Abstract
Whole genome analyses have facilitated the discovery of clinically relevant genetic alterations in a variety of diseases, most notably cancer. A prominent example of this was the discovery of mutations in isocitrate dehydrogenases 1 and 2 (IDH1/2) in a sizeable proportion of gliomas and some other neoplasms. Herein the normal functions of these enzymes, how the mutations alter their catalytic properties, the effects of their d-2-hydroxyglutarate metabolite, technical considerations in diagnostic neuropathology, implications about prognosis and therapeutic considerations, and practical applications and controversies regarding IDH1/2 mutation testing are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mutations in isocitrate dehydrogenases types 1 and 2 (IDH1/2) were first reported in large subsets of gliomas in 2008–2009 [11, 130, 185]. Prior to this discovery there was no reason to think that either gene would be mutated in any tumor, much less gliomas. A 2006 study found an R132C IDH1 mutation in a single colonic adenocarcinoma, but it was understandably not deemed significant at the time [153]. The opinion of the scientific community has clearly changed on the matter, considering that about 500 papers have now been published concerning IDH1/2 mutations and cancer.
The purpose of this review is to distill what we now know about IDH1/2 mutations into a thorough yet concise summary that can help readers keep abreast of this rapidly advancing field. Toward that end, we will discuss the functions of wild-type IDH1 and IDH2, the consequences of mutations on each enzyme’s biochemistry, the downstream effects of these mutations on cancer biology, and practical applications of IDH1/2 mutation testing, specifically in brain cancer.
Wild-type IDH enzyme biochemistry
Three enzymes oxidize isocitrate to alpha-ketoglutarate (α-KG) in human cells: (1) isocitrate dehydrogenase 1 (IDH1); (2) isocitrate dehydrogenase 2 (IDH2); and (3) isocitrate dehydrogenase 3 (IDH3). Despite the similarities of their names, these enzymes differ markedly from each other. IDH1 and IDH2 are single-gene enzymes, respectively, located on 2q33 and 15q26, each existing as homodimers. IDH3 is a heterotetramer composed of two alpha subunits, one beta subunit, and one gamma subunit. These IDH3A, IDH3B, and IDH3G genes are located on chromosomes 15q25, 20p13, and Xq28. IDH1 and IDH2 utilize nicotinamide adenine dinucleotide phosphate (NADP+) as a cofactor, generating NADPH during catalysis. IDH3 uses NAD+ and produces NADH. IDH2 and 3 are located in mitochondria while IDH1 is in the cytosol and peroxisomes (Fig. 1).
Normal functions and subcellular locations of IDH1, IDH2, and IDH3. All three enzymes oxidize isocitrate (ISO) to alpha-ketoglutarate (α-KG). IDH1 and IDH2 are homodimers, whereas IDH3 is a heterotetramer. IDH1 and IDH2 utilize nicotinamide adenine dinucleotide phosphate (NADP+) as a cofactor, generating NADPH. IDH3 uses NAD+ and produces NADH. IDH2 and 3 are located in mitochondria while IDH1 is in the cytosol and peroxisomes. In certain circumstances, IDH1 and IDH2 can reduce α-KG to isocitrate, whereas IDH3 is unidirectional. (The structure in the upper left of the cell depicts a nucleus)
Of the three, only IDH3 appears to participate in the Krebs cycle [188]. The exact role of mitochondrial IDH2 is somewhat unclear, though it may act as a source of NADPH for the mitochondria [71]. IDH1 is the primary source of NADPH reducing equivalents in the cytosol and peroxisomes [14, 83]. Both IDH1 and IDH2 are important in the mitigation of cellular oxidative damage induced by intrinsic metabolism and extrinsic factors like radiation [70, 71, 80–82, 99–102]. It is therefore significant that IDH1 is one of the key genes upregulated in breast cancer stem cells, which have lower levels of reactive oxygen species (ROS) than their progeny and tend to be more radioresistant [37]. IDH1 is also the largest producer of NADPH in the human brain (but not the mouse brain) [9, 14].
IDH3 appears to be a unidirectional enzyme, only capable of oxidizing isocitrate to α-KG. But during hypoxia or mitochondrial dysfunction, IDH1 and IDH2 can reduce α-KG to isocitrate, thereby helping the cell replenish other citric acid cycle intermediates and the fatty acid precursor acetyl-CoA [48, 116, 122, 182].
Mutant IDH enzyme biochemistry
Clinical clues about the function of mutant IDH1/2
IDH1/2 mutations occur in an assortment of seemingly unrelated neoplasms, including gliomas, acute myeloid leukemia (AML), acute lymphocytic leukemia, myelofibrosis, intrahepatic cholangiocarcinoma, melanoma, and chondroid tumors, as well as rare colonic and prostate carcinomas, and even the occasional paraganglioma [6, 17, 49, 77, 107, 152, 163]. Both Ollier disease and Maffucci syndrome—wherein patients develop multiple benign cartilaginous tumors—are caused by somatic mosaic mutations on IDH1 [7].
