Abstract
Introduction
The rotator cuff has a characteristic structure, in that one surface faces articular cartilage and another faces bursa. This structure may produce differences in the healing process between the rotator cuff and other tendons. We investigated the spontaneous healing process of a surgically created supraspinatus tendon tear in rabbits.
Materials and methods
A transverse, full-thickness tear of the supraspinatus tendon was created and its healing examined.
Results
A tear of 12 mm was not repaired within 3 weeks. With a tear of 5 mm, reparative tissue gradually encroached into the defect from the bursal side, and the tear united from the bursal side to the articular side by 12 weeks. The healing rates (width of reparative tissue/width of the tendon×100%) were 32.2%, 52.4%, 58.0%, 88.9%, and 93.8% at 1, 2, 3, 6, and 12 weeks, respectively. The reparative tissue had continuity to the epitenon of the bursal side. Immunohistochemical study showed that at week 1, type III collagen was detected in the reparative tissue and the cutting ends, and the expression gradually decreased. On the other hand, the expression of type I collagen in the reparative tissue was weak at week 1 and increased until week 3. PCNA-positive cells were observed in the reparative tissue.
Conclusion
These results show that the origin of the reparative tissue is the epitenon, and from the bursal side rather than the articular side. This model is very useful for the investigation of the remodeling process of an acute rotator cuff tear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although there are a growing number of reports on tendon healing, only a few experimental studies have been made about the healing process of a rotator cuff tear. Carpenter et al. described an active but inadequate repair response to a defect in the supraspinatus tendon using a rat model [4]. Björkenheim et al. monitored supraspinatus tendon healing by measuring the intra-articular hydrodynamic pressure and observed healing or closure of the defect in a rabbit model [3]. Soslowsky et al. demonstrated that both intrinsic and extrinsic alterations induce changes in the supraspinatus tendon of rats [12]. These studies of a surgically created supraspinatus defect model showed the ability of the tissue to heal histologically and mechanically.
Gelberman et al. reported the healing process of flexor tendon using a dog model. In their study, type I collagen mRNA was localized to epitenon cells on the tendon surface overlying the repair site, and to cells in the gap between the tendon stumps, without detectable expression in tendon fibroblasts [8]. Conversely, Abrahamsson et al. reported that endotenon cells produced collagen and restored the injured surface of the flexor tendon following suturing in a rabbit model [1].
In this study, to analyze mechanisms of healing of a rotator cuff tear, we established a model of surgically created rotator cuff tear in rabbits and demonstrated the histological pictures and the healing rates of a rotator cuff tear during the healing process.
Materials and methods
The 12- to 14-week-old male Japanese white rabbits (weighing 2.6–2.9 kg, n=24) were anesthetized with 50 mg/kg i.v. sodium pentobarbital (Nembutal; Abbott Laboratories). In each rabbit, a full-thickness tear of the right supraspinatus tendon was made according to the method previously described by Kumagai et al. [9]. In brief, the tear was created transversely to the supraspinatus tendon at 4 mm proximal to the insertion of tendon onto the greater tuberosity with a #15 scalpel blade under sterile surgical conditions. The skin wound was closed routinely, and the rabbits were permitted usual cage activity without immobilization. The procedure used in this study was in accordance with the animal experimental ethical review committee of Nagoya University, Japan. In the first experiment, 4 tears of 12 mm in length and 4 tears of 5 mm in length were made, and analyzed at 3 weeks. In the next experiment, 16 tears of 5 mm were made, and 4 tears were analyzed at 1, 2, 6, and 12 weeks, respectively. For immunohistochemical analysis, the supraspinatus tendons attached to the greater tuberosity were obtained. Decalcification was performed at 4°C with 12.5% ethylenediamine tetra-acetic acid solution (pH 7.0) for 2 weeks.
