have been obtained in the suture repair of peripheral meniscus tears in the vascularized zone (red–red-zone), but poor results have been reported when the repair has been performed in the avascular zone [1, 11, 13, 21, 23, 28, 50, 53].
Arnoczky et al. [4] showed in an experimental study on meniscus tears involving the avascular zone in dogs that by introducing a fibrin clot into the vicinity of the meniscal tear, meniscal healing could be enhanced. Port et al. [36] added autologous marrow cells to the fibrin clot. Other authors could show that meniscus healing can be enhanced by an interpositional free synovial autograft [25, 27]. However, none of these techniques that were used in animal models reached a broad acceptance in clinical practice.
Ochi et al. [31] reported that by rasping the edge of meniscal tears in the avascular zone of rabbit meniscus, the expression of specific cytokines (such as platelet-derived growth factor [PDGF] and transforming growth factors-beta [TGF-β]), was increased. These authors concluded that these factors could be responsible for promoting meniscal healing. In recent years, the use of growth factors has been shown to accelerate bone, ligament, and tendon healing to allow for an earlier return to unrestricted activity [30, 42, 43, 45–47, 56].
Since the inner two-thirds of the menisci are avascular and avascularity is considered to be one of the most important factors for the poor healing potential of this region, a growth factor that has the potential to stimulate angiogenesis might be the ideal tool to enhance meniscus healing. The most potent angiogenic factor known is the vascular endothelial growth factor (VEGF), sometimes known as vascular permeability factor (VPF), which was originally identified as a heparin-binding angiogenic peptide secreted by tumour cells [48]. VEGF is a selective endothelial cell mitogen that promotes angiogenesis in vivo and renders the microvasculature hyper-permeable to circulating macromolecules [7, 12, 17, 18]. In addition, VEGF is chemotactic for monocytes and is a procoagulant [17]. The two signalling tyrosine kinase receptors, the Fms-like tyrosine kinase receptor (FLT-1, VEGFR-1) and the kinase insert domain-containing receptor KDR (VEGFR-2/FLK-1), bind VEGF selectively [7, 10, 17, 51]. Recent studies have shown that VEGF plays an important role in angiogenesis in the musculoskeletal system [32, 37–40].
A study by Phillips et al. [41] showed that the formation of new capillaries in avascular tissue might be inducible due to high levels of VEGF. Human VEGF165 was diluted in Dulbecco’s phosphate, dried and implanted in the rabbit cornea. After 5–7 days, capillaries were formed between the limbus and the site where the dried VEGF was implanted. A previous study from our laboratory has shown that VEGF expression plays a role in the healing of meniscus tissue [8]. In the rabbit meniscus, VEGF expression did not achieve levels which were sufficient to cause new vessel formation in the central avascular portion of the meniscus.
Based on the above mentioned studies, we hypothesized that the local application of VEGF via PDLLA (poly- [d,l-lactide] acid)-coated sutures may significantly stimulate blood vessel proliferation and healing of tears in the avascular zone of the menisci. The aim of the present study was to test this hypothesis in a sheep model.
Materials and methods
Animal model
The animal experiment was performed with the permission of the local government animal rights protection authorities in accordance with the National Institute of Health guidelines for the use of laboratory animals.
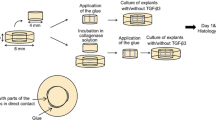
Mature female merino sheep were used in this study. This model was chosen because of the similarities between human and sheep menisci [24]. The animals were assigned randomly into three different treatment groups. Prior to surgery, all animals (average age 2.5 years, average weight approximately 50 kg) were screened to ensure good physical condition. After incubation, anaesthesia was maintained with isoflurane and nitrous oxide. The left hind limb was shaved and prepared in the standard sterile fashion. The knee joint was exposed through an anteromedial incision. The sartorial fascia was incised and reflected to reveal the medial collateral ligament. Then, the meniscus was exposed and a 15-mm longitudinal full thickness tear was created in the avascular zone of the anterior horn of the medial meniscus with a distance of 2 mm to the joint capsule (Fig. 1). The length of the tear was measured with a sterile ruler.