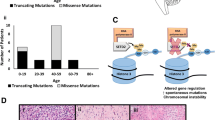
In gliomas, several interesting facts about IDH1/2 are readily apparent. The mutations tend to occur in younger adults, in the 20–60-year range, and are far more common in grades II and III astrocytomas and oligodendrogliomas compared to glioblastomas (GBMs) [15, 130, 185]. IDH1/2-mutant gliomas can occur in the pediatric population, but are more likely in adolescents [72, 135]. In fact, about 60–80 % of those lower-grade gliomas and GBMs that arose from lower-grade gliomas (so-called “secondary” GBMs) have a mutation, while de novo (i.e., “primary”) GBMs rarely do [166, 177, 185] (Fig. 2c). Over 90 % of the mutations involve IDH1, and about 90 % of those IDH1 mutations are CGT > CAT transitions in codon 132, replacing the arginine residue with histidine (R132H IDH1) (Fig. 2). Other point mutations also occur at codon 132, resulting in substitutions like R132C or R132S. Analogous mutations occur at R172 in IDH2, but do not show the same preference for histidine as in mutant IDH1 (Fig. 2b). Other codons can also rarely be affected, including R49, G97, and R100 on IDH1 and R140 on IDH2 [57, 138, 174]. When an IDH1/2 mutation is present, it is virtually always heterozygous, with the tumor retaining the corresponding wild-type allele. Extremely rare cases of concurrent IDH1 and IDH2 mutations have been reported [59], as well as dual R132H and R132C IDH1 mutation in the same tumor [164]. In about 5 % of IDH1/2-mutant gliomas, the mutation is eventually followed by deletion of the wild-type allele [Hai Yan, M.D., Ph.D., personal communication, 12/12/2012] [66, 68]. Rare cases of mutant allele deletion within an area of a glioma have also been reported [138].
IDH1/2 mutations by variant, glioma category, and type of screening. a In a pooled analysis of over 3,400 gliomas from 37 studies in which mutation subtypes were reported, R132H was the most common IDH1 variant, comprising 92 % of all IDH1 mutations (P < 0.0001). The rarest was R132P, occurring in a single case [53]. b In contrast, there was less preference for a specific type of IDH2 mutation, though the R172K variant was present in 60 % of IDH2-mutant tumors (P < 0.0001, N = 89). c Inter-study mutation frequencies significantly differ in grades II–III astrocytomas and grade III oligodendrogliomas, depending on whether the studies tested for both IDH1 and IDH2 mutations (red bars, N = 4,324 gliomas from 26 studies), screened for just IDH1 mutations (purple bars, N = 2,075 gliomas from 12 studies), or only used the R132H IDH1 antibody (blue bars, N = 794 gliomas from 6 studies). Not enough oligodendroglial tumors have been interrogated with R132H IDH1 antibody to be reliably compared with the other columns. Of note, mean mutation frequencies in IDH1-only studies sometimes barely exceeded the frequencies reported in IDH1/2 papers (purple versus red bars in AII and sGBM subgroups). This apparent incongruity can be explained by inter-cohort variations, especially given how rare IDH2 mutations are in astrocytic tumors (see Fig. 4). A list of the studies from which these data are derived is in Supplemental Table 1. All data bars in a-c represent mean ± SEM; statistical analyses were done via Student’s t test or ANOVA with Kruskal–Wallis post hoc test, as appropriate. *P < 0.05; **P < 0.01; ***P < 0.001. AII = grade II diffuse astrocytoma; AIII = grade III anaplastic astrocytoma; pGBM = primary GBM; sGBM = secondary GBM; OII = grade II oligodendroglioma; OIII = grade III anaplastic oligodendroglioma; OAII = grade II oligoastrocytoma; OAIII = grade III anaplastic oligoastrocytoma
Neoenzymatic activity of mutant IDH1/2
The findings that IDH1/2 mutations are heterozygous, nearly always target arginine codons involved in the binding of isocitrate, and are missense mutations implied some sort of dominant inhibition or gain-of-function. An early hypothesis was that it acts as a dominant negative inhibitor of wild-type IDH1/2, causing oncogenesis through lower levels of α-KG, leading to inhibition of α-KG-dependent degradation of hypoxia-inducible factor-1α (Hif-1α) [190]. But other studies have shown no difference in either α-KG or Hif1-α between mutant and wild-type gliomas [34, 76, 117, 140, 181]. Furthermore, dominant inhibition of wild-type IDH1 plus consumption of NADPH by the mutant should result in lower cellular NADPH reducing equivalents. Yet in vitro overexpression of IDH1/2 mutants has no effect on NADPH levels [69], and both oxidized and reduced glutathione are lower in mutant-transfected oligodendroglial cells [140]. It is important to remember that a cell is bound to have ways of compensating for deficits of such critical compounds like α-KG and NADPH, like converting more glutamate into α-KG [98, 140]. Therefore, the effects of IDH1/2 mutations cannot be simply explained by inhibition of their wild-type counterparts, but more likely represent a true gain-of-function.