Immunohistochemistry
Paraffin sections were immunostained for type I collagen, type III collagen, and PCNA by the streptavidin-biotin-peroxidase complex method. Mouse monoclonal antibodies (Fuji Chemical Industry, Toyama, Japan) raised against human type I collagen, type III collagen, or PCNA were used. Then 3.5-μm-thick sections were deparaffinized in xylene and rehydrated in a graded ethanol series. Specimens were then treated with 3% hydrogen peroxide (H2O2) in methanol for 30 min at room temperature to block endogenous peroxidase. After washing with phosphate-buffered saline (PBS, 0.01 M, pH 7.2), the sections were treated with 10% normal rabbit serum for 10 min at room temperature, to eliminate nonspecific staining. Sections were incubated overnight at room temperature with the primary antibody, type I collagen (1:200), type III collagen (1:50), or PCNA (1:200). The sections were washed with PBS, treated with biotinylated link antibody, and reacted with peroxidase-labeled streptavidin. The sections were developed with 3,3′-diaminobenzidine tetrahydrochloride (DAB) solution. The nuclei were counterstained with Mayer’s hematoxylin to improve demonstration of cells. The control studies included substitution of the primary antibody with a nonspecific antibody of the same class. The results of staining were graded to examine differences in histology among the time points according to a previous report [11]. In brief, all features were graded as follows: -, not present; ±, unclear or weak; +, moderate; and ++, strongly present.
Results
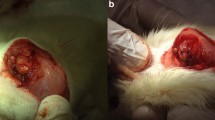
In the first experiment, two different lengths of tears, 5 mm and 12 mm, were analyzed (n=4, respectively). When a tear of 12 mm was made, which included all tendinous fibers of the supraspinatus tendon, it was not repaired at 3 weeks in all samples (Fig. 1A). When a tear of 5 mm was made, the defect was partially healed at 3 weeks (Fig. 1B).
Rotator cuff defects at 3 weeks after creation. Tears of 5 mm (A) and 12 mm (B) in length were created transversely to the supraspinatus tendon. At week 3, the defect was partially repaired in the 5 mm tear (A), but no continuity of torn tendons is observed in the 12 mm tear (B). Arrows indicate the location of the tear of the rotator cuff. R rotator cuff, H humeral head. (Hematoxylin and eosin staining, ×1)
To elucidate the healing process of the supraspinutus tendon tear, we made tears of 5 mm in the supraspinatus tendon in 16 rabbits and analyzed them at 1, 2, 6, and 12 weeks (n=4, respectively). At week 1, the tendon edge was still sharp, and reparative tissue was invading the defect from the bursal side (Fig. 2A). The healing rate of width of reparative tissue/width of the tendon was 32.2% (Fig. 3). At 2 and 3 weeks, the tendon edge became dull, and the defect widened (Fig. 2B,C), and the healing rate increased to 52.4% and 58.0%, respectively. At 6 and 12 weeks, the defect was almost filled with reparative tissue (Fig. 2D,E), and the healing rate was 88.9% and 93.8%. Figure 4 shows the histogram of the time course of the healing rate.
Immunohistochemical staining for type I collagen in the reparative tissue was faint, while staining for type III was relatively strong at 1 week (Fig. 5A). Then, staining for type I collagen increased and type III collagen decreased. Table 1 summarizes the time course of staining for types I and III collagen. Figure 6 shows immunohistochemical staining for PCNA at 1 week. PCNA-positive cells, which indicate proliferation, were mainly observed in the reparative tissue.
Discussion
Rotator cuff tear is one of the most common causes of pain and disability in the upper arm. Although there are a number of reports about the treatment of a rotator cuff tear, including surgery, there are only a few experimental studies of the healing mechanism. In this study, we have demonstrated the time course of the healing of a rotator cuff tear. Recently, using the same experimental model, we have shown the expression and enzymatic activity of MMP-2 and TIMPs during the healing process of a rotator cuff tear [5]. Kumagai et al. have also demonstrated a repair response with type III collagen that was initially formed and gradually replaced with type I collagen in the same model of a rabbit. However, they did not show the microscopic pictures or the healing rates [9].
The structural component of the tendon is type I collagen. In the early phase of tendon healing, however, type III collagen increases and is gradually replaced by type I collagen as the tissue matures. This process is essential for the maintenance of the structure and function of the tendon, and disorder of types I and III collagen production induces fibrosis or scar in various tissues [2, 6, 10, 14, 15]. In the healing of a rotator cuff tear, we found a similar process to tendon healing. The reparative tissue is derived from the bursal side rather than the articular side and has continuity to the epitenon. In the early phase of repair, the reparative tissue produces type III collagen dominantly, then type I collagen increases and replaces it.
