Abstract
The Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW) update 3 recommends that histologic grade II and III IDH-wildtype diffuse astrocytic gliomas that harbor EGFR amplification, the combination of whole chromosome 7 gain and whole chromosome 10 loss (7 + /10 −), or TERT promoter (pTERT) mutations should be considered as glioblastomas (GBM), World Health Organization grade IV. In this retrospective study, we examined the utility of molecular classification based on pTERT status and copy-number alterations (CNAs) in IDH-wildtype lower grade gliomas (LGGs, grade II, and III). The impact on survival was evaluated for the pTERT mutation and CNAs, including EGFR gain/amplification, PTEN loss, CDKN2A homozygous deletion, and PDGFRA gain/amplification. We analyzed 46 patients with IDH-wildtype/pTERT-mutant (mut) LGGs and 85 with IDH-wildtype/pTERT-wildtype LGGs. EGFR amplification and a combination of EGFR gain and PTEN loss (EGFR + /PTEN −) were significantly more frequent in pTERT-mut patients (p < 0.0001). Cox regression analysis showed that the pTERT mutation was a significant predictor of poor prognosis (hazard ratio [HR] 2.79, 95% confidence interval [CI] 1.55–4.89, p = 0.0008), but neither EGFR amplification nor EGFR + /PTEN − was an independent prognostic factor in IDH-wildtype LGGs. PDGFRA gain/amplification was a significant poor prognostic factor in IDH-wildtype/pTERT-wildtype LGGs (HR 2.44, 95% CI 1.09–5.27, p = 0.03, Cox regression analysis). The IDH-wildtype LGGs with either pTERT-mut or PDGFRA amplification were mostly clustered with GBM by DNA methylation analysis. Thus, our study suggests that analysis of pTERT mutation status is necessary and sufficient to diagnose IDH-wildtype diffuse astrocytic gliomas with molecular features of glioblastoma. The PDGFRA status may help further delineate IDH-wildtype/pTERT-wildtype LGGs. Methylation profiling showed that IDH-wildtype LGGs without molecular features of GBM were a heterogeneous group of tumors. Some of them did not fall into existing categories and had significantly better prognoses than those clustered with GBM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 2016 World Health Organization (WHO) classification of central nervous system tumors represents the updates on diagnostic classes, grades, and criteria [16]. However, the classification and grading of IDH-wildtype diffuse astrocytic gliomas remain controversial. The criteria for distinguishing WHO grades II and III have been based on traditional morphologic findings, including mitotic activity and anaplastic nuclear features. Although grades II and III are often collectively called diffuse lower grade gliomas (LGGs), multiple studies have indicated that a substantial subset of IDH-wildtype LGGs based on histologic criteria has an aggressive clinical course, with overall patient survival times equal to or only slightly longer than patients with IDH-wildtype glioblastoma (GBM), WHO grade IV [6, 11, 26]. The Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW) update 3 [5] recommended that histological grade II and III IDH-wildtype diffuse astrocytic gliomas that contain either high-level EGFR amplification, a combination of whole chromosome 7 gain and whole chromosome 10 loss (7 + /10 −), or TERT promoter (pTERT) mutations, be designated as “Diffuse astrocytic glioma, IDH‑wildtype, with molecular features of GBM, WHO grade IV”, because these patients show an aggressive clinical course equivalent to GBM WHO grade IV. We and others have also shown that there is no difference in overall survival (OS) between the IDH-wildtype, pTERT mutant (mut) LGG, and GBM [3]. Thus, molecular delineation of IDH-wildtype diffuse astrocytic tumors has become vital in diagnosing adult glioma and treatment decisions.
The present study aimed to independently evaluate the utility of these molecular prognostic markers, pTERT mutation, EGFR amplification, and 7 + /10 − , and to identify copy-number alterations (CNAs) that could serve as a novel prognostic marker in IDH-wildtype LGGs to predict clinical courses corresponding to GBM, regardless of histologic grade, in a large series of our Japanese cohort. Our study suggested that analysis of pTERT mutation status is necessary and sufficient to diagnose IDH-wildtype diffuse astrocytic gliomas with the molecular features of glioblastoma. The PDGFRA status may help further delineate IDH-wildtype/pTERT-wildtype LGGs. In addition, IDH-wildtype LGGs without molecular features of GBM appeared to be a heterogeneous group of tumors, some of which may have distinct clinical and molecular features.
Materials and methods
Patients
In this study, we included a total of 724 patients with gliomas analyzed in our previous study [3]. The inclusion criteria for the analysis of IDH-wildtype LGGs were as follows: 18 years of age or older, histological diagnosis of grade II and III diffuse glioma originating in the cranium, absence of H3F3A mutation, and clinical data available for survival analysis. Tumors with BRAF V600E mutations were included. There were 151 patients with IDH-wildtype diffuse glioma WHO grade II or III. For survival analysis, 453 patients with IDH-wildtype GBM and 120 with grade II, III IDH-mut, and pTERT-wildtype astrocytoma were used for comparison. Clinical data collected from each institution were as follows: age at diagnosis, sex, preoperative Karnofsky performance status (KPS), the extent of resection, radiation dose, and chemotherapeutic regimen in the initial treatment. Survival data of patients were updated whenever possible. The study was approved by the Institutional Review Board (IRB) of the National Cancer Center (No. 2013–042) and the corresponding local IRB of the participating centers.
Central pathology review
All cases of LGGs and 260 cases of GBM were subjected to central pathology review by three senior neuropathologists (T.K., M.S., and H.S.). Histological diagnosis was made as a consensus between the three pathologists according to the 2007 WHO classification for central nervous system tumors, similar to the histological diagnosis of the 2016 WHO classification. Another 193 cases of GBM were not subjected to histopathological review; therefore, the local diagnoses were the final diagnoses.