In 2009 that gain-of-function was discovered by Dang et al. [34], who published a landmark study showing that the R132H point mutation confers neoenzymatic activity on IDH1. The R132 residue normally helps bind isocitrate substrate, but when it is mutated the enzyme prefers to bind and reduce α-KG to d-2-hydroxyglutarate (hereafter referred to as 2-HG), consuming NADPH in the process (Fig. 3). This neoenzyme is so adept at producing 2-HG that mutant gliomas have 10–100-fold higher levels of 2-HG than their wild-type counterparts, with tissue concentrations ranging from 10 to 30 mM. Similar 2-HG-producing effects have been shown in the other IDH1 and IDH2 mutations, both in gliomas and other cancers [7, 17, 56, 150, 174, 176]. Of note, wild-type IDH1/2 is also capable of producing some 2-HG in the reverse reductive reaction under hypoxic conditions, just not nearly as much as the mutants [182].
Postulated effects of mutant IDH1/2 on gliomagenesis. Unlike mutant IDH2, mutant IDH1 is more efficient when it heterodimerizes with a wild-type partner. Both mutations convert α-KG to 2-HG. This 2-HG compound inhibits some enzymes that use α-KG as a cofactor, including JmjC domain-containing histone demethylases and TET DNA demethylases. The result of this inhibition is a net upregulation of histone and DNA methylation, the former occurring at key amino acid residues that are mutated in some non-IDH1/2-driven gliomas (involving the H3F3A gene encoding histone 3.3). Other proteins involved in chaperoning histone H3.3 include ATRX and DAXX, both of which can be mutated in IDH1/2-wt and IDH1/2-mutant gliomas (though ATRX is far more likely to be mutated than DAXX) [148]. EGLN1-3 prolyl hydroxylases may actually be activated by 2-HG, thereby increasing degradation of Hif-1α. The exact results of all these alterations are not yet clear, but they definitely cause global modifications of gene expression and may promote Alternative Lengthening of Telomeres (ALT). EGLN activation in particular appears to slow down the differentiation of glial precursors, perhaps providing a greater opportunity for additional mutations to arise. Mutant TP53 and/or ATRX tend to produce astrocytomas, whereas 1p/19q codeletion with CIC and/or FUBP1 mutations produce oligodendrogliomas. Asterisks (*) denote mutated proteins
This neoenzymatic activity begs the question as to how key point mutations of R132 and R172 can so radically alter IDH1/2 functionality. As it turns out, wild-type IDH1 activity can be divided into three distinct phases: (a) initial open isocitrate-binding state, (b) closed pre-transition state and (c) catalysis. A mutation at R132 seems to block the shift to a closed pre-transition state [186]. This impedes isocitrate binding, thereby preventing it from inhibiting the reduction of α-KG to 2-HG—a reaction that, to reiterate, weakly occurs even in wild-type IDH1 [134]. An analogous phenomenon likely holds for R172 and IDH2, as well as for some of the rarer mutations on other arginine-encoding codons like R100 in IDH1 and R140 in IDH2.
Since wild-type IDH1 and IDH2 act as homodimers, and mutations virtually always start out heterozygous (even if the tumor eventually loses its wild-type allele), it raises the question as to how (or if) mutant enzymes interact with their wild-type counterparts. Co-precipitation experiments showed that mutant IDH1 heterodimerizes with wild-type IDH1, but mutant IDH2 does not bind very well to wild-type IDH2, again refuting the hypothesis that these mutated enzymes must bind and inhibit their wild-type counterparts [69]. When substrates are supplied in excess during cell-free in vitro experimentation, the R132H:R132H IDH1 homodimer is more efficient at producing 2-HG than a heterodimer [103, 134]. But in a more realistic intracellular environment where substrates are limited, mutant IDH1 produces more 2-HG when it heterodimerizes with wild-type IDH1 [175]. This is consistent with the finding that IDH1-mutant gliomas that lose their wild-type allele produce far less 2-HG than they did before the deletion [68]. The wild-type half of an IDH1 heterodimer grabs isocitrate and converts it into α-KG, which is immediately made available to the mutant half for conversion to 2-HG in a process known as substrate channelling [134]. This allows for efficient 2-HG production across a wider range of local isocitrate concentrations—a feature that could be more important for cytosolic mutant IDH1 than for mutant IDH2, which already resides in the substrate-rich mitochondrion and does not require heterodimerization [175] (Fig. 3).
Thus, it is not surprising that, while virtually all tumor-associated IDH1/2 mutants produce 2-HG, they are not all perfectly identical in either activity or frequency among tumors. For example, R172K IDH2 produces more 2-HG than R140Q IDH2, and after adjusting for subcellular localization, R132H IDH1 is stronger than R172K IDH2 [175]. But among IDH1 mutants, R132H IDH1 appears to be the weakest 2-HG producer whereas there is no significant difference among R172 IDH2 variants [69]. In contrast to the aforementioned strong IDH1 preponderance in gliomas (specifically R132H IDH1), a full one-third of intrahepatic cholangiocarcinomas have IDH2 mutations; even when IDH1 is the mutated enzyme in these tumors, it is more likely to be R132C than R132H [17, 86], just like cartilaginous tumors [6, 7]. Curiously enough, Li-Fraumeni (germline TP53 mutant) gliomas also favor R132C mutations [178]. AMLs are more likely to harbor a mutation in IDH2 than IDH1, specifically at R140 rather than R172 [1, 114, 176]. And in gliomas, when R132C IDH1 is present, it tends to be found in astrocytomas, whereas IDH2 mutations are more frequent in grade III oligodendrogliomas [59] (Fig. 4). All this suggests that, from a microevolutionary standpoint, the amount of optimal 2-HG varies depending on tumor site. And within a given site, differing concentrations of 2-HG might non-randomly affect what kind of tumor is formed.
