Abstract
Amyotrophic lateral sclerosis is characterised by a loss of upper and lower motor neurons and characteristic muscle weakness and wasting, the most common form being sporadic disease with neuronal inclusions containing the tar DNA-binding protein 43 (TDP-43). Frontotemporal lobar degeneration is characterised by atrophy of the frontal and/or temporal lobes, the most common clinical form being the behavioural variant, in which neuronal inclusions containing either TDP-43 or 3-repeat tau are most prevalent. Although the genetic mutations associated with these diseases have allowed various experimental models to be developed, the initial genetic forms identified remain the most common models employed to date. It is now known that these first models faithfully recapitulate only some aspects of these diseases and do not represent the majority of cases or the most common overlapping pathologies. Newer models targeting the main molecular pathologies are still rare and in some instances, lack significant aspects of the molecular pathology. However, these diseases are complex and multigenic, indicating that experimental models may need to be targeted to different disease aspects. This would allow information to be gleaned from a variety of different yet relevant models, each of which has the capacity to capture a certain aspect of the disease, and together will enable a more complete understanding of these complex and multi-layered diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) are linked together by shared genetic and pathologic phenotypes. However, they are both clinically and pathologically diverse. This review will focus on the most common phenotypes of these syndromes in comparison to the animal and cellular models most widely employed in preclinical and molecular studies in order to assist in interpreting the potential utility of these models in understanding pathogenesis.
ALS/FTLD syndromes
Most common clinical ALS/FTLD phenotypes
Amyotrophic lateral sclerosis (ALS) Patients develop signs and symptoms of progressive muscle wasting and atrophy leading to paralysis, with respiratory failure being the predominant mode of death. The incidence of ALS in European populations is 2–16 per 100,000 per year [79]. The main clinical presentations of ALS are (1) limb-onset ALS with a combination of upper motor neuron (UMN) and lower motor neuron (LMN) signs (70%); (2) bulbar onset ALS with subsequent spread to the limbs (25%); and less commonly (3) primary lateral sclerosis (PLS) with pure UMN involvement (<5%) or (4) progressive muscular atrophy (PMA) with pure LMN involvement (<5%) [70]. The median survival is 3 years from symptom onset but this tapers out to 20 years or more in patients with the rarer PLS or PMA forms of disease. ALS occurs sporadically in ~90% of patients (sALS), with only a minority demonstrating familial disease (fALS). Patients with sALS and fALS are clinically indistinguishable, the only exception to this being the minority of patients that have a mutation in either the fused in sarcoma (FUS) gene (~1% of sALS and ~4% of fALS), which is associated with juvenile onset and lower motor neuron syndrome, or the Ala4Val variant in the Cu/Zn superoxide dismutase (SOD1) gene (<2% sALS and <12% fALS), which is linked to rapidly progressive, primarily lower motor neuron syndrome [25, 120]. Cognitive and neuropsychiatric impairment that reaches clinical criteria for behavioural variant frontotemporal dementia (bvFTD) is seen in approximately 15% of patients with ALS [92] and frequently associates with the presence of a hexanucleotide repeat expansion in the C9orf72 gene (~5% of sALS and ~40% of fALS) [18, 25] (Fig. 1a). Mutations in other known ALS genes are very rare (<1% of sALS and ~4% of fALS have a mutation in the TARDBP gene and <1% of sALS and <1% of fALS have a mutation in other known ALS genes) [25] (Fig. 1a). This review will concentrate on the sALS phenotype with UMN and LMN signs and limited cognitive impairment, since this phenotype represents an estimated 75% of ALS cases.
The prevalence of the most common genetic mutations in patients with sporadic/familial ALS and FTLD [25, 32] in comparison to the number of studies that have used transgenic mouse models to study ALS and FTLD. a Most cases with ALS are sporadic, with hexanucleotide repeat expansions in the C9orf72 gene the most common genetic abnormality detected to date. Of the 997 published studies employing mice to study ALS, the majority use SOD1 transgenic manipulations, although more models have been developed and deployed since 2010 (355 published studies prior to 2010, nearly all using SOD1 transgenic models, compared with 642 published studies after 2010, with ~25% now using other transgenes). b Most cases with FTLD are sporadic, with hexanucleotide repeat expansions in the C9orf72 gene the most common genetic abnormality detected to date. Of the 234 published studies that have used mice to study FTLD, the majority use MAPT transgenic manipulations, although more models have been developed and deployed since 2010 (37 published studies prior to 2010, the majority using MAPT transgenic models, compared with 197 published studies after 2010 using a diversity of transgenic models)
Frontotemporal lobar degeneration (FTLD) Patients present clinically with either significant behavioural deterioration (bvFTD) or predominant language decline (primary progressive aphasia, PPA). The estimated prevalence of FTLD is 15–22 per 100,000 individuals per year [88]. Patients with bvFTD (~57%) present with persistent changes in behaviour and interpersonal functioning, which manifest in disinhibition, apathy, stereotypical behaviour, a loss of empathy, altered food preferences and executive deficits [73, 97]. With the exception of ~10% of patients that present with early amnesia [45], episodic memory is relatively spared until the later stages of disease in bvFTD. Based on the language features, patients with PPA (~40%) are further categorized into (1) non-fluent variant primary progressive aphasia (nfv-PPA, ~23%), (2) logopenic variant primary progressive aphasia (lv-PPA, ~32%) or (3) semantic variant primary progressive aphasia (sv-PPA, ~45%) [22, 42, 73]. An autosomal dominant pattern of inheritance is observed in 25–33% of FTLD cases [102, 127], most commonly in patients with bvFTD [106]. Importantly, the clinical phenotype characteristic of each FTLD syndrome is similar across patients with sporadic or familial disease. Approximately 10–15% of FTLD patients develop clinical ALS [80] and frequently associates with the presence of a hexanucleotide repeat expansion in the C9orf72 gene (Fig. 1b). Parkinsonism, including the phenotypes of corticobasal degeneration syndrome and progressive supranuclear palsy, is also observed in patients with FTLD and has been associated with mutations in the genes that encode the microtubule-associated protein tau (MAPT) and progranulin (GRN), which together account for <20% of familial and <20% of sporadic FTLD cases [7] (Fig. 1b).
Survival duration is highly variable across FTLD syndromes, with a median survival of ~3 years in patients with co-existing ALS, and a significant proportion of patients, particularly with sv-PPA, surviving for over a decade [127]. This review will concentrate on the bvFTD phenotype with behavioural and socioemotional features not only because this is the most common clinical syndrome in humans [73], but also because developments in modelling the PPA phenotype are still in their infancy.
Most common pathological ALS/FTLD phenotypes
The most obvious pathological difference between ALS and FTLD is the degree of brain tissue degeneration (Fig. 2a). In ALS, tissue loss is not often apparent, with atrophy in the motor cortex identified in only ~25% of patients [23]. In contrast, FTLD is characterised by significant initial focal tissue loss in the frontal and/or temporal lobes that progresses overtime to involve substantial regions of the brain [14].
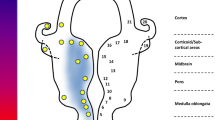
The macroscopic, pathological and biochemical characteristics of patients with bvFTD that have underlying FTLD-TDP pathology, and patients with ALS. a Significant brain tissue degeneration is apparent in patients with bvFTD but not in patients with ALS. b A simplified illustration of the topographical progression of TDP-43 in bvFTD and ALS cases [11, 12] based on the key regions identified as sensitive for discriminating clinical TDP-43 proteinopathy syndromes [115]. In bvFTD, TDP-43 accumulates in the rhinal cortex ± amygdala before progressing to frontotemporal cortices and then targeting the motor systems (motor cortex and hypoglossal nucleus). In ALS, TDP-43 is deposited in the motor system (motor cortex and hypoglossal nucleus) before targeting the frontal and then temporal regions. c An illustration of the most common FTLD-TDP subtypes [82] in bvFTD and the cortical TDP-43 pathology observed in some patients with ALS [114]. Approximately 50–65% of patients with bvFTD have underlying FTLD-TDP type A, 20–30% of patients demonstrate an FTLD-TDP type B and 15–20% of patients have an FTLD-TDP type C. In ALS cases with cortical TDP-43, cases demonstrate TDP-43 neuronal cytoplasmic inclusions with or without short dystrophic neurites across all cortical layers. d Pathological TDP-43 lesions characteristic of FTLD-TDP subtypes and ALS. In FTLD-TDP type A, TDP-43 cytoplasmic inclusions and short dystrophic neurites are observed. In FTLD-TDP type B, TDP-43 cytoplasmic inclusions predominate. In FTLD-TDP type C, long dystrophic neurites are characteristically seen. In ALS cases skein-like inclusions in motor neurons are characteristic. e An illustration of the TDP-43 immunoblot analysis in FTLD-TDP and ALS cortex [114, 119]. Immunoblot analysis of sarkosyl-insoluble phosphorylated TDP-43 revealed similar molecular species of TDP-43 in FTLD-TDP type B and ALS cases, with a predominant 24 kDa band. In FTLD-TDP type C, the 23-kDa band is the most intense. The band pattern of FTLD-TDP type A cases is an intermediate between FTLD-TDP type B/ALS and FTLD-TDP type C
Amyotrophic lateral sclerosis (ALS) is characterised by degeneration of upper and lower motor neurons. In addition to targeted neuronal loss, the normally nuclear occurring TAR DNA-binding protein 43 (TDP-43) accumulates in cytoplasmic inclusions in most surviving motor neurons (Fig. 2d) of almost all autopsied cases of sALS, as well as in the majority of patients with fALS (>84%) (Fig. 3). The only exception to this (~16%) is seen in cases with mutations in the SOD1 or FUS genes, in which cytoplasmic inclusions stain for SOD1 or FUS proteins instead (refer to [120] for a review) (Fig. 3). A recent study staged the progression of TDP-43 pathology in ALS (Fig. 2b) and reported the deposition of TDP-43 pathology in the motor system network of all cases, with TDP-43 progressing to the frontal and temporal lobes in a subset of cases [12].
The main pathological aggregates observed in patients with ALS and FTLD at autopsy. a In ALS, TDP-43 is the most common pathology, and only a minority of patients are found to have SOD1 or FUS aggregates. In FTLD, TDP-43 and tau have a similar prevalence, with FUS aggregates reported in ~10% of patients. b Chart of the most and least common pathological aggregates observed in patients with FTLD with an antemortem diagnosis of behavioural variant frontotemporal dementia (bvFTD), semantic variant of primary progressive aphasia (sv-PPA), non-fluent variant of primary progressive aphasia (nfv-PPA) and logopenic variant of primary progressive aphasia (lv-PPA). TDP-43 pathology is observed in all patients with bvFTD-ALS and is seen at an equal incidence as that of tau pathology in patients with bvFTD without ALS. In patients with PPA, TDP-43 pathology predominates in sv-PPA and is less common in patients with other PPA syndromes. Tau pathology is most common in patients with nfv-PPA and rare in patients with sv-PPA. Pathological Alzheimer’s disease (AD) is the most common pathology observed in patients that present with lv-PPA
Frontotemporal lobar degeneration (FTLD) is a progressive neurodegenerative disease that targets the frontal and temporal lobes (Fig. 2a). Three major proteins have been mechanistically linked to neurodegeneration in FTLD: (1) the TDP-43 protein (FTLD-TDP, ~32–54%), (2) the microtubule-associated protein tau (FTLD-tau, ~43–45%) and (3) in a small proportion of cases, the FUS protein (FTLD-FUS, 3–13%) which, in contrast to ALS, is not associated with mutations in the FUS gene [22, 67] (Fig. 3). In patients with bvFTD, ~60% demonstrate FTLD-TDP and ~40% have FTLD-tau [67] (Fig. 3). In sv-PPA, FTLD-TDP is the predominant pathology (~83%), whereas nfv-PPA is associated predominantly with FTLD-tau (~70%) [67] and lv-PPA is associated with pathological Alzheimer’s disease (AD) [22]. A proportion of FTLD-TDP cases have co-existing ALS (~14%), whereas a proportion of FTLD-tau cases demonstrate extrapyramidalism (57%) [67, 82].