Molecular analysis
Tumor DNA was extracted from frozen tumor tissues for all cases using a DNeasy Blood & Tissue Kit (Qiagen, Tokyo, Japan). The presence of hotspot mutations in IDH1 (R132) and IDH2 (R172), in the pTERT (−124 and −146), at codons 27 and 34 of H3F3A, and codon 600 of BRAF was analyzed by Sanger sequencing or pyrosequencing for all cases, as previously reported [2]. To assess copy-number status, we performed multiplex ligation-dependent probe amplification (MLPA) using the SALSA MLPA KIT P105 (version D2) and P088 (version C2), following the manufacturer’s protocol (MRC Holland, Amsterdam, The Netherlands) [13]. The P105 kit is designed to detect CNAs typically found in gliomas and includes probes against PDGFRA, EGFR, CDKN2A, PTEN, TP53, CDK4, MDM2, and NFKBIA genes. The P088 kit was designed to assess mainly 1p/19q codeletion. The CNAs of 1p and 19q were determined by MLPA, microarray-based comparative genomic hybridization, or microsatellite analysis. The methylation status of the MGMT promoter was analyzed by pyrosequencing after bisulfite modification of genomic DNA extracted from tumor specimens as described [17], with some modifications in the thermal cycling conditions. Based on an outcome-based study to determine an optimal cut-off to judge MGMT promoter methylation in a series of 276 newly diagnosed GBMs, we used a ≥ 16% cut-off for MGMT methylation (Ichimura, manuscript in preparation).
Molecular and clinical data analysis in The Cancer Genome Atlas (TCGA) and Memorial Sloan Kettering Cancer Center (MSKCC)
Independent and extensive molecular data and clinical information, including survival data of IDH-wildtype LGGs in TCGA (n = 94) and MSKCC (n = 73), were collected from cBioPortal for Cancer Genomics (https://cbioportal.org) [10, 12] and the supplemental data of the previous publication by TCGA [7, 9]. In TCGA data, since the pTERT mutation was not examined in every case, only 56 cases with IDH-wildtype LGG were analyzed. CNA was determined based on the log2 copy-number value.
Methylation array analysis
The Infinium MethylationEPIC BeadChip Kit was used to obtain genome-wide DNA methylation profiles for tumor samples according to the manufacturer’s instructions (Illumina, San Diego, USA). For most samples, 500 ng of DNA was used as the input material. The output data (IDAT files) were checked for general quality, as indicated by the manufacturer. IDAT files were uploaded to the Molecular Neuropathology site to perform DNA methylation-based classification of central nervous system tumors (www.molecularneuropathology.org) [8]. The classifier scores and chromosomal copy-number plots were obtained using the methylation classifier.
For further bioinformatics analysis, all computational analyses were performed using R version 4.0.4. Raw signal intensities were obtained from IDAT files of 64 IDH-wildtype LGG samples using the Minfi Bioconductor package version 1.34.0. Unprocessed IDAT files of 2801 samples were downloaded from the NCBI Gene Expression Omnibus (GEO) under accession number GSE109381 and used as reference samples [8]. A correction for the type of material tissue (formalin-fixed paraffin-embedded tissue/frozen) was performed using the removeBatchEffect function (limma package version 3.44.3). The methylated and unmethylated signals were corrected individually, and beta-values were calculated using an offset of 100, as recommended by Illumina. After the probe filtering criteria were applied according to the GitHub repository (https://github.com/mwsill/mnp_training), 428,230 probes were used for the following analysis. To perform unsupervised non-linear dimension reduction, the 1000 most variable probes, according to standard deviation, were selected from 2801 reference samples. The t-distributed stochastic neighbor embedding (t-SNE) [25] plot for 64 IDH-wildtype LGGs and 2801 references was made using the Rtsne package (version 0.15), with 2500 iterations and a perplexity value of 30. The predictions of tumor purity for all cases were calculated using the R package RF_Purify with the method ‘ABSOLUTE’ [14].
Statistical analysis
The characteristics of IDH-wildtype, pTERT-wildtype LGGs, and IDH-wildtype, pTERT mut LGGs were compared. The comparison for age distribution was examined using the Student’s t test. Associations between molecular variables were evaluated using Fisher’s exact test. For survival analysis, OS was defined as the time from the date of initial operation to the date of death from any cause. Patients who were still alive at the last follow-up were censored. OS was estimated using the Kaplan–Meier method. Survival curves were compared using the log-rank test. The hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using the Cox regression model. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using JMP version 15 software (SAS Institute, Cary, NC, USA).
Results
Prognostic value of TERT promoter mutation, EGFR amplification, and EGFR + PTEN− in IDH-wildtype LGGs
In 5 of 140 patients with IDH-wildtype LGGs (three of 49 patients: pTERT-mut, two of 91 patients: pTERT-wildtype), additional genomic DNA was not available for copy-number analysis. Thus, a total of 135 patients with IDH-wildtype LGGs were analyzed for CNAs in our cohort. In 4 of 89 patients with IDH-wildtype/pTERT-wildtype LGGs, there were no mutations, CNA, or MGMT promoter methylation detected in our examination. Considering the possibility of low tumor cell contents in the surgical specimens used for analysis, we excluded these four patients from the subsequent analysis. Thus, 131 patients with IDH-wildtype LGGs were studied. There were 46 IDH-wildtype/pTERT mut LGGs and 85 IDH-wildtype/pTERT-wildtype LGGs. Patient clinical information and molecular status are listed in Supplementary Tables S1 and S2.