In group I (six animals), the menisci were fixed with three conventional 2-0 Ethibond sutures. In group 2, the menisci were fixed with 2-0 Ethibond (Ethicon, Norderstedt, Germany) sutures which have been coated with poly (d,l-lactide) (molecular weight 30 KD, Boehringer Ingelheim KG, Ingelheim, Germany). In group 3, recombinant VEGF 165 (Tebu, Germany) was incorporated into the coating (5% coating mass), resulting in approximately 250 μg growth factor per suture. The coating technique has been described in detail by Schmidmaier et al. [45–47].
The meniscus refixation was performed in a standardized outside-in technique. For each refixation, two stitches were used. For each stitch, five knots were used to fix the refixation securely on the joint capsule. After meniscus refixation, each knee was irrigated and closed in a similar fashion. The animals were then returned to their cages and were allowed to bear full weight. No bracing or immobilization was used.
After 6 weeks, all animals were killed with an overdose of potassium chloride and thiopental-sodium, the knees were harvested, and the menisci and the joint studied. Then, biopsies of different organs were obtained (liver, spleen, kidney, and skin) to find out if the local application of VEGF has any systemic side effects. Blood serum was gained to measure the serum concentration of VEGF. These results will be published in another paper.
Menisci were graded as either a partial or full thickness residual tear, based on modified criteria developed by Henning et al. [20] as healed (<10% cleft), partially healed (<50% cleft), or not healed (>50% cleft). The joint was inspected for cartilage lesions or synovitis.
Then, the menisci were divided into two segments. One segment was prepared for histology and immunohistochemistry and the other was prepared for the biochemical analysis. The biochemical results will be published in another paper.
Histological and immunohistochemical analysis
For immunohistochemical analysis, the menisci were fixed in 3% paraformaldehyde, embedded in paraffin, irradiated at 750 W in a microwave oven with 3% hydrogen peroxide in 0.01 mol/l sodium citrate buffer, pH 6.0 (twice for 5 min), immunostained with anti-Factor VIII antibodies. After rinsing in Tris buffered saline solution, the sections were incubated with biotinylated goat anti-rabbit immunoglobulins (1:200 in Tri Base Buffer, 45 min; DAKO, Glostrup, Denmark), washed in Tris buffered saline solution for 5 min, and incubated with streptavidin–peroxidase (1:50, 30 min; StrepAB Complex/HRP, DAKO, Glostrup, Denmark). The substrate was ABC (DAKO, Glostrup, Denmark). Negative controls were incubated with the secondary antibody and the Strep AB complex alone. Nuclei were counterstained with hemalum (2–3 s), washed in distilled water, and finally covered with Aqua Tex (Merck, Darmstadt, Germany). Histological sections were stained with HE and Toluidine blue.
Histological sections were evaluated using the Image-Pro 4.5 Image Analysis software. The quantitative analysis of the vascularization was expressed as area occupied by positive immunostaining for factor VIII. Five regions of interest adjacent to the tear (500 μm×500 μm) were measured in the external segment of the section at low magnification (×2.5).
Statistics
The Kruskal Wallis test was used for the statistical analysis of the results. The level of significance was set at p<0.05.
Results
Gross observations
During the postoperative period, all animals increased their cage activity gradually and by the second postoperative week, the animals showed only a mild degree of lameness. No lameness was observed after the third postoperative week. There was no difference in the activity between all three groups. No animal showed signs of a local or systemic infection such as local swelling or fever. All animals survived the 6 weeks and were available for necropsy.
Macroscopic evaluation of the knee joints showed no signs of degenerative changes or infection in all specimens. Chondral lesions were not observed.
The menisci were carefully evaluated grossly to assess healing. In the VEGF/PDLLA group (group 3), no meniscus healed. In all menisci of this group, there was a 100% cleft of 15-mm length. The cleft was only bridged by the sutures; however, the adjacent parts of the menisci showed no healing. In the uncoated suture group (group 1) and in the PDLLA-coated-suture group (group 2), partial healing was observed in three animals and complete healing in three animals, respectively (Table 1).