FTLD cases with TDP-43 pathology (FTLD-TDP) are categorised into subtypes based on the morphology and distribution of TDP-43 lesions across the cortical layers (Fig. 2c, d): (1) FTLD-TDP type A (~40% of all pathological cases), which is recognised by the presence of TDP neuronal cytoplasmic inclusions (NCIs) and short dystrophic neurites (DNs) predominantly in the upper cortical layers II/III; (2) FTLD-TDP type B ~35% of all pathological cases), which demonstrates TDP NCIs across all cortical layers; (3) FTLD-TDP type C (~25% of all pathological cases), which is recognised by the presence of long DNs predominantly in the upper cortical layers, and is common in sv-PPA and (4) FTLD-TDP type D, which is very rare due to its association with VCP gene mutations, and is recognised by the presence of neuronal intranuclear inclusions and DNs that are not restricted to any cortical layer [67, 82]. The topographical distribution of TDP-43 deposition in bvFTD (Fig. 2b) was recently assessed and found to first accumulate in the orbitofrontal cortex and amygdala before progressing to the frontal and temporal cortices in all patients, eventually targeting the motor system network and finally the visual cortex and cerebellum in a subset of patients [11].
Based on the tau isoform, morphology and distribution, FTLD cases with tau pathology (FTLD-tau) are subclassified into 3-repeat tau Pick’s disease (PiD, ~30% of all pathological cases), which is recognised by the presence of argyrophilic Pick bodies predominantly located in the dentate gyrus granule cells, hippocampal CA1 pyramidal neurons and frontal and temporal cortices, or 4-repeat tau (Fig. 4). FTLD-tau cases with 4-repeat tau all have neuronal deposition of hyperphosphorylated 4-repeat tau to varying degrees and are further categorised (Fig. 4) into (1) corticobasal degeneration (CBD, ~35% of all pathological cases), which is recognised by the presence of tau-positive astrocytic plaques and threads in the affected neocortex, subcortical white matter and basal ganglia; (2) progressive supranuclear palsy (PSP, ~30% of all pathological cases), distinguished by the presence of tau-positive tufted astrocytes in affected neocortical and subcortical regions; or the recently characterised (3) globular glial tauopathy (GGT), which is recognised by the widespread presence of globular oligodendroglial and astrocytic tau inclusions and is likely to account for ~5–10% of cases categorised as PSP or CBD [1, 19, 67].
The characteristic molecular and morphological FTLD-tau pathologies observed in patients with bvFTD. a In Pick’s disease (PiD), immunoblot analysis demonstrates major molecular tau species of 60 and 64 KDa indicative of 3-repeat tau isoforms. Micrograph and schematic of the typical Pick body inclusions of hyperphosphorylated 3-repeat tau in neurons that characterise PiD. At autopsy, 55–70% of patients with behavioural variant frontotemporal dementia are found to have PiD. b In corticobasal degeneration (CBD), immunoblot analysis demonstrates major molecular tau species of 64 and 68 KDa indicative of 4-repeat tau isoforms. Micrograph and schematic of the characteristic hyperphosphorylated 4-repeat tau aggregated in the end-feet of the astrocytes as astrocytic plaques in CBD. At autopsy, 20–30% of patients with behavioural variant frontotemporal dementia patients are found to have CBD. c In progressive supranuclear palsy (PSP), immunoblot analysis demonstrates major molecular tau species of 64 and 68 KDa indicative of 4-repeat tau isoforms. Micrograph and schematic of the hyperphosphorylated 4-repeat tau aggregated in the processes of the astrocytes in PSP giving them characteristic tufted shape. Au autopsy, <10% of patients with behavioural variant frontotemporal dementia are found to have PSP. d In globular glial tauopathy (GGT), immunoblot analysis demonstrates major molecular tau species of 64 and 68 KDa indicative of 4-repeat tau isoforms. Micrograph and schematic of the hyperphosphorylated 4-repeat tau aggregated in blobs within the cytoplasm of astrocytes in GGT. At autopsy, <10% of patients with behavioural variant frontotemporal dementia are found to have GGT
In patients with bvFTD, FTLD-TDP type A (50–65%) is the predominant FTLD-TDP subtype observed, with FTLD-TDP type B (20–30%) and type C (15–20%) less prevalent (Fig. 2c), although it has been suggested that the type C cases represent misdiagnosed cases of sv-PPA [67]. Patients with bvFTD associated with FTLD-tau predominantly demonstrate PiD (55–70%), and less commonly CBD (20–30%) or other tauopathies (10–15%) [67] (Fig. 4).
Most common biochemical ALS/FTLD phenotypes
In contrast to the major FTLD-TDP cellular subtypes observed, ALS cases are not routinely subcategorised into cellular subtypes and demonstrate a different topographical distribution of similar TDP-43 inclusions (Fig. 2) [11, 12]. Pathological TDP-43 in both ALS and FTLD-TDP is hyperphosphorylated, ubiquitinated and N-terminally truncated [48, 57, 86], concentrating different sized TDP-43 protein species in morphologically distinct inclusions (Fig. 2). Importantly, the TDP-43 pathology identified in ALS brain has been shown to be biochemically indistinguishable from FTLD-TDP type B (which has equal amounts of higher and lower molecular weight species), but distinct from FTLD-TDP type A and FTLD-TDP type C (both with more lower weight molecular species but different higher molecular weight species, Fig. 2e), with the TDP-43 pathology identified in a proportion of Alzheimer’s disease cases biochemically similar to FTLD-TDP type A [114, 119]. While the TDP-43 molecular patterns in different brain and spinal cord regions of individual patients are indistinguishable [119], immunohistochemical analyses suggest more c-terminal compared with n-terminal TDP-43 in the brain versus the spinal cord [57]. These data suggest some convergence of cellular TDP-43 inclusion subtype onto different clinical syndromes, with type A most associated with dementia syndromes, type B associated with ALS syndromes (with some regional differences in the metabolism of TDP-43) and type C mostly associated with sv-PPA [67].
The 3-repeat tauopathy PiD is biochemically and histopathologically distinct from the 4-repeat tauopathies (Fig. 4), with pathological tau inclusions in neurons and glial in all tauopathies hyperphosphorylated [60]. However, within the 4-repeat tauopathies, differences in tau fragmentation patterns have been reported, with immunoblot analyses demonstrating a predominant ~37 kDa tau fragment in CBD, and a prominent ~35 kDa tau fragment in PSP and GGT [1].
Models used to assess ALS/FTLD syndromes
Most common animal models used for clinical assessments
A critical determinant of the differences in clinical phenotypes observed in mammalian models compared to human patients relates to the promoter system employed to transgenically express the pathogenic protein of interest. Accordingly, using pan-neuronal promoters such as the murine Thy1.2 or the hamster PrP promoters confers protein expression to a wide range of neurons and brain regions, including motor control systems that may be variably affected in the different human phenotypes of ALS/FTLD. Nevertheless, different clinical phenotypes (including motor deficits) are considered valuable surrogate readouts of neuronal dysfunction in in vivo models and have been instrumental in testing both symptomatic and disease-modifying treatments [10, 63, 78, 93, 99, 122].
Amyotrophic lateral sclerosis (ALS) Clinical ALS has been modelled in several species (Fig. 5), the most common being rodents (mouse, rats), fish (zebrafish, Danio rerio), nematodes (roundworm, Caenorhabditis elegans) and arthropods (fruit fly, Drosophila melanogaster) (reviewed in [5, 21, 85, 117]). While the neuroanatomical organisation of rodents and anthropoids (monkeys, apes and humans) share general similarities, the cortical architecture in particular (including the motor cortex) has undergone significant evolutionary specialisation in anthropoids [29, 31]. Such differences should be considered when interpreting findings from models of ALS/FTLD. While no ALS model is a perfect clinical replica of the human disease, each can assess a motor weakness phenotype, contributing important clinical insights (Table 1). Nevertheless, mice provide the most closely translatable results to clinical ALS and they represent the most widely employed in vivo ALS model to date (Fig. 5).
Mutant SOD1 mice were the first genetic mouse models of ALS reported in 1995 and have since dominated clinical model studies in the field (Fig. 1a). Various transgenic rodent models of ALS have since been developed [2, 10, 61, 75, 78, 85, 93, 99, 103] and ALS-like phenotypes such as muscle weakness, motor deficits, progressive loss of grip strength, gait abnormalities, hindlimb clasping, reduced survival and significant loss in body fat have been successfully recapitulated in these models (Table 1). Mice are the most frequently used rodent species for ALS animal studies (Fig. 5) with mutant SOD1 mouse lines the most widely used rodent model of ALS (Fig. 1a). This is due to its similar ALS clinical phenotype with regard to onset and progression compared to other transgenic rodent models (see [10, 78, 91, 93, 99] for a review). Briefly, SOD1-G93A mice present with adult onset (~100 days of age), rapidly progressive motor symptoms and muscle wasting and display a mean survival of 130–150 days [47]. In addition to mice, mutant SOD1-expressing rats with different patterns of symptom progression have been reported [84] (Fig. 5). In contrast to human ALS where no significant difference is observed between patients with upper-limb versus lower-limb onset ALS [70], disease progression is significantly faster in forelimb-type compared to hind-limb type SOD1 ALS rats [84]. Importantly, a poorer prognosis is associated with bulbar-onset ALS in humans, but the majority of studies in rodents have focused on phenotyping spinal motor symptoms, with only one group recently reporting impaired orolingual motor function in a SOD1 ALS rat model, highlighting the need to better characterise bulbar symptoms in such animal models of ALS [108].