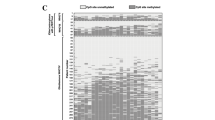
Table 1 shows the characteristics of IDH-wildtype, pTERT-wildtype LGGs, and IDH-wildtype, pTERT mut LGGs. The patients with IDH-wildtype and pTERT mut LGGs were significantly older (p = 0.0017). There were no significant differences in gender, WHO grade, KPS, or MGMT promoter methylation status. The copy numbers of PDGFRA, EGFR, PTEN, and CDKN2A were significantly different between pTERT-wildtype and pTERT mut. Figure 1 shows the distribution of pTERT mutation, EGFR amplification, the combination of EGFR gain and PTEN loss (EGFR + /PTEN −), and PDGFRA gain/amplification. The frequency of PDGFRA gain/amplification was significantly higher in pTERT-wildtype (p = 0.043), while the frequencies of EGFR amplification and EGFR + /PTEN − were significantly higher in pTERT mut (p < 0.0001) (Table 2).
To validate the criteria to define the “Diffuse astrocytic glioma, IDH‑wildtype, with molecular features of GBM, WHO grade IV” recommended in the cIMPACT-NOW update 3, we evaluated the association of TERT promoter mutation, EGFR amplification, and EGFR + /PTEN − with the prognosis (Fig. 2a–c). Of note, although the recommendation was the combined whole chromosome 7 gain and whole chromosome 10 loss (+ 7/ − 10), it was surrogated by the combination of EGFR gain and PTEN loss for practicality. The results showed that pTERT mutation was the only molecular marker significantly associated with a poor prognosis (p < 0.0001). Neither EGFR amplification nor EGFR + /PTEN − was significantly associated with OS, although there was a tendency for poor prognosis in patients with EGFR amplification or EGFR + /PTEN − (p = 0.053 and 0.064, respectively). To further examine the prognostic impact of pTERT mutation, EGFR amplification, and EGFR + /PTEN − , we performed multivariate Cox regression analysis using the model, including the possible confounding variables of age at diagnosis, preoperative KPS, WHO grade, surgical procedure, adjuvant chemoradiotherapy, and MGMT promoter methylation status. The results showed that the pTERT mutation was the sole significant negative predictor of prognosis for the three molecular variables (HR 2.79, 95% CI 1.55–4.89, p = 0.0008) (Table 3). MGMT methylation was significantly associated with a good prognosis (HR 0.39, 95% CI 0.22–0.68, p = 0.0007). Chemoradiotherapy was significantly associated with poor prognosis (HR 2.27; 95% CI 1.05–5.48; p = 0.038). Chemoradiotherapy was possibly selectively administered to patients with progressive astrocytoma. Whether surgical removal or biopsy was not associated with prognosis. We analyzed the published data of IDH-wildtype LGG in TCGA [6] and MSKCC cohorts [15]. In the TCGA data, chromosome 7 + /10- was observed in 1/19 cases (5.3%) of pTERT-wildtype and in 26/37 cases (70.3%) of pTERT mut. EGFR amplification was observed in 3/19 cases (15.8%) of pTERT-wildtype and 24/37 cases (64.9%) of pTERT mut. In the MSKCC data, chromosome 7 + /10− was observed in 2/12 cases (16.7%) of pTERT-wildtype and in 32/49 cases (70.3%) of pTERT mut. EGFR amplification was observed in 4/21 cases (19.0%) of pTERT-wildtype and 20/52 cases (38.5%) of pTERT mut. These data confirmed that chromosome 7 + /10 − (p < 0.0001 in TCGA and MSKCC) and amplification of EGFR (p = 0.0013 in TCGA, p < 0.0001 in MSKCC) were mostly among the pTERT mut cases.
Kaplan–Meier curves of overall survival (OS) in IDH-wildtype lower grade gliomas (LGGs). a IDH-wildtype pTERT mut LGGs (median OS: 16.1 months) showed significantly longer survival than IDH-wildtype pTERT wt LGGs (median OS: 37.0 months) (p < 0.0001). Difference in OS between b IDH-wildtype EGFR amplified (Amp) LGGs (median OS: 22.7 months) and IDH-wildtype non-EGFR Amp LGGs was not significant (median OS: 28.5 months; p = 0.053 months), and c IDH-wildtype non-EGFR + /PTEN-LGGs (median OS: 18.5 months) and IDH-wildtype non-EGFR + /PTEN-LGGs was not significant (median OS: 16.0 months; p = 0.064). d WHO grade III IDH-wildtype astrocytoma (median OS: 22.8 months) showed significantly shorter OS than WHO grade II IDH-wildtype astrocytoma (median OS: 47.2 months; p = 0.00012)
Association of CNAs with WHO histological grade in IDH-wildtype LGGs
In IDH-wildtype LGG, WHO grade III showed a significantly shorter OS than WHO grade II (p = 0.00012) (Fig. 2d). We evaluated the association of CNAs with WHO grade in IDH-wildtype LGGs (Table 4). These results showed that PDGFRA amplification, EGFR gain, PTEN hemizygous deletion, CDK4 amplification, and CDKN2A homozygous deletion were significantly more common in WHO grade III (p = 0.014, 0.011, 0.025, 0.0036, and 0.0046, respectively). There were also significant differences in age at diagnosis, KPS, adjuvant therapy, or MGMT promoter methylation status.
Association of CNAs with overall survival in IDH-wildtype pTERT mut LGGs
We evaluated the association between CNAs and OS in IDH-wildtype pTERT mut LGGs. As shown above, all EGFR amplifications except one case and all combined EGFR + /PTEN − cases were included in the pTERT mut-type in IDH-wildtype LGGs (Fig. 1). Kaplan–Meier curves showed that neither EGFR amplification nor EGFR + /PTEN − was a prognostic predictor (Fig. 3a, b). Homozygous deletion of CDKN2A showed a tendency to predict poor prognosis, although it was not statistically significant (p = 0.079) (Fig. 3c). None of the other CNAs were prognostic predictors.