Histological and immunohistochemical findings
The histological examination confirms the gross findings (Fig. 2). In the PDLLA/VEGF group, there was a 100% cleft in all menisci studied. In the uncoated suture group and in the PDLLA-coated-suture group, partial healing was observed in three animals and complete healing in three animals, respectively. In the partially healed or completely healed clefts, there was a reparative scar tissue.
Factor VIII immunostaining revealed the presence of blood vessels at the repair site of the treated animals. In the VEGF/PDLLA group, factor VIII immunostaining was not restricted to blood vessels or capillaries; single factor VIII positive endothelial cells were distributed in the meniscus tissue (Fig. 3).
The Kruscal Wallis test showed a significant difference in the area covered by factor VIII positive structures (Table 2). The largest area of factor VIII immunostaining was observed in the VEGF/PDLLA group (Fig. 4).
Discussion
The aim of the current study was to evaluate the impact of local application of VEGF via PDLLA-coated sutures on meniscus healing in the avascular zone in a sheep model. We hypothesized that this application may significantly stimulate blood vessel proliferation and healing of tears in the avascular zone of the menisci.
The results of the present study show that the local application of VEGF using a PDLLA-coated suture as delivery tool stimulates the proliferation of endothelial cells but does not enhance meniscus healing. Although factor VIII immunostaining was significantly increased in the VEGF-treated group, none of the menisci of this group healed.
The influence of several growth factors such as basic fibroblast growth factor (bFGF), transforming growth factor beta (TGF-β), insulin like growth factor (IGF), epidermal growth factor (EGF), and platelet-derived growth factor (PDGF) on tendon-, ligament-, and bone healing has been extensively studied [5, 22, 42–47, 54, 55].
To our knowledge, the local application of growth factors to the menisci via coated suture material has not been studied. The delivery tool used in the present study, a low molecular weight poly (d,l lactide), has been shown to be effective in continuously delivering other growth factors such as IGF and TGF-β over a period of 12 weeks with a peak release around the third day [45, 46]. In this phase, first angiogenic reactions would be expected [17]. This technique has been shown to be effective in enhancing fracture healing in rats [45, 46] or in improving anterior cruciate ligament remodelling in a sheep model [54].
Application of VEGF has been shown to be effective in stimulating vessel growth in the treatment of ischemic heart disease and ischemic limb disease [41]. Therefore, we expected that VEGF-induced angiogenesis would also be effective in stimulating healing of lesions in the avascular part of the menisci.
The concentration of VEGF was effective in promoting ACL graft remodelling by application of PDGF in the sheep study performed by Weiler et al. [54]. Nevertheless, we do not know exactly if the dosage used was appropriate to enhance meniscus healing in this model. Further biochemical analysis are planned to examine the VEGF concentration in the meniscus tissue. However, our results show that the dosage used in the present study was obviously high enough to increase the area covered by factor VIII positive structures within the treated menisci.
Factor VIII expression is normally restricted to vascular endothelial cells and therefore factor VIII immunostaining is considered to be a reliable method to detect blood vessels in dense connective tissue [41]. In this study, however, single endothelial cells could be detected in the menisci of the VEGF/PDLLA group. This finding suggests that the application of VEGF might have stimulated proliferation of vascular endothelial cells but the application of VEGF alone was not successful in stimulating the more complex process of vasculogenesis. A combination of growth factors would offer the theoretical advantage of recapitulating at least some of the events leading to correct vessel wall assembly [17]. Recent experimental studies have shown that coadministration of VEGF and angiopoitin-1 has been proposed to result in more normal and less leaky vessels than those induced by VEGF alone (17).