Transgenic mice manipulating the TARDBP gene to produce TDP-43 pathologies were expected with great anticipation to provide ALS models with wider applicability to sALS. These TARDBP models (TDP-43 models) have been growing in use since 2010 (Fig. 1a). The first mutant TDP-43 transgenic line presented with reduced survival, which was initially attributed to motor neuron loss and muscle atrophy [78, 93, 128]. However, follow-up work showed that deaths were rather due to gastrointestinal problems and transgenic expression in the myenteric plexus with gut paralysis [38, 46, 49]. Several constitutively expressing TDP-43 lines have since been developed with different degrees of motor impairments and muscle atrophy [128, 129], yet none model the clinical presentation of ALS as accurately as mutant SOD1 mice (Table 1). Interestingly, heterozygous deletion of the TARDBP [72] or a heterozygous TARDBP mutation that results in premature truncation [98] is associated with minor neurological deficits (clasping, reduced grip strength), while motor neuron-specific conditional TARDBP knockout or iRNA-driven TDP-43 reduction presents with a progressive ALS-like phenotype with muscle atrophy, motor neuron loss and early death [58, 132, 133]. More recently, we and others have established TARDBP lines with controllable transgene expression, complex progressive motor deficits and muscle atrophy, with TDP-43 pathology in both upper and lower motor neurons [69, 125]. Short-term suppression of transgenic TDP-43 expression rapidly reversed functional deficits, supporting a pathogenic role of soluble TDP-43 in neuronal dysfunction [69].
Most recently (Fig. 1a), bacterial artificial chromosome (BAC) transgenic mice expressing the human C9orf72 locus with repeat expansion have been developed but do not yield functional motor changes [87, 89]. A recent C9orf72 BAC transgenic mouse expressing patient-derived repeat expansion also lacked motor deficits, but developed cognitive, behavioural and anxiety phenotypes at 12 months of age, resembling features of FTLD rather than ALS [66]. Interestingly, these deficits were reversible with antisense oligonucleotide treatment [66]. This might suggest that these C9orf72 mouse models may provide a greater understanding of FTLD rather than ALS features (Table 1). However, in a small subset of a new BAC transgenic strain using patient-derived C9orf72 gene constructs an ALS-like phenotype can be observed, with progressive weight loss, reduced activity, breathing problems and reduced survival [77]. Using adeno-associated viruses [24], FTD/ALS-like deficits including hyperactivity, anxiety, antisocial behaviour and motor problems have been reproduced when 66 GGGGCC repeats were expressed in mice, indicating that mice are suitable to recapitulate deficits of FTD/ALS linked to C9orf72 (Table 1). Together, these C9orf72 mutant models with overlapping ALS/FTLD features are likely to be informative for the clinical continuum between these disorders [17].
Another vertebrate species with a motor system that has been used to model ALS is the zebrafish (Danio rerio) (Fig. 5). A major advantage of the zebrafish is the ease of performing imaging due to its transparency and external development. However, as covered in a review performed by Babin and colleagues, the absence of corticospinal and rubrospinal tracts in the zebrafish nervous system renders the zebrafish a homologous model only for lower motor neuron disorders and, therefore, only partially models ALS [5].
The C. elegans and fruit fly have been established as the most useful invertebrate systems for studying ALS (Fig. 5). Both these models have well-characterised and easily accessible nervous systems that contain similar basic peripheral motor neural circuitry as humans. In addition to this, they have a short generation time, making them fast and inexpensive models to generate for studying the basic biochemical processes targeted in ALS. The ALS phenotype has been predominantly modelled in C. elegans by expressing mutant or wild-type SOD1, TDP-43 or FUS proteins, resulting in locomotion defects and impaired neuronal transmission (reviewed in [117]). In comparison to the C. elegans, the fruit fly has been more extensively used for studying ALS, with a larger range of gene knock-out, mutant and wild-type expression models developed to date (reviewed in [21]). Reduced locomotion (walking and/or climbing), decreased lifespan and occasional degeneration of indirect flight muscles have been variably reported in these ALS fruit flies [21]. Although these models have the advantage of studying the basic biochemical processes, the C. elegans and fruit flies cannot adequately represent the complexity of the mammalian motor system. Locomotor deficits and reduced lifespan suggest progressive loss of motor function and eventual death characteristic of ALS, but specific aspects of the ALS phenotype such as the site and progression of motor dysfunction cannot be recapitulated in C. elegans and require some inference in fruit flies (climbing versus flying). As such, while their strength is in studying molecular processes and signalling, the simplicity of these invertebrate nervous systems and limited functional readouts remain a disadvantage, and knowledge garnered from these models should be verified in mammalian systems such as rodents.
Behavioural variant frontotemporal dementia (bvFTD) Given the limited ability to model behavioural characteristics of bvFTD in invertebrates, rodents remain the most widely studied models of bvFTD (Table 1). As for ALS, mice are the predominant rodent species used (Fig. 5). Shortly after the discovery of MAPT mutations in familial FTLD in 1998 [55], P301L mutant tau-expressing transgenic lines were generated [43, 76]. These were the first to reproduce the hyperphosphorylation and neuronal accumulation of tau in animals. Since then, a large number of MAPT transgenic (tau transgenic) mice have been generated, expressing different isoforms of tau together with different pathogenic FTLD MAPT mutations [10, 61, 99]. Tau transgenic mice have long dominated animal studies into FTLD and remain the most frequently used FTD models to date (Fig. 5). Phenotypically, tau transgenic mice often present with motor neuron and memory deficits (Table 1), both of different degrees and penetrance. This stands in contrast to the lack of motor neuron and memory deficits in patients with FTLD-tau (see above). However, several tau transgenic lines display disinhibition and risk-taking behaviour (Table 1), features more closely resembling bvFTD [27, 28, 35, 95, 113, 121, 126]. Furthermore, changes to activity consistent with apathy have been reported in some tau transgenic lines [71, 126].
Mouse models of other genes involved in FTLD have more recently entered the scene (Fig. 1b). For example, GRN knock-out mice have been effective at modelling behavioural abnormalities reminiscent of patients with bvFTD such as social deficits, aggression, depression-like behaviour and disinhibition in the absence of motor function impairment [41, 68, 135]. Behavioural deficits have been recorded in other rodent models of FTD [27, 54, 103, 110, 111, 118]. However, it is important to differentiate which of these ‘behavioural’ models have utilised Alzheimer-related tests of hippocampal-dependent learning and memory tasks, such as the Morris water maze [54, 103, 110, 118] and which of these demonstrate preservation of hippocampal-dependent learning and memory until late in the disease [135]. This is of importance since memory deficits in early bvFTD are very rare [97] and have been linked to atrophy of the anterior thalamus instead of the hippocampus [53]. Overlapping motor neuron and behavioural deficits have been recapitulated in several rodent models [54, 90, 110, 118], possibly mimicking aspects of the human ALS/FTLD disease continuum [17]. Accordingly, TDP-43 transgenic lines with both motor neuron impairments and muscular atrophy, and behavioural changes including disinhibition have been reported (Table 1), linking them to both ALS and FTLD [69, 125].
Animal models recapitulating the most common ALS/FTLD pathologies
Neuropathological changes such as neuronal loss and the formation of pathognomonic inclusions have been reproduced with relative accuracy in animal models (Fig. 6). For example, SOD1-G93A transgenic mice showed 90% loss of spinal cord motor neurons, comparable to the cell loss in ALS [47], and more recent models expressing TDP-43 variants present with significant neurodegeneration, often with early onset [56, 69]. In contrast, neuronal loss is not a common feature of mutant MAPT transgenic mouse models of FTLD-tau [44] and has only been reported in models with very high expression levels of the transgene [10, 78, 93, 99, 103]. In addition to gross neuropathological changes, cellular inclusions characteristic of FTLD and ALS have been recapitulated in mice (Table 1). Tau inclusions in transgenic mice usually have NFT-like morphology even if the pathogenic mutant protein expressed is associated with distinct types of inclusions in humans. There are some exceptions such as the Pick body-like inclusions found in the K369I mutant tau expressing K3 mice as shown in Fig. 6 [63, 100]. Tau inclusions have a similar appearance in most tau transgenic mouse models even though mice can form different types of tau aggregate inclusions per se. This is seen when recipient mice are injected with tau preparations from FTLD-tau subtypes and in such mice the disease-typical patterns of tau pathologies are maintained [26]. This suggests that additional factors are required for the disease-type specific tau inclusions. As observed for tau inclusions in tau transgenic mice, TDP-43 transgenic mice produce (if at all) cytoplasmic inclusions similar to those observed in ALS rather than FTLD.
A comparison of the pathology observed in patients and mouse models of ALS/FTLD-TDP and FTLD-tau. a The modified Bielschowsky silver stain showing a characteristic Pick body in the amygdala of a bvFTD patient compared to a Pick body in the amygdala of a 5-month-old MAPT K369I mutation mouse [63]. b Immunohistochemistry of cross-sectional cervical spinal cord sections using phospho-TDP-43 antibodies (in humans CAC-TIP-PTD-M01 from Cosmo Bio, in mice 60019-2-Ig from Proteintech) showing a characteristic phosphorylated TDP-43 inclusion in an upper motor neuron of a patient with ALS compared to a phosphorylated TDP-43 inclusion observed in an upper motor neuron of a 1-month-old inducible TARDBP A315T mutation mouse [69] (lower neuron has the cytoplasmic TDP-43 inclusion compared with a nuclear location in the neuron above). c Immunohistochemistry of transverse sections of the medulla oblongata using phospho-TDP-43 antibodies (in humans CAC-TIP-PTD-M01 from Cosmo Bio, in mice 60019-2-Ig from Proteintech) showing a characteristic phosphorylated TDP-43 inclusion a hypoglossal motor neuron of a patient with ALS compared to the location of phosphorylated TDP-43 in the cytoplasm of hypoglossal neurons in a 1-month-old inducible TARDBP A315T mutation mouse [69]
Amyotrophic lateral sclerosis (ALS) Although mutant SOD1 rodent models replicate aspects of the clinical ALS symptoms most accurately of all animal models, SOD1 models fall short in producing the most common neuropathological feature of ALS, the cytoplasmic inclusions formed by TDP-43 [12]. While this is in line with the absence of TDP-43 pathology in fALS with SOD1 mutations [81], it limits the utility of SOD1-based animal models for studying generalised pathomechanisms and understanding the neuropathological changes of the sALS (Table 1). Reproducing TDP-43 pathology in mice has been of mixed success (Fig. 6b, c) and, as stated earlier, is driven by particular promoter systems and transgenic protein species. Insoluble TDP-43 species have been isolated from several TDP-43 transgenic mouse lines, using biochemical methods, typically sequential extraction of brain tissue with RIPA and urea-based buffers [69]. However, in most transgenic mice overexpressing TDP-43 protein (with or without mutations), cytoplasmic inclusions are only rarely found, despite marked cell loss in some models [56, 69]. The only model with more frequent neuronal TDP-43 inclusions was achieved by overexpressing a non-disease human TDP-43 variant that lacked nuclear localization sequences [125]. In contrast, a similar model with expression of TDP-43 together with the pathogenic A315T mutation did not result in overt inclusion formation [69]. This may indicate that the formation of TDP-43 inclusions is a lengthy process and characterises neurons still present at autopsy in human ALS, with not sufficient time to develop during the life span of mice. Alternatively, additional factors contributing to the deposition of TDP-43 may not be found in mice, or neurons displaying these features may be lost too quickly to be seen in mice, with either scenario suggesting that mice may not as yet provide robust mechanistic models for human ALS pathology [64].