Kaplan–Meier curves of overall survival (OS) in IDH-wildtype pTERT-mutant LGGs. Difference in OS in a EGFR amplification (amp) (median OS: 20.8 months) vs. non-EGFR amp (median OS: 16.0 months; p = 0.91), b EGFR + /PTEN (median OS: 18.5 months) vs. non-EGFR + /PTEN (median OS: 16.0 months; p = 0.69), and c CDKN2A homozygous deletion (HD) (median OS: 13.3 months) vs. non-CDKN2A HD (median OS: 21.2 months; p = 0.079)
Association of CNAs with overall survival in IDH-wildtype pTERT-wildtype LGGs
Next, we evaluated the association between CNAs and OS in IDH-wildtype pTERT-wildtype LGGs. As shown in Table 1, copy-number gain or amplification of PDGFRA was observed significantly more frequently in pTERT-wildtype, IDH-wildtype LGGs. In addition, copy-number gain or amplification of PDGFRA was a significant predictor of poor prognosis in IDH-wildtype pTERT-wildtype LGGs (p = 0.021) (Fig. 4a). None of the other CNAs were associated with survival. To examine the prognostic effect of copy-number gain or amplification of PDGFRA, we performed multivariate Cox regression analysis using the model, including the variables of age at diagnosis, preoperative KPS, WHO grade, surgical procedure, adjuvant chemoradiotherapy, and MGMT promoter methylation status. The results showed that PDGFRA gain or amplification was an independent predictor of poor prognosis (HR 2.44, 95% CI 1.09–5.27, p = 0.030) in addition to age, adjuvant therapy, and MGMT methylation (Table 5).
Kaplan–Meier curves of overall survival (OS). a IDH-wildtype pTERT wt LGGs with PDGFRA gain/amplification (median OS: 32.5 months) showed significantly longer OS than those without PDGFRA gain or amp (median OS: 45.1 months; p = 0.021). b OS of each molecular group. IDH-wildtype, pTERT-wildtype, PDGFRA gain or Amp LGG had OS equivalent to IDH-wildtype, pTERT-wildtype GBM (median OS: 21 months), or IDH-wildtype, pTERT-mutant LGG (median OS: 16.1 months) or IDH-wildtype, pTERT-mutant GBM (median OS: 16.1 months). *p = 0.044, **p = 0.0062 c OS of each molecular group. Median OS was not reached for Group A. Group C (median OS: 21.7 months) showed significantly shorter survival than Group B (median OS: 45.1 months; p < 0.0001). Group A, IDH-mutant grade II, III astrocytoma; Group B, IDH-wildtype LGG without any of the poor prognostic factors; Group C, IDH-wildtype LGG with any of the poor prognostic factors
Association between molecular classification and prognosis of LGGs
Based on these results, we divided IDH-wildtype LGGs into three groups: IDH-wildtype, pTERT-wildtype, no PDGFRA gain/amplification; IDH-wildtype, pTERT-wildtype, PDGFRA gain/amplification; IDH-wildtype, pTERT mut, with or without PDGFRA gain/amplification. We compared the OS of IDH-wildtype pTERT-wildtype PDGFRA gain/amplification LGGs with IDH-wildtype pTERT mut LGGs, IDH-wildtype pTERT-wildtype GBM, or IDH-wildtype pTERT mut GBM. The results showed that the IDH-wildtype, pTERT-wildtype, and PDGFRA gain/amplification groups showed a short OS equivalent to IDH-wildtype pTERT mut LGGs, IDH-wildtype pTERT-wildtype GBM, or IDH-wildtype pTERT mut GBM (Fig. 4b).
Since the copy-number gain or amplification of PDGFRA was found to be a predictor of poor prognosis in IDH-wildtype LGGs, we brought together IDH-wildtype LGGs with any of the predictors of poor prognoses, such as pTERT mut, PDGFRA gain/amplification, EGFR amplification, or EGFR + /PTEN − (Group C astrocytoma), although almost all EGFR amplification and EGFR + /PTEN − were included in pTERT mut-LGGs. We defined grade II and III IDH-mut astrocytoma as Group A astrocytoma, and IDH-wildtype LGGs without any poor prognostic predictors as Group B astrocytoma. Comparison of the characteristics between Group B and C astrocytomas showed that the frequencies of elderly patients, WHO grade III, or MGMT methylation were significantly higher in Group C astrocytoma (p < 0.001, p = 0.016 and 0.023, respectively) (Supplementary Table S3). In Group B astrocytoma, patients with WHO grade III showed significantly shorter overall survival than those with WHO grade II (p = 0.024) (Supplementary Fig. S1a). In Group C astrocytoma, WHO grade was not significantly associated with OS (p = 0.087) (Supplementary Fig. S1b). There was a significant difference in OS between Group A and B astrocytomas (p < 0.0001). The OS of Group C astrocytoma was significantly shorter than that of Group A or B astrocytoma (p < 0.0001) and equivalent to IDH-wildtype GBM (Fig. 4c).
Methylation array analysis of IDH-wildtype LGGs
A total of 54 Group B astrocytomas, for which additional genomic DNA was available, were analyzed for genome-wide DNA methylation analysis. Selected ten Group C astrocytomas, including four tumors with IDH-wildtype, pTERT-wildtype, PDGFRA amplification LGGs, and six tumors with IDH-wildtype pTERT mut LGGs, were also analyzed (Fig. 1). There was no discrepancy between chromosomal copy-number profiles obtained from the DNA methylation array and the CNA results obtained using MLPA.