Frequencies of non-R132H IDH1 mutations differ among subtypes of grade II-III gliomas. Combining data from multiple studies that included details on mutation subtypes by histologic diagnosis (see Fig. 2), about 89 % of IDH1/2 mutations in grade II–III astrocytic tumors were R132H IDH1 (a, d), with another 10 % being other IDH1 mutations. Only 0.5–1 % of those gliomas had an IDH2 mutation. In contrast, 7–8 % of grade III oligodendrogliomas and oligoastrocytomas were IDH2-mutant (e, f). But this increased proportion of IDH2 mutations was only seen in grade III tumors; 94–97 % of grade II oligodendrogliomas and oligoastrocytomas were R132H IDH1, and only 1 % was IDH2. A list of the studies from which these data are derived is in Supplemental Table 1
Parenthetically, to date no cancer-associated IDH3 mutations have been found [91], suggesting that it is difficult to confer comparable neoenzymatic activity in this heterotrimeric complex via a single point mutation.
Effects of d-2-hydroxyglutarate
2-Hydroxyglutaric aciduria
Proving that R132H IDH1 produces d-2-HG [34] was a significant advancement in oncology, not only for solving the question of what the mutation does at a biochemical level but also for spurring a whole new avenue of research aimed at discovering the impact of 2-HG in cancer. Prior to 2009, 2-HG studies focused mostly on a very rare inborn metabolic disease called 2-hydroxyglutaric aciduria. The disease was first described in 1980 and was immediately recognized as existing in two forms: l-2-HG and d-2-HG, with each isomer producing its own phenotype [27, 42]. l-2-HG aciduria manifests in early childhood and is slowly progressive, featuring leukodystrophy, psychomotor retardation, cerebellar ataxia, and seizures [155]. In contrast, d-2-HG aciduria can present either as a severe encephalopathy with cardiomyopathy and dysmorphisms affecting the face and other structures, or as a milder variant featuring developmental delay and hypotonia [157]. Remarkably, some siblings of d-2-HG patients also have high serum and urine d-2-HG levels without any symptoms at all. Causative germline inactivating mutations in l-2-hydroxyglutarate dehydrogenase and d-2-hydroxyglutarate dehydrogenase had been known for some time [155, 157], but the genetic defect in a subset of patients with d-2-HG aciduria remained a mystery. In a nice example of symbiotic mutualism between the metabolic and cancer literatures, the discovery of mutant IDH1/2 in tumors prompted a search for similar germline mutations in that idiopathic subset of d-2-HG aciduria. Sure enough, in 15 of 17 patients with no dehydrogenase mutations, R140Q or R140G IDH2 mutations were detected [90]. Those patients had higher urinary concentrations of d-2-HG compared to those with dehydrogenase mutations, although there does not appear to be a correlation between d-2-HG levels and disease severity [157]. No 2-HG dehydrogenase mutations have yet been identified in gliomas [20, 91]. Interestingly, four patients have been described with both d-2-HG aciduria and chondromatosis; two of them had somatic IDH1 mutations [170].
Because of these organic acidurias, studies on the effects of 2-HG predate the discovery of IDH1/2 mutations. In slices of rat cortex, 2-HG generates free radicals, causes oxidative stress, inhibits cytochrome c oxidase and ATP synthase, and lowers the rate of aerobic glycolysis [96, 97]. But a great deal of evidence now implicates the epigenome as a critical target of 2-HG in cancer, though this aspect of IDH1/2 research is particularly fast-moving and requires some background knowledge to appreciate its significance.
IDH1/2 and epigenetics
Histone activity can be modified via attachment of different moieties—such as acetyl or methyl groups—to specific amino acid residues on the histone. These modifications dictate how histones interact with chromatin, thereby affecting things like genomic imprinting, DNA repair, and gene expression [165]. Histone methylation occurs on lysine or arginine residues. The Jumonji C (JmjC) domain family of histone demethylases removes methyl groups from lysine residues. (“Jumonji” is Japanese for cruciform, derived from the abnormal cross-shaped formation of the neural plate and neural groove in mice with the prototypic JMJ gene mutation [162].) Each of these histone demethylases contains a Jumonji C DNA-binding domain, requiring Fe2+ and α-KG cofactors. Three subfamilies include JHDM1, JHDM2, and JMJD2, each of which contains enzymes that demethylate different histone lysine residues. JHDM1 targets H3K36, JHDM1D is for H3K27, and JHDM2 enzymes demethylate H3K9. JMJD2 is comprised of four enzymes—JMJD2A, 2B, 2C and 2D—all of which demethylate H3K9 or H3K36 [162].