Many in vitro studies have shown that growth factors have not only one single effect; VEGF stimulates also the expression of tissue degrading enzymes such as metalloproteinases (MMPs) and has an inhibiting effect on the expression of tissue inhibitors of matrix metalloproteinases (TIMP) [40]. Tissue degradation is a necessary process during angiogenesis because it enables invasion of the endothelial cells into the matrix [17]. Recent studies have shown that VEGF is not as selective for endothelial cells as it was believed [10, 40]. Under pathological conditions such as in degenerative joint disease or during a healing response, other cell types have the potential to express the VEGF receptor KDR [37–39]. In a previous study, we could show that the VEGF receptor KDR is expressed by fibrochondrocytes during meniscus healing in the rabbit [8]. in vitro studies have shown that VEGF can stimulate chondrocytes to proliferate but also to express MMP-13 via HIF1-α induction [40]. Therefore, the induction of MMP expression might be one factor which inhibits healing despite increased angiogenesis. Further studies have to examine the influence of locally applied VEGF on MMP expression in the meniscus.
There are several limitations to consider when interpreting the results of this study. First, this study was carried out using a sheep model and we could not mimic the arthroscopic technique of meniscus refixation as performed in human patients. The dimensions of sheep menisci are smaller than human menisci [24] and as in all animal models a well-controlled rehabilitation could not be applied postoperatively [6]. However, the sheep meniscus has been shown to have a peripheral vascular and inner avascular zone that mount a healing response to injury similar to that of the human meniscus through their ability or inability to heal [24].
We investigated only one time point (6 weeks after surgery). This time point is relatively early in the healing process but from a clinical point of view it seems to be interesting, because many rehabilitation protocols limit restricted weight bearing or brace protection to 6 weeks [6].
In conclusion, our data suggest that growth factors have not always beneficial effects on soft tissue healing. The results demonstrate that further knowledge about meniscus healing is necessary to understand the possible interactions between the several cytokines which are expressed during this process. Our results show that VEGF alone might not be the ideal candidate for the treatment of meniscus lesions. However, the ability of the delivery system to stimulate angiogenesis in the meniscus tissue via VEGF application justifies further research with other growth factors or growth factor combinations.
References
Albrecht-Olsen P, Kristensen G, Burgaard P et al (1999) The arrow versus horizontal suture in arthroscopic meniscus repair: a prospective randomized study with arthroscopic evaluation. Knee Surg Sports Traumatol Arthrosc 7:268–273
Arnoczky SP, Warren RF (1982) Microvasculature of the human meniscus. Am J Sports Med 10:90–95
Arnoczky SP, Warren RF (1983) The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am J Sports Med 11:131–141
Arnoczky SP, Warren RF, Spivak JM (1988) Meniscal repair using an exogenous fibrin clot. An experimental study in dogs. J Bone Joint Surg Am 70:1209–1217
Batten ML, Hansen JC, Dahnes LE (1996) Influence of dosage and timing of application of platelet derived growth factor on early healing of the rat medial collateral ligament. J Orthop Res 14:736–741
Barber FA (1999) Accelerated rehabilitation for meniscus repairs. Arthroscopy 10:206–210
Barleon SG, Martiny-Baron G, Weindel K, Herzog C, Marme D (1997) Vascular endothelial growth factor up-regulates its receptor fms-like tyrosine kinase 1 (FLT-1) and a soluble variant of FLT-1 in human vascular endothelial cells. Cancer Res 57:5421–5422
Becker R, Pufe P, Giessmann N, Neumann W, Mentlein R, Petersen W (2004) Expression of the vascular endothelial growth factor (VEGF) during meniscus healing in a rabbit model. J Bone Joint Surg (in press)