Table 6 summarizes the results of the DNA methylation-based classification, and Supplementary Table S4 shows the details (www.molecularneuropathology.org). Among the 54 Group B astrocytomas, 23 cases (42.6%) matched with one of the existing reference groups, including 4 GBM, IDH-wildtype, 3 Glioma, IDH-mutant, 2 anaplastic pilocytic astrocytoma (ANA_PA), 1 anaplastic pleomorphic xanthoastrocytoma (PXA), one ganglioglioma (GG), one CNS high-grade neuroepithelial tumor, one medulloblastoma, and ten control tissues. Among the 31 no-match cases (57.4%), 23 showed a calibrated score above 0.3, and were classified as follows: 11 Glioblastoma, IDH-wildtype, 1 Glioma, IDH-mutant, 1 diffuse midline glioma H3 K27M mutant, 2 dysembryoplastic neuroepithelial tumor, 1 GG, 1 pilocytic astrocytoma, 1 ependymoma, posterior fossa group B, 1 plexus tumor, and 4 control tissue. Eight Group B astrocytomas showed calibrated scores below 0.3 and were not classified as any of the existing categories. Among the Group C astrocytomas, all six IDH-wildtype and pTERT-mutant LGGs were classified as Glioblastoma, IDH-wildtype, with a calibrated score of above 0.9, except for one case (0.41). One of the four IDH-wildtype, pTERT-wildtype, and PDGFRA amplified LGGs were classified as Glioblastoma, IDH-wildtype, with a calibrated score above 0.9, while the other three were not matched (classified as Glioblastoma, IDH-wildtype, and calibrated scored < 0.9).
The methylation-based t-SNE distribution of IDH-wildtype, pTERT-wildtype LGGs mainly formed two clusters, with several exceptions (Fig. 5a). One group of tumors formed a cluster within methylation class family Glioblastoma, IDH-wildtype, mainly between subclass midline and subclass RTK I (“GBM” cluster, Fig. 5b). All IDH-wildtype, pTERT mut, IDH-wildtype, pTERT-wildtype, and PDGFRA amplification LGGs were located within the GBM cluster, except one. Another group of tumors formed a cluster separate from any of the existing reference groups near the methylation class control tissue and several subtypes of LGGs, including LGG_GG (Fig. 5c). We tentatively defined this cluster as an “LGG” cluster. We then compared the OS of GBM cluster cases with LGG cluster cases. Results showed that the LGG cluster cases had significantly longer OS than the GBM cluster cases (Fig. 5d). When the cases classified in methylation class control tissue were excluded from the LGG cluster, the remaining LGG cluster cases still showed significantly longer OS than the GBM cluster cases (Fig. 5e).
DNA methylation-based unsupervised clustering of IDH-wildtype astrocytomas. a Our cohort (n = 64) and the reference cohort (n = 2801) from 91 methylation classes [GSE109381] were plotted using t-distributed stochastic neighbor embedding (t-SNE) dimensionality reduction. Individual samples of reference cohorts relevant to our cohorts (n = 22) were color-coded according to the respective matched class colors. The other 60 reference methylation classes that were not associated with our cohorts and 9 methylation class control tissues were compiled and collectively indicated as dark gray and light gray plots, respectively, to highlight the relevant classes (the class names are indicated). The clusters highlighted in b and c are indicated by squares. b Of the 64 samples from our cohort, 20 were clustered within the glioblastoma cluster, including four PDGFRA amplified cases and four pTERT-mutant cases. c Twenty-nine tumors clustered close but not overlapping with the LGG and control tissue clusters. All but one such cases were categorized as Group B IDH-wildtype astrocytoma which were devoid of any of the markers, including PDGFRA amplification and pTERT mutation, which defined molecular GBM. d Kaplan–Meier curves of overall survival (OS) in LGG cluster (median OS: 37.0 months) vs. GBM cluster (median OS: 23.8 months; p = 0.025). e Kaplan–Meier curves of overall survival (OS) in LGG cluster except the control match cases (median OS: 35.5 months) vs. GBM cluster (median OS: 23.8 months; p = 0.042). The class abbreviations were as follows: A_IDH; methylation class IDH glioma, subclass astrocytoma: A_IDH_HG; methylation class IDH glioma, subclass high-grade astrocytoma: O_IDH; methylation class IDH glioma, subclass 1p/19q codeleted oligodendroglioma: GBM_MES; methylation class glioblastoma, IDH-wildtype, subclass mesenchymal: GBM_RTK_I; methylation class glioblastoma, IDH-wildtype, subclass RTK I: GBM_RTK_II; methylation class glioblastoma, IDH-wildtype, subclass RTK II: GBM_RTK_III; methylation class glioblastoma, IDH-wildtype, subclass RTK III: GBM_MID; methylation class glioblastoma, IDH-wildtype, subclass midline: GBM_MYCN; methylation class glioblastoma, IDH-wildtype, subclass MYCN: LGG_GG; methylation class low-grade glioma, ganglioglioma: LGG_PA_GG_ST; methylation class low-grade glioma, subclass hemispheric pilocytic astrocytoma and ganglioglioma: LGG_DNT; methylation class low-grade glioma, dysembryoplastic neuroepithelial tumor: LGG_RGNT; methylation class low-grade glioma, rosette forming glioneuronal tumor: LGG_PA_PF; methylation class low-grade glioma, subclass posterior fossa pilocytic astrocytoma: ANA_PA; methylation class anaplastic pilocytic astrocytoma: PXA; methylation class (anaplastic) pleomorphic xanthoastrocytoma: DMG_K27; methylation class diffuse midline glioma H3 K27M mutant: MB_G3; methylation class medulloblastoma, subclass group 3: MB_G4; methylation class medulloblastoma, subclass group 4: EPN_PF_A; methylation class ependymoma, posterior fossa group A: EPN_PF_B; methylation class ependymoma, posterior fossa group B: HGNET_MN1; methylation class CNS high-grade neuroepithelial tumor with MN1 alteration: PDGFRAamp; PDGFRA amplified: TERTmut; pTERT mutant
Discussion
This study showed that almost all cases with EGFR amplification or combined EGFR + /PTEN− in IDH-wildtype LGGs also had pTERT mutations. Among the molecular characteristics that defined “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of GBM (WHO grade IV),” that is, pTERT mutation, EGFR amplification, and EGFR + /PTEN−, the only independent prognostic predictor in IDH-wildtype LGGs was pTERT mutation. Neither EGFR amplification nor EGFR + /PTEN − was significantly associated with OS in IDH-wildtype LGGs. In other words, the prognosis of IDH-wildtype LGGs with EGFR amplification or EGFR + /PTEN − was dependent on the status of the pTERT mutation. In IDH-wildtype pTERT-wildtype LGGs, gain or amplification of PDGFRA was observed significantly more frequently than in pTERT mut tumors. The gain or amplification of PDGFRA was a significant predictor of poor prognosis in IDH-wildtype pTERT-wildtype LGGs.