These JmjC domain-containing demethylases and their histone targets had previously been known to regulate processes like cell proliferation and androgen sensitivity, but multiple groups have now shown that the 2-HG product of mutant IDH1/2 inhibits JHDM1A, JMJD2A, and JMJD2C, specifically by competing with α-KG cofactor [30, 87, 111, 184] (Fig. 3). As a result of inhibiting these demethylases, methylation of H3K9, H3K27, and H3K36 is higher when IDH1/2 mutations are present. However, 2-HG does not equally inhibit all JmjC domain-containing demethylases, much less all α-KG-dependent enzymes. This is where 2-HG in cancer becomes very complicated and confusing. For example, one group showed that 2-HG does not inhibit JMJD2D histone demethylase [87]. Another group suggested that 2-HG inhibits prolyl hydroxylase-2, leading to elevated Hif-1α [184], but 2-HG inhibition of prolyl hydroxylase-2 is at least an order of magnitude weaker than for other targets [30]. Surprisingly, 2-HG may actually promote the activity of EGLN1-3 prolyl hydroxylases (derived from “EGg-Laying defective Nine” C. elegans), leading to degradation of Hif-1α and facilitation of transformation in astrocytes and hematopoietic cells [87, 108] (Fig. 3). Furthermore, the two enantiomers of 2-HG differ in their potency, with l-2-HG generally being a better inhibitor of α-KG-dependent enzymes than d-2-HG [30, 87, 184]. This could account for why l-2-HG aciduria and d-2-HG aciduria have different phenotypes. It could also account for why neoplastic mutations make d-2-HG and not l-2-HG—perhaps a mutation that produced l-2-HG would be too potent for effective oncogenesis in most situations. Though if this is indeed the case, it begs the question as to why tumors have been seen only in l-2-HG aciduria patients, and not in d-2-HG aciduria (see “Oncogenesis” section below).
The fact that histone methylation is such an important target of 2-HG dovetails nicely with new research showing the importance of histones in cancer, especially gliomas. Histone H3.3 is encoded by H3F3A and is associated with open, active chromatin at telomeres (and probably elsewhere, too) [3, 110]. H3.3 activity is controlled by modifications at key amino acid residues: methylation of K9 and K27 inhibits transcription, whereas K36 methylation generally promotes transcription. For H3.3 to target and open up telomeric chromatin, a complex including alpha thalassemia/mental retardation syndrome X-linked protein (ATRX) and death-associated protein 6 (DAXX) needs to form with H3.3. This is important for the phenomenon of Alternative Lengthening of Telomeres (ALT), a process by which cancer cells avoid telomere shortening and senescence via homologous recombination of telomeric DNA [41]. ALT is necessary in the minority of tumors that, for whatever reason, either cannot or do not upregulate telomerases. There is now a clear connection between histones, ALT, and gliomagenesis, since (a) ALT is associated with H3.3/ATRX/DAXX mutations in gliomas [110], (b) nearly half of pediatric GBMs have inactivating mutations in the H3.3/ATRX/DAXX complex [148], and (c) the majority of diffuse intrinsic pontine gliomas (DIPGs) have K27M mutations on H3.3 [79, 183]. ALT and H3.3/ATRX/DAXX also have several strong links to 2-HG. First, methylation of K9, K27, and K36 in H3.3 is increased by 2-HG via inhibition of the aforementioned JmjC demethylases [30, 40, 184]. Second, histone mutations appear to be mutually exclusive with IDH1/2 mutations [158]. Third, ALT is more common in IDH1/2-mutant astrocytomas than in wild-type astrocytomas [78, 115, 124, 142]. Fourth, methylation of H3K9—the same histone lysine whose methylation is increased by 2-HG—leads to reduced hTR and hTERT telomerases in cancer cell lines [10]. Finally, ATRX mutations are far more common in IDH1/2-mutant versus wild-type adult gliomas [78, 106]. While more precise details are still emerging, it thus appears that H3.3 modulation is a critical part of ALT and oncogenesis in a significant proportion of gliomas. In pediatric tumors it happens directly via H3F3A mutations, in adults it occurs indirectly via 2-HG, and additional ATRX/DAXX mutations contribute to the process in both groups (Fig. 3).
In addition to blocking histone demethylases, 2-HG inhibits DNA demethylases, including the α-KG-dependent AlkB homologue 2 DNA demethylase and TET1/2 methylcytosine hydroxylases [30, 47, 184] (Fig. 3). Inhibition of DNA demethylation would be expected to promote global hypermethylation, which is exactly what is seen in neoplasms with IDH1/2 mutations [47, 93, 126, 167]. Consistent with this, TET2 inactivating mutations or TET2 promoter methylation are mutually exclusive with IDH1/2 mutations, suggesting that both accomplish the same thing [29, 47, 85, 133]. But not all global hypermethylation is equal; profiles will differ depending on which driver gene is altered [5, 133]. It is also interesting (and surprising) to note that levels of the presumed precursor of nucleotide demethylation, 5-hydroxymethylcytosine (5hmC), are not lower in IDH1-mutant astrocytomas compared to wild-type [68, 123].