Neuropathologically, C9orf72 carriers are characterised by neuronal TDP-43 pathology [120]. Of the C9orf72 BAC transgenic mice recently reported [66, 77, 87, 89], only the line with an ALS-like phenotype had some TDP-43 pathology [77], although the more artificial adeno-associated virus-mediated expression of C9orf72 repeats produces RNA foci and dipeptide inclusions together with TDP-43 pathology [24]. This suggests that murine TDP-43 is capable of forming inclusions per se. However, in line with disease-mutant TDP-43 transgenic mice that fail to reliably produce widespread ALS-like neuronal inclusions despite high TARBP transgene levels, developing widespread TDP-43 inclusions may remain a more unique feature of human neuropathology. Alternatively, perhaps virus-associated inflammation is a necessary co-factor required for such TDP-43 pathology.
Frontotemporal lobar degeneration (FTLD) In contrast to the limited success with reproducing TDP-43 lesions in ALS/FTLD animal models (Fig. 6b, c), reproducing neuronal tau accumulation in tau transgenic mice has been remarkably efficacious and is the most common model used to study neuronal FTLD pathology (Fig. 1b). While neuronal expression of full-length human tau in mice results only in pre-tangle formation with aberrantly phosphorylated tau, pathogenic FTLD mutations produce more pathological forms of neuronal tau that form inclusions [76]. Similar to neuronal tau pathology, astrocytosis and microgliosis found in FTLD-tau cases have been recapitulated in mutant tau transgenic mice with neuronal tau pathology [123], although due to the use of neuronal promoters, the characteristic glial tau inclusions are not observed (Table 1). Most tau transgenic mouse models express one of the three 4-repeat human tau isoforms, providing models for 4-repeat dominant forms of FTLD-tau (Fig. 4). In contrast, the 3-repeat tau-containing Pick bodies (Fig. 4) that dominate in patients with bvFTD have only been reproduced in one tau transgenic mouse model to date. Rockenstein and colleagues generated mice with neuronal expression of 3-repeat human tau containing a L266V/G272V double MAPT mutation, which has been identified in familial PiD [15, 52, 100]. These mice develop PiD-like tau inclusions [100]. Interestingly, we produced mice with expression of 4-repeat human tau containing the pathogenic PiD MAPT mutation K369I, which similarly resulted in ovoid neuronal inclusions (Fig. 6a) typical for PiD [63]. While more research on the pathogenesis of 3-repeat versus 4-repeat tau inclusions is required, these observations suggest that in familial PiD, distinct mutations contribute to the typical tau lesions.
Several mouse lines using other FTLD genes have been generated over the past years (Fig. 1b). A number of GRN knockout mice showed age-related neuroinflammation with microgliosis and astrogliosis [68, 83, 90, 130, 135], the development of ubiquitinated inclusions and some TDP-43 phosphorylation [130, 134, 135]. GRN knockout mice have been instrumental in showing that the progranulin protein regulates maintenance of synapses, and loss of progranulin results in microglia-mediated loss of inhibitory synapses [77]. Furthermore, GRN deficient mice present with lysosomal dysfunction [116]. This highlights the value of GRN knockout mice for understanding specific disease mechanisms, with a molecular link between loss of progranulin and TDP-43 remaining to be established. Expression of human mutant VCP resulted in the development of VCP-negative, TDP-43-positive inclusions, with nuclear clearance of TDP-43 [30, 101, 136]. However, despite mature TDP-43 pathology no neurodegeneration was found in VCP-expressing animal lines, and functional deficits were mild [6, 30, 101]. Reduced VCP-mediated degradation of TDP-43 by the proteasome in VCP transgenic mice may have contributed to TDP-43 pathology [101], further supporting the hypothesis that development of TDP-43 inclusions requires multiple hits. Expression of a truncated variant of CHMP2B produced ubiquitin and p62-positive inclusions that were TDP-43-negative, resembling the human pathology of CHMP2B carriers [40]. Taken together, transgenic mice with FTLD genes other than MAPT have reproduced aspects of different pathologies associated with the less common forms of FTLD.
In vitro human induced pluripotent stem cell models of the most common pathogenic mechanisms of ALS/FTLD
The recent breakthrough in successfully generating induced pluripotent stem cells (iPSCs) from human somatic cells [112] has ushered in a new era in which researchers can now study disease mechanisms in patient-derived neurons in vitro. Significant advantages of human iPSCs is that they can be generated from patients with sporadic disease and of advanced age, and they also have the human-specific complexity of RNA biology likely to be most perturbed in these disorders [13]. Major disadvantages of iPSC-derived neurons is the cellular and epigenetic heterogeneity within and across cell lines (reviewed in [50, 74, 94]), and that they have been shown to resemble foetal rather than adult neurons [51], disadvantages found in many other in vivo cell model systems. We focus on comparing the pathologies identified in the most common ALS and FTLD iPSCs with the characteristic pathological hallmarks in patients at autopsy. Only studies in which cellular assessments of pathological lesions were made will be included.
Amyotrophic lateral sclerosis (ALS) As discussed above, TDP-43 is a normally occurring nuclear protein and the mislocalization of TDP-43 to form cytoplasmic aggregates is the histopathological hallmark of almost all patients with ALS. In iPSC-derived motor neurons from patients with fALS and mutations in the TARDBP gene, cellular TDP-43 aggregates and decreased cell survival have been reported, suggesting that this model is representative of human disease [9, 36]. However, in contrast to that seen in patients, these TDP-43 aggregates in TARDBP iPSC motor neurons are predominantly nuclear. This was also observed in iPSC-derived motor neurons from patients with sALS. Of the iPSC-derived motor neurons generated from sixteen patients with sALS, TDP-43 aggregates were identified in three patient cell lines, all of which only occurred within the nucleus [16]. In an independent study of iPSC-derived neurons from a patient with fALS/FTLD carrying a TARDBP mutation, specific sensitivity of patient-derived neurons to straurosporine-induced stress was found, with exposure to this cellular stressor reported to increase the percentage of neurons with mislocalized cytoplasmic TDP-43 aggregates [137]. Together these studies highlight the phenotypic variability, which may be attributed to different reprogramming methods used across research groups (reviewed in [50, 74, 94]). Although it may be argued that the predominance of TDP-43 in the nucleus of iPSC neurons may represent an earlier disease process that is not captured in histopathological findings of end-stage autopsies, it is important to note that even in in vitro models of ALS, it is the levels of cytoplasmic rather than nuclear TDP-43 that correlate with cellular toxicity [8].
In the iPSC-derived astroglia from a patient with fALS and a TARDBP mutation, significantly increased amounts of cytoplasmic TDP-43 and decreased astrocytic cell survival were observed [107]. However, the increased cytoplasmic TDP-43 in these mutant astrocytes was not accompanied by a corresponding depletion of nuclear TDP-43, suggesting increased stability and/or lower clearance of TDP-43 and also suggesting that the loss of nuclear TDP-43 is a later event in the disease process [107]. These mutant astrocytes were also not found to impact survival of co-cultured iPSC-derived motor neurons generated from the same patient, contradicting the non-cell-autonomous neurodegeneration identified in in vitro SOD1 models [33]. Importantly, cytoplasmic TDP-43 inclusions have only been identified in oligodendroglia and not in astrocytes in patients with ALS [12].
Frontotemporal lobar degeneration (FTLD) TDP-43 cytoplasmic inclusions are present in patients with sporadic FTLD and patients with mutations in the GRN gene, with no obvious differences in the amount of TDP-43 cytoplasmic aggregates deposited between these two groups [82]. In contrast to this, a significantly higher percentage of cytoplasmic TDP-43 inclusions have been reported in iPSC neurons generated from a patient with a GRN S116X mutation compared to a patient with sporadic FTLD and a normal control [3]. In contrast to these GRN S116X neurons, nuclear TDP-43 aggregates in the absence of cytoplasmic TDP-43 inclusions were reported in iPSC-derived FTLD cortical neurons from a patient with a GRN IVS1+5G>C mutation that employed a similar iPSC generation method, despite the characteristic GRN haploinsufficiency identified across patients with mutations in the GRN gene [3, 96]. Variability across iPSCs derived from C9orf72-carriers has also been found, with the characteristic dipeptide repeat protein inclusions seen in humans [4] identified by one group [34], but not another [104].
Consistent with that seen in many patients with mutant FTLD-tau at autopsy, significantly higher levels of 4-repeat tau have been reported in iPSC neurons and glial cells derived from patients with splice-site mutations in the MAPT gene [37, 59, 109, 131]. However, in contrast to the cytoplasmic aggregates observed within neurons and axonal projections in patients, phosphorylated tau in iPSC-derived MAPT neurons demonstrate a punctate expression pattern mainly localised to the axonal compartment, with minimal perinuclear staining, which is similar to that seen in control iPSC neurons [37, 39, 59]. These data indicate that the characteristic neuropathological lesions of MAPT mutation-carriers with increased 4-repeat tau are not replicated in iPSCs. iPSCs from patients with 3-repeat tau have yet to be reported.
Conclusions regarding comparison between the most common ALS/FTLD phenotypes and models of these disease phenotypes
The data suggest that experimental models of ALS/FTLD recapitulate different aspects of the diverse phenotypes observed in ALS/FTLD, with different models recapitulating some disease aspects, but no model faithfully recapitulating all disease aspects. This may be expected given the complexity of these syndromes in general, but it should be noted that many of the current models being pursued are driven by genetic mutations either observed in only a small minority of patients, or that are known to have divergent disease mechanisms (for example, C9orf72). If our expectation of a ‘good’ model is to be a perfect replica of human ALS/FTLD in the majority of cases, then all models will continue to fail in this aspect. The data presented show reasonable comparisons of feature phenotypes that we suggest could be used as surrogate readouts to layer knowledge of the complex neuronal or neuronal network dysfunction observed in patients. For example, many tau transgenic mice have predominant motor neuron phenotypes that may appear to remotely resemble symptoms of only a small proportion of FTLD-tau cases [63]. However, these motor symptoms have been instrumental for testing drug candidates as surrogate markers of neuronal dysfunction, which then have translated to other more complex testing paradigms for behavioural and cognitive deficits [122]. In summary, rethinking expectations for experimental models to fully recapitulate the most common human ALS/FTLD phenotypes will improve translation of molecular concepts that appear to have relevance.
Functional whole animal analyses that use transgenic and/or focal ablation models are underutilised in this field and may provide more information of network interactions in whole animals. The direct assessment of neuronal network dysfunction in animal models may provide more ‘neutral’ readouts that do not rely on attributable behavioural traits and give better comparability. For example, electroencephalography (EEG) and spectral analysis of recorded frequencies have revealed neuronal network aberrations in Alzheimer’s disease mice with amyloid-β formation [62]. This includes compromised measures that have been linked to cognition and memory formation in mammals, including humans. While EEG aberrations indicative of neuronal network disturbances have also been reported in patients with FTLD [20, 65], EEG studies in FTD mouse models remain limited [105]. Hyperexcitability of upper motor neurons is an emerging theme in human ALS [124] and it would be interesting to see if similar network activity changes are reproduced in ALS mouse models.