We previously reported that pTERT mutation was a significant predictor of poor prognosis in WHO grade II–IV IDH-wildtype glioma, independent of the MGMT methylation status [3]. In addition, several studies have shown the significance of pTERT mutation as a poor prognostic predictor in IDH-wildtype glioma [11, 18]. In cIMPACT-NOW update 3, histological grade II and III IDH-wildtype diffuse astrocytic gliomas that contain high-level EGFR amplification, the combination of whole chromosome 7 gain and whole chromosome 10 loss, or pTERT mutations were recommended as WHO grade IV. Our cohort showed that IDH-wildtype pTERT mut LGGs have a worse prognosis than IDH-wildtype pTERT-wildtype GBM. This result indicated that pTERT status was a more significant prognostic marker than a histologic diagnosis in IDH-wildtype astrocytic gliomas. Almost all EGFR amplification and EGFR + /PTEN − cases in IDH-wildtype LGGs were among the pTERT mut cases. These results indicate that the most significant prognostic marker in IDH-wildtype LGGs was the pTERT mutation.
A genome-wide DNA methylation analysis matched five out of the six IDH-wildtype pTERT mut LGGs (no EGFR amplification, no EGFR + /PTEN −) with the methylation class family Glioblastoma, IDH-wildtype (Supplementary Table S4). Taken together, it appears that the presence of pTERT mutations alone in IDH-wildtype LGG may, in most cases, fulfill the criteria proposed by cIMPACT-NOW update 3 to define “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of GBM, WHO grade IV”. This has a significant consequence in clinical practice where the resources for a multimodal approach for molecular diagnostics, such as methylation profiling, are limited. Nonetheless, caution is required when interpreting isolated pTERT mutations, as this genotype may occasionally be observed in PXA [5, 23]. As has been emphasized in cIMPACT-NOW update 3, these molecular criteria should only be applied to morphologically verified diffusely infiltrative glioma with astrocytic lineage, such as in our cases.
Our study examined the CNAs of EGFR and PTEN using MLPA in all cases, and the whole of chromosomes 7 and 10 were examined in 64 of 131 cases using DNA methylation analysis. According to a study by Stichel et al. [23], the most common type of chromosome 7 and 10 CNA in IDH-wildtype LGGs was whole chromosome 7 + /10 − , which was 75.8% of any 7/10 CNA. The frequencies of whole 7 + /10q − and 7p + /whole 10 − were 7.0% and 2.3%, respectively. The presence of EGFR + /PTEN − indicated the existence of either 7 + /10 − or 7 + /10q − or 7p + /10 − . Therefore, EGFR + /PTEN − represented whole 7 + /10 − with a probability of over 90%. If it was not EGFR + /PTEN−, there was no whole 7 + /10 − either.
All cases with EGFR + /PTEN − were included in the pTERT mut cases. In addition, all EGFR amplifications except one case were observed among the pTERT mut cases. Copy-number plots obtained from DNA methylation array analysis were consistent with the results of the CNAs obtained from MLPA. There were no whole chromosome 7 + /10 − cases in Group B astrocytomas. These results were consistent with previous studies regarding LGG [1] and GBM [24]. Aoki et al. reported that the gain of 7p, loss of 10q, and pTERT mutation were strongly mutually associated with IDH-wildtype LGGs. Umehara et al. reported that association or tendency toward co-occurrence was observed among the pTERT mutation, EGFR gain/amplification, and PTEN deletion.
These findings were largely confirmed in the TCGA and MSKCC datasets. However, these datasets have limitations: the number of cases in which all necessary genetic data were available, particularly the pTERT status, was relatively small, and the patients’ follow-up period was not sufficient to allow the assessment of the prognostic impact of the genotype. Validation in a larger number of cases with a complete set of data and longer follow-up periods are warranted.
We showed that the gain or amplification of PDGFRA was a significant predictor of poor prognosis in IDH-wildtype/pTERT-wildtype LGGs. Although PDGFRA amplification has been reported to be associated with significantly worse OS in IDH1 mutant GBM [19], there is no large-scale study evaluating CNAs of PDGFRA in IDH-wildtype LGGs. The gain or amplification of PDGFRA was observed in 21.4% of IDH-wildtype LGG and associated with shorter OS (Fig. 4a). Gain or amplification of PDGFRA was observed significantly more frequently (p = 0.043, Table 2) in pTERT-wildtype (27.1%) cases than in pTERT-mut cases (10.9%) in IDH-wildtype LGGs. Umehara et al. reported that the amplification of PDGFRA tended to be mutually exclusive to pTERT mutation in GBM data [24].
To further elucidate the significance of PDGFRA gain/amplification in IDH-wildtype/pTERT-wildtype LGGs, a genome-wide DNA methylation analysis was performed in four selected cases. One of them was classified as Glioblastoma, IDH-wildtype by the DKFZ methylation classifier, with a calibration score above 0.9, while the other three tumors did not match, although the methylation class of Glioblastoma, IDH-wildtype was suggested (Supplementary Table S4). Methylation-based t-SNE distribution analysis clustered all four IDH-wildtype, pTERT-wildtype, PDGFRA gain/amplification LGGs within the GBM cluster between GBM_MID and GBM_RTKIII (Fig. 5b). Thus, PDGFRA gain/amplification is likely to serve as an additional marker to molecularly define GBM in IDH-wildtype gliomas, although its biology requires further exploration.