Oncogenesis
Despite these effects of 2-HG on DNA and histone methylation, there is a growing consensus that, while obviously important, IDH1/2 mutations are insufficient to drive neoplasia. A transgenic mouse model engineered to express mutant IDH1 in hematopoietic cells showed increased progenitor cells in the bone marrow, but no leukemias [147]. That same group also developed a nestin-R132H IDH1 mouse model, expressing the mutant in neural stem cells [146]. Most mice died in utero, and the ones that made it to birth soon died from severe intracranial bleeding. This bleeding was caused by 2-HG inhibiting the α-KG-dependent prolyl hydroxylation of type IV collagen, leading to disorganized basement membrane structures around blood vessels. But not only were no tumors found, no histone methylation was detected. While disappointing to those searching for a robust IDH1/2-mutant transgenic tumor model, patient data had already hinted that such a model might be difficult to generate. For although l-2-HG aciduria patients sometimes develop brain tumors [2, 187], no tumors have yet been described in over 85 patients with d-2-HG aciduria [90]. (Remember that clinically relevant IDH1/2 mutations only produce the d-isomer of 2-HG.) Because 2-HG can slow down cellular differentiation [111], mutant IDH1/2 might not directly trigger oncogenesis, but rather extend the opportunity for other tumor-promoting mutations to occur in undifferentiated cells (Fig. 3). For example, IDH1/2 mutations are strongly associated with TP53 mutations in astrocytomas and 1p/19q codeletion with CIC and/or FUBP1 mutations in oligodendrogliomas [13, 51, 84, 92, 144, 189], although IDH1/2 mutations appear to precede both TP53 mutation and 1p/19q codeletion [177].
Application of IDH1/2 to cancer
Diagnostics
An attractive feature of IDH1/2 mutations is that, even though our understanding of their full significance is obviously incomplete, there is already considerable utility in screening for their presence in the setting of a brain lesion. These mutations are present in 60–80 % of WHO grades II and III astrocytomas and oligodendrogliomas (as well as grade IV secondary GBMs) (Fig. 2c), and are never seen in mimickers of glioma like vasculitis, encephalitis, demyelinating disease, or reactive gliosis [21, 63]. Likewise, non-infiltrative gliomas including pilocytic astrocytoma, dysembryoplastic neuroepithelial tumor, and ganglioglioma do not contain IDH1/2 mutations [21, 61, 63, 88]. In fact, tumors that were diagnosed as gangliogliomas based on histopathological appearance, but harbored these mutations, ended up behaving like diffusely infiltrative gliomas [64]. Thus, the presence of a mutation, even in an otherwise equivocal biopsy, can be considered as solid evidence of an infiltrating glioma, i.e., WHO grade >I. Mutation screening can also help differentiate between anaplastic oligodendrogliomas or glioblastomas with an oligodendroglial component (GBM-O, a.k.a. mixed oligoastrocytoma grade IV) and the more aggressive (and IDH1/2 wild-type) small cell GBM [73]. Frontal and temporal lobes are the most common locations, whereas infratentorial tumors only rarely have mutations [44, 158]. In fact, the subventricular zone in the supratentorium may be the preferred site for IDH1/2 mutant gliomas [51]. They can even be found in type I (no discrete enhancing mass lesion) and II (mass lesion present) gliomatosis cerebri [36, 149].
When pooling all IDH1/2-mutant infiltrative gliomas together, over 90 % will have the R132H IDH1 variant (Fig. 2a). This observation makes a R132H mutation-specific antibody effective as a rapid immunohistochemical screen on formalin-fixed, paraffin-embedded tissues [22, 24, 25]. Two such antibodies exist, DIA-H09 and Imab-1; in a head-to-head comparison, the DIA-H09 antibody was slightly more specific, with less background staining than the Imab-1 antibody [137]. For the immunostain to be interpreted as positive, one should see dark brown cytoplasmic staining that extends out to the tumor processes [61]. Weak staining of neurons or labelling of red blood cells does not count. Sometimes nuclear staining is also seen, but it is not yet clear whether this represents actual abnormal nuclear localization or just antigen diffusion [137]. Another controversy is intratumoral homogeneity of R132H IDH1 expression; some have found 100 % homogeneity in all cases [22], whereas others reported that about 15 % of mutant gliomas have a subset of tumor cells that are negative [137]. Even so, when interpreted correctly, the degree of concordance between R132H IDH1 immunohistochemistry and sequencing is very high, as the immunostain will match sequencing results 98 % of the time [137]. And in biopsies with only a few infiltrating glioma cells amidst mostly non-neoplastic tissue, the antibody has a better shot at detecting mutated cells than does any PCR/sequencing technique [32, 61, 143]. Thus, this antibody has already become a staple of brain tumor workup.
Although the R132H IDH1 immunostain is an excellent first-line screen in brain biopsies, it obviously will not detect less common IDH1 mutations or IDH2 mutations. This is a non-trivial issue, since the frequency of those other mutations is uneven between glioma subtypes. In both primary and secondary GBMs, well over 95 % of IDH1/2 mutations will be of the R132H IDH1 variety, but there is variation at the WHO grade II–III level (Fig. 4). For example, about 10 % of IDH1-mutant grade II and III astrocytomas will have a non-R132H IDH1 mutation, most commonly R132C (Fig. 4a, d). Prior work suggested that IDH2 mutations are more likely to occur in oligodendroglial tumors [59], but combining results from several studies suggests that this is true only at the grade III level (Fig. 4e, f), and that WHO grade II oligodendrogliomas have just as strong a predilection for R132H IDH1 as do GBMs (Fig. 4b). What all this means regarding gliomagenesis is unclear, but considering we now know that not all IDH1/2 mutations are identical in their 2-HG production capacity and subcellular localization, such differences cannot be dismissed out of hand. At the very least, it underscores why it is often necessary to do follow-up sequencing of R132H IDH1 immunonegative cases, especially when dealing with a suspected grade II or III glioma (Fig. 2c).