Search strategies
We examined recent literature on amyotrophic lateral sclerosis and frontotemporal lobar degeneration, targeting full text English language studies published since 1990. We selected articles on the basis of our personal knowledge and Pubmed database searches using the following search structure: “disease” and “organism” and “gene”, whereby the terms for “disease” were either “amyotrophic” or “frontotemporal”, the terms for “organism” were either “mouse”, “rat”, “fruitfly”, “C. elegans”, “zebrafish” or “iPSC”, and the terms for “gene” were either “MAPT”, “FUS”, “SOD1”, “C9orf72”, “TARDBP”, “GRN”, “VCP” or “CHMP2B”. The final selection of references was based on our judgment of relevance, completeness, and compatibility with recent clinical, pathological, and genetic models.
References
Ahmed Z, Bigio EH, Budka H, Dickson DW, Ferrer I, Ghetti B, Giaccone G, Hatanpaa KJ, Holton JL, Josephs KA, Powers J, Spina S, Takahashi H, White CL 3rd, Revesz T, Kovacs GG (2013) Globular glial tauopathies (GGT): consensus recommendations. Acta Neuropathol 126:537–544. doi:10.1007/s00401-013-1171-0
Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M (2002) Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci 22:9340–9351
Almeida S, Zhang ZJ, Coppola G, Mao WJ, Futai K, Karydas A, Geschwind MD, Tartaglia MC, Gao FY, Gianni D, Sena-Esteves M, Geschwind DH, Miller BL, Farese RV, Gao FB (2012) Induced pluripotent stem cell models of progranulin-deficient frontotemporal dementia uncover specific reversible neuronal defects (vol 2, pg 789, 2012). Cell Rep 2:1471. doi:10.1016/j.celrep.2012.11.006
Ash PEA, Bieniek KF, Gendron TF, Caulfield T, Lin WL, DeJesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW, Rademakers R, Boylan KB, Dickson DW, Petrucelli L (2013) Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 77:639–646. doi:10.1016/j.neuron.2013.02.004
Babin PJ, Goizet C, Raldua D (2014) Zebrafish models of human motor neuron diseases: advantages and limitations. Prog Neurobiol 118:36–58. doi:10.1016/j.pneurobio.2014.03.001
Badadani M, Nalbandian A, Watts GD, Vesa J, Kitazawa M, Su HL, Tanaja J, Dec E, Wallace DC, Mukherjee J, Caiozzo V, Warman M, Kimonis VE (2010) VCP associated inclusion body myopathy and paget disease of bone knock-in mouse model exhibits tissue pathology typical of human disease. PLoS One 5. doi:10.1371/journal.pone.0013183
Baizabal-Carvallo JF, Jankovic J (2016) Parkinsonism, movement disorders and genetics in frontotemporal dementia. Nat Rev Neurol 12:175–185. doi:10.1038/nrneurol.2016.14
Barmada SJ, Skibinski G, Korb E, Rao EJ, Wu JY, Finkbeiner S (2010) Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. J Neurosci 30:639–649. doi:10.1523/Jneurosci.4988-09.2010
Bilican B, Serio A, Barmada SJ, Nishimura AL, Sullivan GJ, Carrasco M, Phatnani HP, Puddifoot CA, Story D, Fletcher J, Park IH, Friedman BA, Daley GQ, Wyllie DJA, Hardingham GE, Wilmut I, Finkbeiner S, Maniatis T, Shaw CE, Chandran S (2012) Mutant induced pluripotent stem cell lines recapitulate aspects of TDP-43 proteinopathies and reveal cell-specific vulnerability. Proc Natl Acad Sci USA 109:5803–5808. doi:10.1073/pnas.1202922109
Boxer AL, Gold M, Huey E, Gao FB, Burton EA, Chow T, Kao A, Leavitt BR, Lamb B, Grether M, Knopman D, Cairns NJ, Mackenzie IR, Mitic L, Roberson ED, Van Kammen D, Cantillon M, Zahs K, Salloway S, Morris J, Tong G, Feldman H, Fillit H, Dickinson S, Khachaturian Z, Sutherland M, Farese R, Miller BL, Cummings J (2013) Frontotemporal degeneration, the next therapeutic frontier: molecules and animal models for frontotemporal degeneration drug development. Alzheimers Dement 9:176–188. doi:10.1016/j.jalz.2012.03.002
Brettschneider J, Del Tredici K, Irwin DJ, Grossman M, Robinson JL, Toledo JB, Lee EB, Fang L, Van Deerlin VM, Ludolph AC, Lee VMY, Braak H, Trojanowski JQ (2015) Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD) (vol 127, pg 423, 2014). Acta Neuropathol 129:929. doi:10.1007/s00401-015-1428-x
Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, Kwong L, Lee EB, Elman L, McCluskey L, Fang LB, Feldengut S, Ludolph AC, Lee VMY, Braak H, Trojanowski JQ (2013) Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 74:20–38. doi:10.1002/ana.23937
Briggs JA, Wolvetang EJ, Mattick JS, Rinn JL, Barry G (2015) Mechanisms of long non-coding RNAs in mammalian nervous system development, plasticity, disease, and evolution. Neuron 88:861–877. doi:10.1016/j.neuron.2015.09.045
Broe M, Hodges JR, Schofield E, Shepherd CE, Kril JJ, Halliday GM (2003) Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology 60:1005–1011
Bronner IF, ter Meulen BC, Azmani A, Severijnen LA, Willemsen R, Kamphorst W, Ravid R, Heutink P, van Swieten JC (2005) Hereditary Pick’s disease with the G272 V tau mutation shows predominant three-repeat tau pathology. Brain 128:2645–2653. doi:10.1093/brain/awh591
Burkhardt MF, Martinez FJ, Wright S, Ramos C, Volfson D, Mason M, Garnes J, Dang V, Lievers J, Shoukat-Mumtaz U, Martinez R, Gai H, Blake R, Vaisberg E, Grskovic M, Johnson C, Irion S, Bright J, Cooper B, Nguyen L, Griswold-Prenner I, Javaherian A (2013) A cellular model for sporadic ALS using patient-derived induced pluripotent stem cells. Mol Cell Neurosci 56:355–364. doi:10.1016/j.mcn.2013.07.007
Burrell JR, Halliday GM, Kril JJ, Ittner LM, Gotz J, Kiernan MC, Hodges JR (2016) The frontotemporal dementia-motor neuron disease continuum. Lancet 388:919–931. doi:10.1016/S0140-6736(16)00737-6
Byrne S, Elamin M, Bede P, Shatunov A, Walsh C, Corr B, Heverin M, Jordan N, Kenna K, Lynch C, McLaughlin RL, Iyer PM, O’Brien C, Phukan J, Wynne B, Bokde AL, Bradley DG, Pender N, Al-Chalabi A, Hardiman O (2012) Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurol 11:232–240. doi:10.1016/S1474-4422(12)70014-5
Cairns NJ, Bigio EH, Mackenzie IRA, Neumann M, Lee VMY, Hatanpaa KJ, White CL, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DMA (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22. doi:10.1007/s00401-007-0237-2
Carlino E, Frisaldi E, Rainero I, Asteggiano G, Cappa G, Tarenzi L, Vighetti S, Pollo A, Pinessi L, Benedetti F (2014) Nonlinear analysis of electroencephalogram in frontotemporal lobar degeneration. NeuroReport 25:496–500. doi:10.1097/Wnr.0000000000000123
Casci I, Pandey US (2015) A fruitful endeavor: modeling ALS in the fruit fly. Brain Res 1607:47–74. doi:10.1016/j.brainres.2014.09.064
Chare L, Hodges JR, Leyton CE, McGinley C, Tan RH, Kril JJ, Halliday GM (2014) New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry 85:865–870. doi:10.1136/jnnp-2013-306948
Chen ZY, Ma L (2010) Grey matter volume changes over the whole brain in amyotrophic lateral sclerosis: a voxel-wise meta-analysis of voxel based morphometry studies. Amyotroph Lateral Scler 11:549–554. doi:10.3109/17482968.2010.516265
Chew J, Gendron TF, Prudencio M, Sasaguri H, Zhang YJ, Castanedes-Casey M, Lee CW, Jansen-West K, Kurti A, Murray ME, Bieniek KF, Bauer PO, Whitelaw EC, Rousseau L, Stankowski JN, Stetler C, Daughrity LM, Perkerson EA, Desaro P, Johnston A, Overstreet K, Edbauer D, Rademakers R, Boylan KB, Dickson DW, Fryer JD, Petrucelli L (2015) Neurodegeneration. C9ORF72 repeat expansions in mice cause TDP-43 pathology, neuronal loss, and behavioral deficits. Science 348:1151–1154. doi:10.1126/science.aaa9344
Cirulli ET, Lasseigne BN, Petrovski S, Sapp PC, Dion PA, Leblond CS, Couthouis J, Lu YF, Wang Q, Krueger BJ, Ren Z, Keebler J, Han Y, Levy SE, Boone BE, Wimbish JR, Waite LL, Jones AL, Carulli JP, Day-Williams AG, Staropoli JF, Xin WW, Chesi A, Raphael AR, McKenna-Yasek D, Cady J, Vianney de Jong JM, Kenna KP, Smith BN, Topp S, Miller J, Gkazi A, Consortium FS, Al-Chalabi A, van den Berg LH, Veldink J, Silani V, Ticozzi N, Shaw CE, Baloh RH, Appel S, Simpson E, Lagier-Tourenne C, Pulst SM, Gibson S, Trojanowski JQ, Elman L, McCluskey L, Grossman M, Shneider NA, Chung WK, Ravits JM, Glass JD, Sims KB, Van Deerlin VM, Maniatis T, Hayes SD, Ordureau A, Swarup S, Landers J, Baas F, Allen AS, Bedlack RS, Harper JW, Gitler AD, Rouleau GA, Brown R, Harms MB, Cooper GM, Harris T, Myers RM, Goldstein DB (2015) Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 347:1436–1441. doi:10.1126/science.aaa3650
Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M, Ghetti B, Goedert M, Tolnay M (2013) Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci USA 110:9535–9540. doi:10.1073/pnas.1301175110
Cook C, Dunmore JH, Murray ME, Scheffel K, Shukoor N, Tong J, Castanedes-Casey M, Phillips V, Rousseau L, Penuliar MS, Kurti A, Dickson DW, Petrucelli L, Fryer JD (2014) Severe amygdala dysfunction in a MAPT transgenic mouse model of frontotemporal dementia. Neurobiol Aging 35:1769–1777. doi:10.1016/j.neurobiolaging.2013.12.023
Cook C, Kang SS, Carlomagno Y, Lin WL, Yue M, Kurti A, Shinohara M, Jansen-West K, Perkerson E, Castanedes-Casey M, Rousseau L, Phillips V, Bu GJ, Dickson DW, Petrucelli L, Fryer JD (2015) Tau deposition drives neuropathological, inflammatory and behavioral abnormalities independently of neuronal loss in a novel mouse model. Hum Mol Genet 24:6198–6212. doi:10.1093/hmg/ddv336
Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, Maier I, Martin J, Nudo RJ, Ramon-Cueto A, Rouiller EM, Schnell L, Wannier T, Schwab ME, Edgerton VR (2007) Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med 13:561–566. doi:10.1038/nm1595
Custer SK, Neumann M, Lu H, Wright AC, Taylor JP (2010) Transgenic mice expressing mutant forms VCP/p97 recapitulate the full spectrum of IBMPFD including degeneration in muscle, brain and bone. Hum Mol Genet 19:1741–1755. doi:10.1093/hmg/ddq050
Defelipe J (2011) The evolution of the brain, the human nature of cortical circuits, and intellectual creativity. Front Neuroanat 5:29. doi:10.3389/fnana.2011.00029
DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GYR, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256. doi:10.1016/j.neuron.2011.09.011
Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC (2008) Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell 3:637–648. doi:10.1016/j.stem.2008.09.017
Donnelly CJ, Zhang PW, Pham JT, Heusler AR, Mistry NA, Vidensky S, Daley EL, Poth EM, Hoover B, Fines DM, Maragakis N, Tienari PJ, Petrucelli L, Traynor BJ, Wang JO, Rigo F, Bennett CF, Blackshaw S, Sattler R, Rothstein JD (2013) RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron 80:415–428. doi:10.1016/j.neuron.2013.10.015
Dumont M, Stack C, Elipenahli C, Jainuddin S, Gerges M, Starkova NN, Yang LC, Starkov AA, Beal F (2011) Behavioral deficit, oxidative stress, and mitochondrial dysfunction precede tau pathology in P301S transgenic mice. Faseb J 25:4063–4072. doi:10.1096/fj.11-186650
Egawa N, Kitaoka S, Tsukita K, Naitoh M, Takahashi K, Yamamoto T, Adachi F, Kondo T, Okita K, Asaka I, Aoi T, Watanabe A, Yamada Y, Morizane A, Takahashi J, Ayaki T, Ito H, Yoshikawa K, Yamawaki S, Suzuki S, Watanabe D, Hioki H, Kaneko T, Makioka K, Okamoto K, Takuma H, Tamaoka A, Hasegawa K, Nonaka T, Hasegawa M, Kawata A, Yoshida M, Nakahata T, Takahashi R, Marchetto MCN, Gage FH, Yamanaka S, Inoue H (2012) Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Transl Med 4. doi:10.1126/scitranslmed.3004052
Ehrlich M, Hallmann AL, Reinhardt P, Arauzo-Bravo MJ, Korr S, Ropke A, Psathaki OE, Ehling P, Meuth SG, Oblak AL, Murrell JR, Ghetti B, Zaehres H, Scholer HR, Sterneckert J, Kuhlmann T, Hargus G (2015) Distinct neurodegenerative changes in an induced pluripotent stem cell model of frontotemporal dementia linked to mutant TAU protein. Stem Cell Rep 5:83–96. doi:10.1016/j.stemcr.2015.06.001
Esmaeili MA, Panahi M, Yadav S, Hennings L, Kiaei M (2013) Premature death of TDP-43 (A315T) transgenic mice due to gastrointestinal complications prior to development of full neurological symptoms of amyotrophic lateral sclerosis. Int J Exp Pathol 94:56–64. doi:10.1111/iep.12006
Fong H, Wang CZ, Knoferle J, Walker D, Balestra ME, Tong LM, Leung L, Ring KL, Seeley WW, Karydas A, Kshirsagar MA, Boxer AL, Kosik KS, Miller BL, Huang YD (2013) Genetic correction of tauopathy phenotypes in neurons derived from human induced pluripotent stem cells. Stem Cell Rep 1:226–234. doi:10.1016/j.stemcr.2013.08.001
Ghazi-Noori S, Froud KE, Mizielinska S, Powell C, Smidak M, Fernandez de Marco M, O’Malley C, Farmer M, Parkinson N, Fisher EM, Asante EA, Brandner S, Collinge J, Isaacs AM (2012) Progressive neuronal inclusion formation and axonal degeneration in CHMP2B mutant transgenic mice. Brain 135:819–832. doi:10.1093/brain/aws006
Ghoshal N, Dearborn JT, Wozniak DF, Cairns NJ (2012) Core features of frontotemporal dementia recapitulated in progranulin knockout mice. Neurobiol Dis 45:395–408. doi:10.1016/j.nbd.2011.08.029
Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M (2011) Classification of primary progressive aphasia and its variants. Neurology 76:1006–1014. doi:10.1212/WNL.0b013e31821103e6
Gotz J, Chen F, van Dorpe J, Nitsch RM (2001) Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 293:1491–1495. doi:10.1126/science.1062097
Gotz J, Ittner LM (2008) Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev Neurosci 9:532–544. doi:10.1038/nrn2420
Graham A, Davies R, Xuereb J, Halliday G, Kril J, Creasey H, Graham K, Hodges J (2005) Pathologically proven frontotemporal dementia presenting with severe amnesia. Brain 128:597–605. doi:10.1093/brain/awh348
Guo Y, Wang Q, Zhang K, An T, Shi P, Li Z, Duan W, Li C (2012) HO-1 induction in motor cortex and intestinal dysfunction in TDP-43 A315T transgenic mice. Brain Res 1460:88–95. doi:10.1016/j.brainres.2012.04.003
Gurney ME, Pu HF, Chiu AY, Dalcanto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen WJ, Zhai P, Sufit RL, Siddique T (1994) Motor-neuron degeneration in mice that express a human Cu, Zn superoxide-dismutase mutation. Science 264:1772–1775. doi:10.1126/science.8209258
Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H (2008) Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 64:60–70. doi:10.1002/ana.21425
Hatzipetros T, Bogdanik LP, Tassinari VR, Kidd JD, Moreno AJ, Davis C, Osborne M, Austin A, Vieira FG, Lutz C, Perrin S (2014) C57BL/6J congenic Prp-TDP43A315T mice develop progressive neurodegeneration in the myenteric plexus of the colon without exhibiting key features of ALS. Brain Res 1584:59–72. doi:10.1016/j.brainres.2013.10.013
Hedges EC, Mehler VJ, Nishimura AL (2016) The use of stem cells to model amyotrophic lateral sclerosis and frontotemporal dementia: from basic research to regenerative medicine. Stem Cells Int. doi:10.1155/2016/9279516
Ho R, Sances S, Gowing G, Amoroso MW, O’Rourke JG, Sahabian A, Wichterle H, Baloh RH, Sareen D, Svendsen CN (2016) ALS disrupts spinal motor neuron maturation and aging pathways within gene co-expression networks. Nat Neurosci 19:1256–1267. doi:10.1038/nn.4345
Hogg M, Grujic ZM, Baker M, Demirci S, Guillozet AL, Sweet AP, Herzog LL, Weintraub S, Mesulam MM, LaPointe NE, Gamblin TC, Berry RW, Binder LI, de Silva R, Lees A, Espinoza M, Davies P, Grover A, Sahara N, Ishizawa T, Dickson D, Yen SH, Hutton M, Bigio EH (2003) The L266V tau mutation is associated with frontotemporal dementia and Pick-like 3R and 4R tauopathy. Acta Neuropathol 106:323–336. doi:10.1007/s00401-003-0734-x
Hornberger M, Wong S, Tan R, Irish M, Piguet O, Kril J, Hodges JR, Halliday G (2012) In vivo and post-mortem memory circuit integrity in frontotemporal dementia and Alzheimer’s disease. Brain 135:3015–3025. doi:10.1093/brain/aws239
Huang C, Zhou HX, Tong JB, Chen H, Liu YJ, Wang DA, Wei XT, Xia XG (2011) FUS transgenic rats develop the phenotypes of amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Plos Genet 7. doi:10.1371/journal.pgen.1002011
Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393:702–705. doi:10.1038/31508
Igaz LM, Kwong LK, Lee EB, Chen-Plotkin A, Swanson E, Unger T, Malunda J, Xu Y, Winton MJ, Trojanowski JQ, Lee VMY (2011) Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice. J Clin Investig 121:726–738. doi:10.1172/Jci44867
Igaz LM, Kwong LK, Xu Y, Truax AC, Uryu K, Neumann M, Clark CM, Elman LB, Miller BL, Grossman M, McCluskey LF, Trojanowski JQ, Lee VM (2008) Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Am J Pathol 173:182–194. doi:10.2353/ajpath.2008.080003
Iguchi Y, Katsuno M, Niwa J, Takagi S, Ishigaki S, Ikenaka K, Kawai K, Watanabe H, Yamanaka K, Takahashi R, Misawa H, Sasaki S, Tanaka F, Sobue G (2013) Loss of TDP-43 causes age-dependent progressive motor neuron degeneration. Brain 136:1371–1382. doi:10.1093/brain/awt029
Iovino M, Agathou S, Gonzalez-Rueda A, Velasco-Herrera MD, Borroni B, Alberici A, Lynch T, O’Dowd S, Geti I, Gaffney D, Vallier L, Paulsen O, Karadottir RT, Spillantini MG (2015) Early maturation and distinct tau pathology in induced pluripotent stem cell-derived neurons from patients with MAPT mutations. Brain 138. doi:10.1093/brain/awv222
Iqbal K, Liu F, Gong CX (2016) Tau and neurodegenerative disease: the story so far. Nat Rev Neurol 12. doi:10.1038/nrneurol.2015.225
Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM (1999) Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron 24:751–762
Ittner AA, Gladbach A, Bertz J, Suh LS, Ittner LM (2014) p38 MAP kinase-mediated NMDA receptor-dependent suppression of hippocampal hypersynchronicity in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun 2:149. doi:10.1186/s40478-014-0149-z
Ittner LM, Fath T, Ke YD, Bi M, van Eersel J, Li KM, Gunning P, Gotz J (2008) Parkinsonism and impaired axonal transport in a mouse model of frontotemporal dementia. Proc Natl Acad Sci USA 105:15997–16002. doi:10.1073/pnas.0808084105
Ittner LM, Halliday GM, Kril JJ, Gotz J, Hodges JR, Kiernan MC (2015) OPINION FTD and ALS-translating mouse studies into clinical trials. Nat Rev Neurol 11:360–366. doi:10.1038/nrneurol.2015.65
Iyer PM, Egan C, Pinto-Grau M, Burke T, Elamin M, Nasseroleslami B, Pender N, Lalor EC, Hardiman O (2015) Functional connectivity changes in resting-state EEG as potential biomarker for amyotrophic lateral sclerosis. PLoS One 10:e0128682. doi:10.1371/journal.pone.0128682
Jiang J, Zhu Q, Gendron TF, Saberi S, McAlonis-Downes M, Seelman A, Stauffer JE, Jafar-Nejad P, Drenner K, Schulte D, Chun S, Sun SY, Ling SC, Myers B, Engelhardt J, Katz M, Baughn M, Platoshyn O, Marsala M, Watt A, Heyser CJ, Ard MC, De Muynck L, Daughrity LM, Swing DA, Tessarollo L, Jung CJ, Delpoux A, Utzschneider DT, Hedrick SM, de Jong PJ, Edbauer D, Van Damme P, Petrucelli L, Shaw CE, Bennett CF, Da Cruz S, Ravits J, Rigo F, Cleveland DW, Lagier-Tourenne C (2016) Gain of toxicity from ALS/FTD-linked repeat expansions in c9orf72 is alleviated by antisense oligonucleotides targeting GGGGCC-containing RNAs. Neuron 90:535–550. doi:10.1016/j.neuron.2016.04.006
Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, Mann DM, Dickson DW (2011) Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol 122:137–153. doi:10.1007/s00401-011-0839-6
Kayasuga Y, Chiba S, Suzuki M, Kikusui T, Matsuwaki T, Yamanouchi K, Kotaki H, Horai R, Iwakura Y, Nishihara M (2007) Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav Brain Res 185:110–118. doi:10.1016/j.bbr.2007.07.020
Ke YD, van Hummel A, Stevens CH, Gladbach A, Ippati S, Bi MA, Lee WS, Kruger S, van der Hoven J, Volkerling A, Bongers A, Halliday G, Haass NK, Kiernan M, Delerue F, Ittner LM (2015) Short-term suppression of A315T mutant human TDP-43 expression improves functional deficits in a novel inducible transgenic mouse model of FTLD-TDP and ALS. Acta Neuropathol 130:661–678. doi:10.1007/s00401-015-1486-0
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC (2011) Amyotroph Lateral Scler. Lancet 377:942–955. doi:10.1016/S0140-6736(10)61156-7
Koss DJ, Robinson L, Drever BD, Plucinska K, Stoppelkamp S, Veselcic P, Riedel G, Platt B (2016) Mutant Tau knock-in mice display frontotemporal dementia relevant behaviour and histopathology. Neurobiol Dis 91:105–123. doi:10.1016/j.nbd.2016.03.002
Kraemer BC, Schuck T, Wheeler JM, Robinson LC, Trojanowski JQ, Lee VMY, Schellenberg GD (2010) Loss of murine TDP-43 disrupts motor function and plays an essential role in embryogenesis. Acta Neuropathol 119:409–419. doi:10.1007/s00401-010-0659-0
Lanata SC, Miller BL (2016) The behavioural variant frontotemporal dementia (bvFTD) syndrome in psychiatry. J Neurol Neurosurg Psychiatry 87:501–511. doi:10.1136/jnnp-2015-310697
Lee S, Huang EJ (2015) Modeling ALS and FTD with iPSC-derived neurons. Brain Res. doi:10.1016/j.brainres.2015.10.003
Leroy K, Bretteville A, Schindowski K, Gilissen E, Authelet M, De Decker R, Yilmaz Z, Buee L, Brion JP (2007) Early axonopathy preceding neurofibrillary tangles in mutant tau transgenic mice. Am J Pathol 171:976–992. doi:10.2353/ajpath.2007.070345
Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M (2000) Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet 25:402–405. doi:10.1038/78078
Liu Y, Pattamatta A, Zu T, Reid T, Bardhi O, Borchelt DR, Yachnis AT, Ranum LP (2016) C9orf72 BAC mouse model with motor deficits and neurodegenerative features of ALS/FTD. Neuron 90:521–534. doi:10.1016/j.neuron.2016.04.005
Liu YC, Chiang PM, Tsai KJ (2013) Disease animal models of TDP-43 proteinopathy and their pre-clinical applications. Int J Mol Sci 14:20079–20111. doi:10.3390/ijms141020079
Logroscino G, Traynor BJ, Hardiman O, Chio A, Mitchell D, Swingler RJ, Millul A, Benn E, Beghi E, Eurals (2010) Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg psychiatry 81:385–390. doi:10.1136/jnnp.2009.183525
Lomen-Hoerth C, Anderson T, Miller B (2002) The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology 59:1077–1079
Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, Kwong LK, Forman MS, Ravits J, Stewart H, Eisen A, McClusky L, Kretzschmar HA, Monoranu CM, Highley JR, Kirby J, Siddique T, Shaw PJ, Lee VM, Trojanowski JQ (2007) Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol 61:427–434. doi:10.1002/ana.21147
Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122:111–113. doi:10.1007/s00401-011-0845-8
Martens LH, Zhang J, Barmada SJ, Zhou P, Kamiya S, Sun B, Min SW, Gan L, Finkbeiner S, Huang EJ, Farese RV Jr (2012) Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J Clin Invest 122:3955–3959. doi:10.1172/JCI63113
Matsumoto A, Okada Y, Nakamichi M, Nakamura M, Toyama Y, Sobue G, Nagai M, Aoki M, Itoyama Y, Okano H (2006) Disease progression of human SOD1 (G93A) transgenic ALS model rats. J Neurosci Res 83:119–133. doi:10.1002/jnr.20708
McGoldrick P, Joyce PI, Fisher EMC, Greensmith L (2013) Rodent models of amyotrophic lateral sclerosis. Bba-Mol Basis Dis 1832:1421–1436. doi:10.1016/j.bbadis.2013.03.012
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VMY (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133. doi:10.1126/science.1134108
O’Rourke JG, Bogdanik L, Muhammad AKMG, Gendron TF, Kim KJ, Austin A, Cady J, Liu EY, Zarrow J, Grant S, Ho R, Bell S, Carmona S, Simpkinson M, Lall D, Wu K, Daughrity L, Dickson DW, Harms MB, Petrucelli L, Lee EB, Lutz CM, Baloh RH (2015) C9orf72 BAC transgenic mice display typical pathologic features of ALS/FTD. Neuron 88:892–901. doi:10.1016/j.neuron.2015.10.027
Onyike CU, Diehl-Schmid J (2013) The epidemiology of frontotemporal dementia. Int Rev Psychiatry 25:130–137. doi:10.3109/09540261.2013.776523
Peters OM, Cabrera GT, Tran H, Gendron TF, McKeon JE, Metterville J, Weiss A, Wightman N, Salameh J, Kim J, Sun H, Boylan KB, Dickson D, Kennedy Z, Lin Z, Zhang YJ, Daughrity L, Jung C, Gao FB, Sapp PC, Horvitz HR, Bosco DA, Brown SP, de Jong P, Petrucelli L, Mueller C, Brown RH Jr (2015) Human C9ORF72 hexanucleotide expansion reproduces RNA foci and dipeptide repeat proteins but not neurodegeneration in BAC transgenic mice. Neuron 88:902–909. doi:10.1016/j.neuron.2015.11.018
Petkau TL, Neal SJ, Milnerwood A, Mew A, Hill AM, Orban P, Gregg J, Lu G, Feldman HH, Mackenzie IRA, Raymond LA, Leavitt BR (2012) Synaptic dysfunction in progranulin-deficient mice. Neurobiol Dis 45:711–722. doi:10.1016/j.nbd.2011.10.016
Philips T, Rothstein JD (2015) Rodent models of amyotrophic lateral sclerosis. Curr Protoc Pharmacol 69:5.67.1–21. doi:10.1002/0471141755.ph0567s69
Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, Lynch C, Pender N, Hardiman O (2012) The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry 83:102–108. doi:10.1136/jnnp-2011-300188
Picher-Martel V, Valdmanis PN, Gould PV, Julien JP, Dupre N (2016) From animal models to human disease: a genetic approach for personalized medicine in ALS. Acta Neuropathol Commun 4. doi:10.1186/s40478-016-0340-5
Preza E, Hardy J, Warner T, Wray S (2016) Review: induced pluripotent stem cell models of frontotemporal dementia. Neuropathol Appl Neurobiol 42:497–520. doi:10.1111/nan.12334
Przybyla M, Stevens CH, van der Hoven J, Harasta A, Bi M, Ittner A, van Hummel A, Hodges JR, Piguet O, Karl T, Kassiou M, Housley GD, Ke YD, Ittner LM, Eersel J (2016) Disinhibition-like behavior in a P301S mutant tau transgenic mouse model of frontotemporal dementia. Neurosci Lett 631:24–29. doi:10.1016/j.neulet.2016.08.007
Raitano S, Ordovas L, De Muynck L, Guo WT, Espuny-Camacho I, Geraerts M, Khurana S, Vanuytsel K, Toth BI, Voets T, Vandenberghe R, Cathomen T, Van Den Bosch L, Vanderhaeghen P, Van Damme P, Verfaillie CM (2015) Restoration of progranulin expression rescues cortical neuron generation in an induced pluripotent stem cell model of frontotemporal dementia. Stem Cell Rep 4:16–24. doi:10.1016/j.stemcr.2014.12.001
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134:2456–2477. doi:10.1093/brain/awr179
Ricketts T, McGoldrick P, Fratta P, de Oliveira HM, Kent R, Phatak V, Brandner S, Blanco G, Greensmith L, Acevedo-Arozena A, Fisher EM (2014) A nonsense mutation in mouse Tardbp affects TDP43 alternative splicing activity and causes limb-clasping and body tone defects. PLoS One 9:e85962. doi:10.1371/journal.pone.0085962
Roberson ED (2012) Mouse models of frontotemporal dementia. Ann Neurol 72:837–849. doi:10.1002/ana.23722
Rockenstein E, Overk CR, Ubhi K, Mante M, Patrick C, Adame A, Bisquert A, Trejo-Morales M, Spencer B, Masliah E (2015) A novel triple repeat mutant tau transgenic model that mimics aspects of pick’s disease and fronto-temporal tauopathies. PLoS One 10. doi:10.1371/journal.pone.0121570
Rodriguez-Ortiz CJ, Hoshino H, Cheng D, Liu-Yescevitz L, Blurton-Jones M, Wolozin B, LaFerla FM, Kitazawa M (2013) Neuronal-specific overexpression of a mutant valosin-containing protein associated with IBMPFD promotes aberrant ubiquitin and TDP-43 accumulation and cognitive dysfunction in transgenic mice. Am J Pathol 183:504–515. doi:10.1016/j.ajpath.2013.04.014
Rohrer JD, Warren JD (2011) Phenotypic signatures of genetic frontotemporal dementia. Curr Opin Neurol 24:542–549. doi:10.1097/WCO.0b013e32834cd442
SantaCruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH (2005) Tau suppression in a neurodegenerative mouse model improves memory function. Science 309:476–481. doi:10.1126/science.1113694
Sareen D, O’Rourke JG, Meera P, Muhammad AKMG, Grant S, Simpkinson M, Bell S, Carmona S, Ornelas L, Sahabian A, Gendron T, Petrucelli L, Baughn M, Ravits J, Harms MB, Rigo F, Bennett CF, Otis TS, Svendsen CN, Baloh RH (2013) Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci Transl Med 5. doi:10.1126/scitranslmed.3007529
Scott L, Kiss T, Kawabe TT, Hajos M (2016) Neuronal network activity in the hippocampus of tau transgenic (Tg4510) mice. Neurobiol Aging 37:66–73. doi:10.1016/j.neurobiolaging.2015.10.002
Seelaar H, Kamphorst W, Rosso SM, Azmani A, Masdjedi R, de Koning I, Maat-Kievit JA, Anar B, Kaat LD, Breedveld GJ, Dooijes D, Rozemuller JM, Bronner IF, Rizzu P, van Swieten JC (2008) Distinct genetic forms of frontotemporal dementia. Neurology 71:1220–1226. doi:10.1212/01.wnl.0000319702.37497.72