It is controversial whether the subacromial bursa should be preserved when an operation of a rotator cuff tear is performed. Uhthoff et al. examined specimens with a rotator cuff rupture and insisted that the main source of the reparative tissue was the wall of the subacromial bursa. They recommended preserving the subacromial bursa as much as possible [13]. However, to our knowledge, there is no evidence that preservation of the subacromial bursa promotes the healing of a rotator cuff tear. On the other hand, with an arthroscopic method recently used for rotator cuff repair [7], the subacromial bursa was resected to improve visualization, but the results were as good as open surgery in which the subacromial bursa was preserved. In the present study, histological examination revealed that the rotator cuff has a spontaneous healing capacity. The reparative cells encroached from the bursal side to the articular side, gradually filling the defects, and were positive for PCNA, which indicates that the cells were proliferating. The reparative tissue had continuity to the epitenon of the bursal side. Taken together with clinical results, the origin of the reparative tissue is the epitenon of the bursal side surface of rotator cuff rather than the wall of the bursa. In addition, we could not find continuity of the reparative tissue to the subacromial bursa. Thus, it may not be necessary to preserve the subacromial bursa in a rotator cuff tear. However, further investigation is necessary in both experimental and clinical studies.
References
Abrahamsson SO, Lundborg G, Lohmander LS (1992) Restoration of the injured flexor tendon surface: a possible role for endotenon cells. A morphological study of the rabbit tendon in vivo. J Hand Surg Br 17:553–560
Birk DE, Mayne R (1997) Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur J Cell Biol 72:352–361
Björkenheim JM, Paavolainen P, Ahovuo J, Slatis P (1990) Resistance of a defect of the supraspinatus tendon to intraarticular hydrodynamic pressure: an experimental study on rabbits. J Orthop Res 8:175–179
Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ (1998) Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg 7:599–605
Choi HR, Kondo S, Hirose K, Ishiguro N, Hasegawa Y, Iwata H (2002) Expression and enzymatic activity of MMP-2 during healing process of the acute supraspinatus tendon tear in rabbits. J Orthop Res 20:927–933
Fan L, Sarkar K, Franks DJ, Uhthoff HK (1997) Estimation of total collagen and types I and III collagen in canine rotator cuff tendons. Calcif Tissue Int 61:223–229
Gartsman GM (2001) All arthroscopic rotator cuff repairs. Orthop Clin North Am 32:501–510
Gelberman RH, Amiel D, Harwood F (1992) Genetic expression for type I procollagen in the early stages of flexor tendon healing. J Hand Surg Am 17:551–558
Kumagai J, Sarkar K ,Uhthoff HK (1993) Repair process of surgically produced rotator cuff tear (abstract). Trans Orthop Res Soc 18:315
Kumagai J, Sarkar K, Uhthoff HK, Okawara Y, Ooshima A (1994) Immunohistochemical distribution of type I, II and III collagens in the rabbit supraspinatus tendon insertion. J Anat 185:279–284
Sano H, Kumagai J, Sawai T (2002) Experimental fascial autografting for the supraspinatus tendon defect: remodeling process of the grafted fascia and the insertion into bone. J Shoulder Elbow Surg 11:166–173
Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR (1996) Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg 5:383–392
Uhthoff HK, Sarkar K (1991) Surgical repair of rotator cuff ruptures. The importance of the subacromial bursa. J Bone Joint Surg Br 73:399–401
Von der Mark K (1981) Localization of collagen types in tissues.Int Rev Connect Tissue Res 9:265–324
Williams IF, McCullagh KG, Silver IA (1984) The distribution of types I and III collagen and fibronectin in the healing equine tendon. Connect Tissue Res 12:211–227
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirose, K., Kondo, S., Choi, HR. et al. Spontaneous healing process of a supraspinatus tendon tear in rabbits. Arch Orthop Trauma Surg 124, 374–377 (2004). https://doi.org/10.1007/s00402-004-0663-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-004-0663-8