Bullough PG, Munuera L, Murphy J et al (1979) The strength of the menisci of the knee as it relates to their fine structure. J Bone Joint Surg Br 52:564–570
Clauss WH, Breier G, Knies U, Rockl W, Waltenberger J, Risau W (1996) The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem 271:17629–17634
DeHaven KE (1999) Meniscus repair. Am J Sports Med 27:242–250
Dumont, Fong GH, Puri MC, Gradwohl G, Alitalo K, Breitman ML (1995) Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn 203:80–92
Ellermann, Siebold R, Buelow JU et al (2002)Clinical evaluation of meniscus repair with a bioabsorbable arrow: a 2- to 3-year follow-up study. Knee Surg Sports Traumatol Arthrosc 10:289–293
Erricsson E (2003) Meniscus repair. Knee Surg Sports Traumatol Arthrosc 11(1):1
Fairbanks TJ (1948) Knee joint changes after meniscectomy. J Bone Joint Surg Br 30:664–670
Fukubayashi T, Kurosawa H (1980) The contact area and pressure distribution pattern of the knee. A study of normal and osteoarthrotic knee joints. Acta Orthop Scand 51(6):871–879
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25(4):581–611
Gerber, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N (1999) VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 5:623–628
Greis PE, Bardana DD, Holmstrom MC et al (2002) Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg 10(3):168–176
Henning CE, Lynch MA, Yearout KM, Vequist SW, Stallbaumer RJ, Decker KA. (1990) Arthroscopic meniscal repair using an exogenous fibrin clot. Clin Orthop 252:64–72
Howell JR, Handoll HH (2000) Surgical treatment for meniscal injuries of the knee in adults. Cochrane Database Syst Rev 2:1353
Hildebrandt KA, Woo SL, Smith DW (1998) The effects of platelet derived growth factor BB on healing of the rabbit medial collateral ligament: an in vitro study. Am J Sports Med 26:549–554
Ikeuchi H (1979) Meniscus surgery using the Watanabe arthroscope. Orthop Clin North Am 10(3):629–642
Joshi MD, Suh JK, Woo SL (1995) Interspecies variation of compressive biomechanical properties of the meniscus. J Biomed Mater Res 29:823–828
Jitsuiki, Ochi M, Ikuta Y (1994) Meniscal repair enhanced by an interpositional free synovial autograft: an experimental study in rabbits. Arthroscopy 10:659–666
Klimkiewicz JJ, Shaffer B (2002) Meniscal surgery update: indications and techniques for resection, repair, regeneration, and replacement. Arthroscopy 18(9):14–25
Kobuna, Shirakura K, Niijima M (1995) Meniscal repair using a flap of synovium. An experimental study in the dog. Am J Knee Surg 8:52–55
Laprell H, Stein V, Petersen W (2002) Arthroscopic all-inside meniscus repair using a new refixation device: a prospective study. Arthroscopy 18(4):387–393
Letson K, Dahners LE (1994) The effect of combinations of growth factor on ligament healing. Clin Orthop 308:207–212
Nakamura N, Shino K, Natsuume T (1998) Early biological gene transfer of platelet derived growth factor (PDGF-B) into healing patella ligament. Gene Ther 5:1165–1170
Ochi, Uchio Y, Okuda K, Shu N, Yamaguchi H, Sakai Y (2001) Expression of cytokines after meniscal rasping to promote meniscal healing. Arthroscopy 17:724–731
Pauwels F (1960) Eine neue Theorie über den Einfluß mechanischer Reize auf die Differenzierung der Stützgewebe. Zehnter Beitrag zur funktionellen Anatomie und kausalen Morphologie des Stützapparates. Zeitschrift Anatomie Entwicklungsgeschichte 121:478–515
Petersen W, Tillmann B (1998) Collagenous fibril texture of the human knee joint menisci. Anat Embryol 197:317–324
Petersen W, Tillmann B (1995) Age-related blood and lymph supply of the knee menisci. A cadaver study. Acta Orthop Scand 66:308–312
Petersen W, Tillmann B (1999) Structure and vascularization of the knee joint menisci. Z Orthop Ihre Grenzgeb 137:31–39
Port, Jackson DW, Lee TQ, Simon TM (1996) Meniscal repair supplemented with exogenous fibrin clot and autogenous cultured marrow cells in the goat model. Am J Sports Med 24:547–555
Pufe T, Petersen W, Tillmann B, Mentlein R (2001) Splice variants VEGF121 and VEGF165 of the angiogenic peptide vascular endothelial cell growth factor are expressed in the synovial tissue of patients with rheumatoid arthritis. J Rheumatol 28:1482–1485