Thus, it is very helpful to supplement R132H IDH1 immunohistochemistry with molecular methods that catch other variants. Multiple assays can do this, with sensitivity well beyond the 20 % mutant allele limit of traditional PCR and Sanger sequencing [46, 62, 109, 119, 129, 169]. But regardless of the method used, critical sources of inter-laboratory variability are optimization of DNA extraction and PCR product purification [169]. Of course, the absence of an IDH1/2 mutation does not exclude the possibility of a glioma, especially if the tissue only contains a few scattered infiltrating neoplastic cells. Follow-up testing of immunonegative cases is probably worthwhile in grades II–III gliomas and secondary GBMs, in patients between 20 and 60 years, in those whose tumors presented with seizures, and in any GBM with minimal necrosis [94, 125, 156].
In particular, the special association between IDH1/2 gliomas and seizures deserves special mention. Some have used a 2-HG octyl ester to increase cell permeability [30, 108, 184], but others have shown effects by exogenous 2-HG even without esterification [140]. And recent work showed that exogenous unmodified 2-HG can enter cells via a sodium-dependent dicarboxylate transporter [19]. Since mutant cells secrete 2-HG into culture medium in vitro or into the extracellular space in vivo [34, 45, 69, 90, 105, 150], its effects may extend to admixed non-neoplastic cells in the tumor. For example, seizures are not only a feature of the 2-HG acidurias but are also more likely in IDH1/2-mutant gliomas than their wild-type counterparts [67, 156]. It is therefore possible that seizures in such patients are not merely sequelae of tumor infiltration or mass effect, but instead represent a direct local activity of 2-HG on non-neoplastic brain cells.
Finally, instead of directly testing for the mutation in biopsied tissue, it is possible to detect elevated 2-HG in IDH1/2-mutant brain tumors using proton magnetic resonance spectroscopy [8, 28, 43, 98], though it is not yet clear whether this approach is sensitive and specific enough for routine use. The compound can also be detected in paraffin tissue blocks containing mutant tumor [12]. In IDH1/2-mutant leukemias, quantification of 2-HG in serum or even urine is feasible and correlates with disease recurrence [12, 45, 56, 150]. However, a similar approach does not work very well in gliomas, presumably because insufficient 2-HG ends up in the systemic circulation [23]. Likewise, direct detection of mutant IDH1/2 in the systemic circulation of glioma patients is a relatively insensitive biomarker [16].
Prognosis
Even when a histologic diagnosis of infiltrative glioma is certain, and there is no question about its WHO grade, there is still a very good reason to test for IDH1/2 mutations—tumors with the mutation tend to be far less aggressive than their WHO grade-matched wild-type counterparts [38, 125, 130, 145, 179, 180, 185]. This is clearly true at the grade III–IV level, where grade III tumors lacking the mutations are just as lethal as wild-type grade IV tumors [58, 185]. In fact, one series of wild-type anaplastic astrocytomas showed progression to classic ring-enhancing GBMs within just a few months of initial resection, raising the possibility that many such tumors were in fact GBMs at presentation, albeit without necrosis or microvascular proliferation on the initial specimen [32, 128]. And one reason why advanced patient age is an unfavorable prognostic marker is because IDH1/2 mutant tumors are less likely to occur in older people [54, 58, 185]. IDH1/2 mutations are not only characteristic of many proneural GBMs, specifically those with global hypermethylation [126, 158, 166], but also they comprise the subset of proneural GBMs that actually have a better prognosis [126].
IDH1/2 mutational status is also a very good triaging tool for subsequent 1p/19q testing in suspected oligodendrogliomas, insofar as true whole-arm codeletion hardly ever happens in the absence of a mutation [92, 190]. Thus, a wild-type glioma probably does not need to be tested for codeletion, because even an apparent codeletion (e.g., by FISH) cannot be trusted [33].
As is the case with other aspects of IDH1/2, however, there are controversies about its prognostic power in certain contexts. For example, it is not at all clear whether IDH1/2 status effectively stratifies grade II gliomas, especially astrocytomas; some have suggested a better prognosis [104, 118, 127, 139] while others found no difference [4, 51, 52, 65, 75, 76, 84, 161]. Since grade I gliomas do not have IDH1/2 mutations, some tumors that appear to be grade II histologically but are IDH1/2 wild-type might actually represent “overgraded” grade I gliomas. If so, inclusion of such cases would mask the prognostic effects of IDH1/2 mutations in true diffusely infiltrative grade II gliomas. For example, one study that showed no survival difference in grade II gliomas also reported a 33 % higher rate of gross total excision in wild-type tumors, raising the possibility that at least some of their grade II tumors might have been grade I in a biological sense—i.e., essentially non-infiltrative—a point the authors themselves suggested [4]. A different group found that their grade II astrocytomas actually had worse progression-free survival than matched wild-type tumors, but better postrecurrence survival [164]. This incongruity might be explained by accidental inclusion of some wild-type grade I tumors that did not recur, but among tumors that did recur (which were more likely to be real infiltrative gliomas), IDH1/2-mutation was favorable.