In addition, we showed that almost all CNAs analyzed in this study were more frequently observed in WHO grade III than WHO grade II in IDH-wildtype LGG. There were 26/28 cases (92.9%) of WHO grade III in IDH-wildtype LGG with PDGFRA gain or amplification. These results indicated that copy-number gain or amplification of PDGFRA in IDH-wildtype LGG was associated with a higher WHO grade. The prognosis of WHO grade II and III IDH-wildtype LGG was significantly different in our Kaplan–Meier and multivariate Cox regression analyses. However, in IDH-wildtype/pTERT-wildtype LGG, WHO grade was not a significant prognostic predictor in multivariate Cox regression analysis. Whether the apparent poorer prognosis of grade III patients than grade II in IDH-wildtype/pTERT-wildtype LGGs can be attributed to molecular alterations such as CNA, including PDGFRA gain/amplification, remains to be seen.
It has been reported that CDKN2A/B homozygous deletion is a poor prognostic factor in IDH-mut WHO grade III astrocytoma [4, 22]. However, there is no large cohort evaluating the prognostic value of CDKN2A/B in IDH-wildtype astrocytoma. In our study, although CDKN2A homozygous deletion was not a statistically significant prognostic predictor in IDH-wildtype LGGs, there was a tendency to predict poor prognosis in IDH-wildtype/pTERT mut LGGs (p = 0.079). Again, an independent validation with a larger cohort is warranted.
It is noteworthy that IDH-wildtype LGG without any of the above predictors for poor prognosis (Group B, Fig. 4c) showed significantly longer OS than IDH-wildtype GBM or IDH-wildtype LGG with one or more of the poor prognostic predictor (Group C, Fig. 4c) while showing significantly shorter OS than IDH-mut LGG (Group A, Fig. 4c). Furthermore, patients with WHO grade II astrocytoma had significantly longer survival than those with grade III astrocytoma in Group B (Supplementary Fig. S1a). In Group C, no significant difference in overall survival between grades II and III was observed (Supplementary Fig. S1b). However, WHO grade III was significantly enriched in Group C, most likely reflecting that this group of tumors was biologically equivalent to GBM.
We then analyzed 54 Group B astrocytomas for genome-wide DNA methylation. When the DKFZ DNA methylation-based classification was applied, 23 Group B cases matched with some of the existing reference groups, including 4 methylation class family Glioblastoma, IDH-wildtype, and 10 methylation class control tissue. Among them, one PXA and one GG had BRAF V600E mutations. Thirty-one cases of Group B astrocytoma did not reach the cut-off of ≥ 0.9, and were interpreted as no match. More than half of the unmatched Group B astrocytomas formed a separate cluster nearby but did not overlap with other existing LGG reference groups (Supplementary Table S4 and Fig. 5c). None of them had BRAF mutation. These cases (defined as “LGG”) showed significantly longer OS than those that were clustered with GBM (Fig. 5d, e). Of note, no patients belonged to the age group that was typically observed among pediatric-type diffuse low-grade gliomas. These findings suggest that bona fide IDH-wildtype diffuse astrocytoma may exist as a separate entity from Group C IDH-wildtype astrocytomas, which are molecularly equivalent to GBM. The presence of “true” IDH-wildtype low-grade astrocytomas that show significantly longer OS than GBM has also been suggested elsewhere [21]. This group of tumors deserves further investigation to refine the classification of diffuse astrocytic tumors.
Group B IDH-wildtype astrocytomas were presented as a heterogeneous group of tumors by methylation profiling. Apart from the molecularly defined GBM and the newly defined LGG, as described above, isolated cases were classified into other existing entities such as ANA_PA (Supplementary Table S4), as has been previously reported [20]. However, three tumors that were classified as Glioma, IDH-mutant by the methylation classifier, were devoid of IDH mutations, as determined by Sanger sequencing or pyrosequencing. The tumor classified as Medulloblastoma group 3 and 4 was a supratentorial tumor developed in a 74-year-old patient who was diagnosed with anaplastic astrocytoma WHO grade III based on central pathology review. Ten tumors were classified as methylation class control tissue. Although under-sampling is an important issue in molecular analysis, tumor cell content, estimated using the methylation array data in these tumors, ranged from 30 to 60%, which was comparable to those in other tumors. Some of these tumors showed elevated MGMT methylation, suggesting that they contained a certain proportion of tumor cells. Furthermore, the prognosis of these cases was equivalent to that of the other cases of the LGG cluster defined above (Fig. 5d). Thus, IDH-wildtype astrocytomas without molecular features of GBM may not necessarily fall into existing categories and therefore need better classification. Our data are also in agreement with another study in which a considerable number of IDH-wildtype grade II and III astrocytomas were interpreted as no prediction in DNA methylation-based classification [23].
This study inevitably has inherent limitations as a retrospective analysis susceptible to selection biases, such as introducing the initial inclusion criteria for molecular data availability. Another limitation is the different molecular techniques utilized in our cohorts, including TCGA and MSKCC, where a more comprehensive analysis was performed. On the other hand, MLPA, which we used to evaluate CNAs, is a reliable and cost-effective method utilized in routine diagnosis. These results were consistent with the CNA profiles obtained from the methylation array analysis. It may serve as a powerful tool to efficiently detect typical CNA to delineate the poor prognosis group in adult gliomas.
In conclusion, we report here that pTERT mutation is the most useful marker to predict molecularly defined GBM with the poorest prognosis in IDH-wildtype LGGs. It is the most critical marker to identify the newly proposed entity of “Diffuse astrocytic glioma, IDH‑wildtype, with molecular features of GBM, WHO grade IV” among the IDH-wildtype diffuse astrocytomas. It is noteworthy that there were IDH-wildtype diffuse astrocytomas with a significantly better prognosis than GBM, suggesting that IDH-wildtype diffuse astrocytomas are a highly heterogeneous group of tumors that need to be further molecularly delineated by additional methods including methylation profiling.