Pufe T, Petersen W, Tillmann B, Mentlein R (2001) The splice variants VEGF121 and VEGF189 of the angiogenic peptide vascular endothelial growth factor are expressed in osteoarthritic cartilage. Arthritis Rheum 44:1082–1088
Pufe T, Petersen W, Tillmann B, Mentlein R (2001) Detection of the vascular endothelial growth factor in fetal, degenerative and normal human tendons. Arthritis Rheum (in press)
Pufe T, Harde V, Petersen W, Goldring MB, Tillmann B, Mentlein R (2004) Vascular endothelial growth factor (VEGF) induces matrix metalloproteinase expression in immortalized chondrocytes. J Pathol 202(3):367–374
Phillips, Stone AM, Jones BD, Schultz JC, Whitehead RA, Knighton DR (1994) Vascular endothelial growth factor (rhVEGF165) stimulates direct angiogenesis in the rabbit cornea. In Vivo 8:961–965
Raschke M, Kolbeck S, Bail H, Schmidmaier G, Flyvbjerg A, Lindner T, Dahne M, Roenne IA, Haas N (2002) Homologous growth hormone accelerates healing of segmental bone defects. Bone 29(4):368
Raschke M, Wildemann B, Inden P, Bail H, Flyvbjerg A, Hoffmann J, Haas NP, Schmidmaier G (2002) Insulin-like growth factor-1 and transforming growth factor-beta1 accelerates osteotomy healing using polylactide-coated implants as a delivery system: a biomechanical and histological study in minipigs. Bone 30(1):144–151
Scapinelli R (1969) Vascular anatomy of the human cruciate ligaments and surrounding structures. Clin Anat 10(3):151–162
Schmidmaier G, Wildemann B, Stemberger A, Haas N, Raschke M (2001) Biodegradable poly (d,l-lactide) coating of implants for continuous release of growth factors. J Biomed Mater Res 58:449–455
Schmidmaier G, Wildemann B, Heeger J, Gabelein T, Flyvbjerg A, Bail HJ, Raschke M (2002) Improvement of fracture healing by systemic administration of growth hormone and local application of insulin-like growth factor-1 and transforming growth factor-beta1. Bone 31(1):165–172
Schmidmaier G, Wildemann B, Cromme F, Kandziora F, Haas NP, Raschke M (2002) Bone morphogenetic protein-2 coating of titanium implants increases biomechanical strength and accelerates bone remodeling in fracture treatment: a biomechanical and histological study in rats. Bone 30(6):816–822
Senger, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF (1988) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219:983–985
Spindler, Mayes CE, Miller RR, Imro AK, Davidson JM (1995) Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB). J Orthop Res 13:201–207
Tenuta JJ, Arciero RA (1994) Arthroscopic evaluation of meniscal repairs. Factors that effect healing. Am J Sports Med 22:797–802
Thomas KA (1996) Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem 271:603–606
Veth RPH (1985) Clinical significance of knee joint changes after meniscectomy. Clin Orthop Relat Res 198:56–60
Warren RF (1985) Arthroscopic meniscus repair. Arthroscopy 1:170–172
Weiler A, Förster C, Hunt P, Falk R, Jung T, Unterhauser F, Bergman V, Schmidmaier G, Haas N (2004) The influence of locally applied platelet derived growth factor-BB on free tendon graft remodeling after anterior cruciate ligment reconstruction. Am J Sports Med 32:881–891
Yasuda K, Tomita F, Yamazaki S, minami A, Tohyama H (2004) The effect of growth factors—on the biomechanical properties of the bone-patellar tendon-bone graft after anterior cruciate ligament reconstruction. Am J Sports Med 32:870–880
Woo SL, Suh JK, Parsons IM (1998) Biologic intervention in ligament healing. Sports Med Arthrosc Rev 6:74–82
Acknowledgements
The study was supported by a grant from the “Deutschsprachige Arbeitsgemeinschaft für Arthroskopie (AGA)”. None of the authors received direct financial support of any commercial party.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petersen, W., Pufe, T., Stärke, C. et al. The effect of locally applied vascular endothelial growth factor on meniscus healing: gross and histological findings. Arch Orthop Trauma Surg 127, 235–240 (2007). https://doi.org/10.1007/s00402-005-0024-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-005-0024-2