Serio A, Bilican B, Barmada SJ, Ando DM, Zhao C, Siller R, Burr K, Haghi G, Story D, Nishimura AL, Carrasco MA, Phatnani HP, Shum C, Wilmut I, Maniatis T, Shaw CE, Finkbeiner S, Chandran S (2013) Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. P Natl Acad Sci USA 110:4697–4702. doi:10.1073/pnas.1300398110
Smittkamp SE, Spalding HN, Brown JW, Gupte AA, Chen J, Nishimune H, Geiger PC, Stanford JA (2010) Measures of bulbar and spinal motor function, muscle innervation, and mitochondrial function in ALS rats. Behav Brain Res 211:48–57. doi:10.1016/j.bbr.2010.03.007
Sposito T, Preza E, Mahoney CJ, Seto-Salvia N, Ryan NS, Morris HR, Arber C, Devine MJ, Houlden H, Warner TT, Bushell TJ, Zagnoni M, Kunath T, Livesey FJ, Fox NC, Rossor MN, Hardy J, Wray S (2015) Developmental regulation of tau splicing is disrupted in stem cell-derived neurons from frontotemporal dementia patients with the 10 + 16 splice-site mutation in MAPT. Hum Mol Genet 24:5260–5269. doi:10.1093/hmg/ddv246
Swarup V, Phaneuf D, Bareil C, Robertson J, Rouleau GA, Kriz J, Julien JP (2011) Pathological hallmarks of amyotrophic lateral sclerosis/frontotemporal lobar degeneration in transgenic mice produced with TDP-43 genomic fragments. Brain 134:2610–2626. doi:10.1093/brain/awr159
Sydow A, Van der Jeugd A, Zheng F, Ahmed T, Balschun D, Petrova O, Drexler D, Zhou LP, Rune G, Mandelkow E, D’Hooge R, Alzheimer C, Mandelkow EM (2011) Tau-Induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic tau mutant. J Neurosci 31:2511–2525. doi:10.1523/Jneurosci.5245-10.2011
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. doi:10.1016/j.cell.2007.11.019
Takeuchi H, Iba M, Inoue H, Higuchi M, Takao K, Tsukita K, Karatsu Y, Iwamoto Y, Miyakawa T, Suhara T, Trojanowski JQ, Lee VMY, Takahashi R (2011) P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS One 6. doi:10.1371/journal.pone.0021050
Takeuchi R, Tada M, Shiga A, Toyoshima Y, Konno T, Sato T, Nozaki H, Kato T, Horie M, Shimizu H, Takebayashi H, Onodera O, Nishizawa M, Kakita A, Takahashi H (2016) Heterogeneity of cerebral TDP-43 pathology in sporadic amyotrophic lateral sclerosis: evidence for clinico-pathologic subtypes. Acta Neuropathol Commun 4:61. doi:10.1186/s40478-016-0335-2
Tan RH, Kril JJ, Fatima M, McGeachie A, McCann H, Shepherd C, Forrest SL, Affleck A, Kwok JB, Hodges JR, Kiernan MC, Halliday GM (2015) TDP-43 proteinopathies: pathological identification of brain regions differentiating clinical phenotypes. Brain 138:3110–3122. doi:10.1093/brain/awv220
Tanaka Y, Chambers JK, Matsuwaki T, Yamanouchi K, Nishihara M (2014) Possible involvement of lysosomal dysfunction in pathological changes of the brain in aged progranulin-deficient mice. Acta Neuropathol Commun 2:78. doi:10.1186/s40478-014-0078-x
Therrien M, Parker JA (2014) Worming forward: amyotrophic lateral sclerosis toxicity mechanisms and genetic interactions in Caenorhabditis elegans. Front Genet 5:85. doi:10.3389/fgene.2014.00085
Tsai KJ, Yang CH, Fang YH, Cho KH, Chien WL, Wang WT, Wu TW, Lin CP, Fu WM, Shen CKJ (2010) Elevated expression of TDP-43 in the forebrain of mice is sufficient to cause neurological and pathological phenotypes mimicking FTLD-U. J Exp Med 207:1661–1673. doi:10.1084/jem.20092164
Tsuji H, Arai T, Kametani F, Nonaka T, Yamashita M, Suzukake M, Hosokawa M, Yoshida M, Hatsuta H, Takao M, Saito Y, Murayama S, Akiyama H, Hasegawa M, Mann DM, Tamaoka A (2012) Molecular analysis and biochemical classification of TDP-43 proteinopathy. Brain 135:3380–3391. doi:10.1093/brain/aws230
Turner MR, Hardiman O, Benatar M, Brooks BR, Chio A, de Carvalho M, Ince PG, Lin C, Miller RG, Mitsumoto H, Nicholson G, Ravits J, Shaw PJ, Swash M, Talbot K, Traynor BJ, Van den Berg LH, Veldink JH, Vucic S, Kiernan MC (2013) Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol 12:310–322. doi:10.1016/S1474-4422(13)70036-X
Van der Jeugd A, Vermaercke B, Halliday GM, Staufenbiel M, Gotz J (2016) Impulsivity, decreased social exploration, and executive dysfunction in a mouse model of frontotemporal dementia. Neurobiol Learn Mem 130:34–43. doi:10.1016/j.nlm.2016.01.007
van Eersel J, Ke YD, Liu X, Delerue F, Kril JJ, Gotz J, Ittner LM (2010) Sodium selenate mitigates tau pathology, neurodegeneration, and functional deficits in Alzheimer’s disease models. Proc Natl Acad Sci USA 107:13888–13893. doi:10.1073/pnas.1009038107
van Eersel J, Stevens CH, Przybyla M, Gladbach A, Stefanoska K, Chan CK, Ong WY, Hodges JR, Sutherland GT, Kril JJ, Abramowski D, Staufenbiel M, Halliday GM, Ittner LM (2015) Early-onset axonal pathology in a novel P301S-Tau transgenic mouse model of frontotemporal lobar degeneration. Neuropathol Appl Neurobiol 41:906–925. doi:10.1111/nan.12233
Vucic S, Nicholson GA, Kiernan MC (2008) Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain 131:1540–1550. doi:10.1093/brain/awn071
Walker AK, Spiller KJ, Ge GH, Zheng A, Xu Y, Zhou M, Tripathy K, Kwong LK, Trojanowski JQ, Lee VMY (2015) Functional recovery in new mouse models of ALS/FTLD after clearance of pathological cytoplasmic TDP-43. Acta Neuropathol 130:643–660. doi:10.1007/s00401-015-1460-x
Warmus BA, Sekar DR, McCutchen E, Schellenberg GD, Roberts RC, McMahon LL, Roberson ED (2014) Tau-mediated NMDA receptor impairment underlies dysfunction of a selectively vulnerable network in a mouse model of frontotemporal dementia. J Neurosci 34:16482–16495. doi:10.1523/Jneurosci.3418-14.2014
Warren JD, Rohrer JD, Rossor MN (2013) Clinical review. Frontotemporal dementia. BMJ 347:f4827. doi:10.1136/bmj.f4827
Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH (2009) TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci USA 106:18809–18814. doi:10.1073/pnas.0908767106
Wils H, Kleinberger G, Janssens J, Pereson S, Joris G, Cuijt I, Smits V, Ceuterick-de Groote C, Van Broeckhoven C, Kumar-Singh S (2010) TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci USA 107:3858–3863. doi:10.1073/pnas.0912417107
Wils H, Kleinberger G, Pereson S, Janssens J, Capell A, Van Dam D, Cuijt I, Joris G, De Deyn PP, Haass C, Van Broeckhoven C, Kumar-Singh S (2012) Cellular ageing, increased mortality and FTLD-TDP-associated neuropathology in progranulin knockout mice. J Pathol 228:67–76. doi:10.1002/path.4043
Wren MC, Zhao J, Liu CC, Murray ME, Atagi Y, Davis MD, Fu Y, Okano HJ, Ogaki K, Strongosky AJ, Tacik P, Rademakers R, Ross OA, Dickson DW, Wszolek ZK, Kanekiyo T, Bu GJ (2015) Frontotemporal dementia-associated N279K tau mutant disrupts subcellular vesicle trafficking and induces cellular stress in iPSC-derived neural stem cells. Mol Neurodegener 10. doi:10.1186/s13024-015-0042-7
Wu LS, Cheng WC, Shen CK (2012) Targeted depletion of TDP-43 expression in the spinal cord motor neurons leads to the development of amyotrophic lateral sclerosis-like phenotypes in mice. J Biol Chem 287:27335–27344. doi:10.1074/jbc.M112.359000
Yang C, Wang H, Qiao T, Yang B, Aliaga L, Qiu L, Tan W, Salameh J, McKenna-Yasek DM, Smith T, Peng L, Moore MJ, Brown RH Jr, Cai H, Xu Z (2014) Partial loss of TDP-43 function causes phenotypes of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 111:E1121–E1129. doi:10.1073/pnas.1322641111
Yin F, Banerjee R, Thomas B, Zhou P, Qian L, Jia T, Ma X, Ma Y, Iadecola C, Beal MF, Nathan C, Ding A (2010) Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J Exp Med 207:117–128. doi:10.1084/jem.20091568
Yin FF, Dumont M, Banerjee R, Ma Y, Li HH, Lin MT, Beal MF, Nathan C, Thomas B, Ding AH (2010) Behavioral deficits and progressive neuropathology in progranulin-deficient mice: a mouse model of frontotemporal dementia. Faseb J 24:4639–4647. doi:10.1096/fj.10-161471
Yin HZ, Nalbandian A, Hsu CI, Li S, Llewellyn KJ, Mozaffar T, Kimonis VE, Weiss JH (2012) Slow development of ALS-like spinal cord pathology in mutant valosin-containing protein gene knock-in mice. Cell Death Dis 3:e374. doi:10.1038/cddis.2012.115
Zhang ZJ, Almeida S, Lu YB, Nishimura AL, Peng LT, Sun DQ, Wu B, Karydas AM, Tartaglia MC, Fong JC, Miller BL, Farese RV, Moore MJ, Shaw CE, Gao FB (2013) Downregulation of MicroRNA-9 in iPSC-derived neurons of FTD/ALS patients with TDP-43 mutations. PLoS One 8. doi:10.1371/journal.pone.0076055
Acknowledgements
The authors wish to thank Ms. Heidi Cartwright for assistance with preparation of figures.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by funding to Forefront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neurone disease, from NHMRC of Australia program Grant (#1037746) and the ARC Centre of Excellence in Cognition and its Disorders Memory Node (#CE110001021). RT is an NHMRC-ARC Dementia Research Development Award Fellow (#APP1110369). YK is an ARC Discovery Early Career Researcher Award Fellow (#DE130101591). LI is a NHMRC Senior Research Fellow (#1003083). GH is a NHMRC Senior Principal Research Fellow (#630434).
Rights and permissions
About this article
Cite this article
Tan, R.H., Ke, Y.D., Ittner, L.M. et al. ALS/FTLD: experimental models and reality. Acta Neuropathol 133, 177–196 (2017). https://doi.org/10.1007/s00401-016-1666-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-016-1666-6