References
Aoki K, Nakamura H, Suzuki H, Matsuo K, Kataoka K, Shimamura T et al (2018) Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol 20:66–77. https://doi.org/10.1093/neuonc/nox132
Arita H, Narita Y, Takami H, Fukushima S, Matsushita Y, Yoshida A et al (2013) TERT promoter mutations rather than methylation are the main mechanism for TERT upregulation in adult gliomas. Acta Neuropathol 126:939–941. https://doi.org/10.1007/s00401-013-1203-9
Arita H, Yamasaki K, Matsushita Y, Nakamura T, Shimokawa A, Takami H et al (2016) A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol Commun 4:79. https://doi.org/10.1186/s40478-016-0351-2
Brat DJ, Aldape K, Colman H, Figrarella-Branger D, Fuller GN, Giannini C et al (2020) cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol 139:603–608. https://doi.org/10.1007/s00401-020-02127-9
Brat DJ, Aldape K, Colman H, Holland EC, Louis DN, Jenkins RB et al (2018) cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV.” Acta Neuropathol 136:805–810. https://doi.org/10.1007/s00401-018-1913-0
Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372:2481–2498. https://doi.org/10.1056/NEJMoa1402121
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR et al (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477. https://doi.org/10.1016/j.cell.2013.09.034
Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D et al (2018) DNA methylation-based classification of central nervous system tumours. Nature 555:469–474. https://doi.org/10.1038/nature26000
Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA et al (2016) Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 164:550–563. https://doi.org/10.1016/j.cell.2015.12.028
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA et al (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401–404. https://doi.org/10.1158/2159-8290.CD-12-0095
Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H et al (2015) Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372:2499–2508. https://doi.org/10.1056/NEJMoa1407279
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1. https://doi.org/10.1126/scisignal.2004088
Jeuken J, Cornelissen S, Boots-Sprenger S, Gijsen S, Wesseling P (2006) Multiplex ligation-dependent probe amplification: a diagnostic tool for simultaneous identification of different genetic markers in glial tumors. J Mol Diagn 8:433–443. https://doi.org/10.2353/jmoldx.2006.060012
Johann PD, Jager N, Pfister SM, Sill M (2019) RF_Purify: a novel tool for comprehensive analysis of tumor-purity in methylation array data based on random forest regression. BMC Bioinform 20:428. https://doi.org/10.1186/s12859-019-3014-z
Jonsson P, Lin AL, Young RJ, DiStefano NM, Hyman DM, Li BT et al (2019) Genomic correlates of disease progression and treatment response in prospectively characterized gliomas. Clin Cancer Res 25:5537–5547. https://doi.org/10.1158/1078-0432.CCR-19-0032
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Mulholland S, Pearson DM, Hamoudi RA, Malley DS, Smith CM, Weaver JM et al (2012) MGMT CpG island is invariably methylated in adult astrocytic and oligodendroglial tumors with IDH1 or IDH2 mutations. Int J Cancer 131:1104–1113. https://doi.org/10.1002/ijc.26499
Pekmezci M, Rice T, Molinaro AM, Walsh KM, Decker PA, Hansen H et al (2017) Adult infiltrating gliomas with WHO 2016 integrated diagnosis: additional prognostic roles of ATRX and TERT. Acta Neuropathol 133:1001–1016. https://doi.org/10.1007/s00401-017-1690-1
Phillips JJ, Aranda D, Ellison DW, Judkins AR, Croul SE, Brat DJ et al (2013) PDGFRA amplification is common in pediatric and adult high-grade astrocytomas and identifies a poor prognostic group in IDH1 mutant glioblastoma. Brain Pathol 23:565–573. https://doi.org/10.1111/bpa.12043
Reuss DE, Kratz A, Sahm F, Capper D, Schrimpf D, Koelsche C et al (2015) Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol 130:407–417. https://doi.org/10.1007/s00401-015-1454-8
Richardson TE, Hatanpaa KJ, Walker JM (2021) Molecular characterization of “true” low-grade IDH-wildtype astrocytomas. J Neuropathol Exp Neurol 80:431–435. https://doi.org/10.1093/jnen/nlab023
Shirahata M, Ono T, Stichel D, Schrimpf D, Reuss DE, Sahm F et al (2018) Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol 136:153–166. https://doi.org/10.1007/s00401-018-1849-4
Stichel D, Ebrahimi A, Reuss D, Schrimpf D, Ono T, Shirahata M et al (2018) Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol 136:793–803. https://doi.org/10.1007/s00401-018-1905-0
Umehara T, Arita H, Yoshioka E, Shofuda T, Kanematsu D, Kinoshita M et al (2019) Distribution differences in prognostic copy number alteration profiles in IDH-wild-type glioblastoma cause survival discrepancies across cohorts. Acta Neuropathol Commun 7:99. https://doi.org/10.1186/s40478-019-0749-8
van der Maaten L, Hinton G (2008) Visualizing data using t-SNE. J Mach Learn Res 9:2579–2605
Weller M, Weber RG, Willscher E, Riehmer V, Hentschel B, Kreuz M et al (2015) Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol 129:679–693. https://doi.org/10.1007/s00401-015-1409-0
Acknowledgements
The authors thank all clinicians who took care of the patients and contributed to this study by providing specimens and clinical information. We would like to thank Editage (www.editage.com) for English language editing. This work was supported partially by JSPS KAKENHI Grant Number 25462283 (K.I.) and Practical Research for Innovative Cancer Control program of the Japan Agency for Medical Research and Development, Grant/Award Number: 17ck0106140 h0003 (K.I.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fujimoto, K., Arita, H., Satomi, K. et al. TERT promoter mutation status is necessary and sufficient to diagnose IDH-wildtype diffuse astrocytic glioma with molecular features of glioblastoma. Acta Neuropathol 142, 323–338 (2021). https://doi.org/10.1007/s00401-021-02337-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-021-02337-9