Another point of debate is whether IDH1/2 mutations are better prognostic markers than other molecular markers. Several multivariate analyses have shown IDH1/2 to be more powerful than 1p/19q codeletion and even O6-Methylguanine-DNA methyltransferase (MGMT) promoter methylation [50, 74, 145, 154, 180], though some have suggested MGMT might still be stronger [26, 66, 84, 120]. Given the prominence of MGMT in the workup of GBMs, its relationship with IDH1/2 mutations deserves particular attention.
IDH1/2 and MGMT
MGMT is a DNA repair protein that removes alkyl groups from the O6 position of guanine in DNA, making cells resistant to the alkylating agent temozolomide [160]. When its promoter is methylated, MGMT expression decreases and temozolomide sensitivity increases. As a result of a seminal study by Hegi et al. [60], MGMT promoter methylation testing is standard for the workup of GBMs. But that same study also showed that methylated GBMs responded better to a radiation-only regimen, even though MGMT is not known to have a role in repairing the kind of DNA damage induced by radiation. However, considering that IDH1/2 mutations promote global hypermethylation, including methylation of the MGMT promoter [31, 50, 121, 126, 145, 166], it is possible that some of the MGMT-methylated GBMs in the Hegi study also had IDH1/2 mutations, and that it is the latter defect which promotes radiosensitivity. In support of this, some have shown that IDH1/2 mutations correlate with radiosensitivity [105, 127, 168], though even this has been disputed [39, 139].
In clinical studies, it is difficult to weigh the relative importance of IDH1/2 versus MGMT, because although around half of IDH1/2 wild-type gliomas will still have MGMT promoter methylation [58, 145], the reverse situation—IDH1/2-mutant tumors without methylation—is uncommon. Exactly how uncommon seems to depend on the cohort and on how methylation is being tested. For example, one group using methylation-specific PCR reported 15–20 % of grade III astrocytomas, and 2 % of GBMs, as having IDH1/2 mutation but not MGMT promoter methylation [58]. This is consistent with another study of grade II–IV gliomas using a similar method [145]. In contrast, a different group tested methylation via pyrosequencing of 16 CpG sites in the MGMT promoter. They found that, in their cohort of over 400 grade II–IV gliomas, virtually all IDH1/2-mutant tumors also had methylation regardless of WHO grade [121]. Others have reported similar degrees of concordance [159, 166, 168].
This does not mean, however, that MGMT is now irrelevant. A recent study of GBMs in the elderly (in which IDH1/2 mutations are uncommon) showed that MGMT promoter methylation was associated with better response to temozolomide-containing regimens, but not to radiotherapy alone [113]. It is also unclear whether IDH1/2 mutations affect temozolomide response independent of MGMT [159]. MGMT promoter methylation is probably a favorable marker independent of IDH1/2, but only in regimens containing temozolomide. IDH1/2 mutations, on the other hand, may be relevant to a broader spectrum of adjuvant therapies. Thus, having both molecular alterations is likely more favorable than either in isolation. MGMT testing is still, therefore, useful in the workup of GBMs that are wild-type for IDH1/2, but in the opinion of this author, if an IDH1/2 mutation is detected at any grade of glioma, it can safely be assumed that MGMT promoter methylation is also present, and proving it would, therefore, be unnecessary.
IDH1/2 variants and outcome
Earlier, differences in 2-HG production among IDH1/2 variants were discussed; this raises the question as to whether different variants have different outcomes. This is difficult to study in gliomas, where there is a marked preponderance for R132H IDH1 (Fig. 2), but in AML there is less enrichment for a specific mutation. In those cancers, mutations on IDH1 seem to associate with a worse prognosis than IDH2 [1, 29, 114, 132]. Even variants within the same gene might have differing prognostic significance, as R140 mutations on IDH2 might be somewhat more favorable than R172 [55, 114, 131]. Similarly, R132C IDH1 might be more favorable than R132H [89]. Extremely large cohorts adjusting for other variables like WHO grade, 1p/19q status, and treatment would be needed to reliably determine if similar associations exist in gliomas, although one study has already suggested that there are no survival differences between R132H and non-R132H IDH1 variants [53].
Yet another layer of complexity regarding IDH1/2 mutations and prognosis is the phenomenon of monoallelic gene expression (MAE), wherein only one allele of a gene is expressed even though both alleles are present. This of course happens during imprinting and X-inactivation, but it can also occur in otherwise non-imprinted genes. One study found that about 15 % of IDH1-mutant gliomas had monoallelic gene expression of IDH1, that it was usually directed toward the normal allele (i.e., the mutant allele was not expressed), and, as expected, survival was worse in such cases [172]. This could help explain the aforementioned R132H IDH1 immunohistochemical heterogeneity and false-negativity seen in some gliomas [137], and raises the possibility that sequencing might not always be adequate to identify tumors that are functionally IDH1/2 mutant.
A final word on IDH1/2 and prognosis is from a report that an rs11554137:C >T single-nucleotide polymorphism (SNP) is present on codon 105 of the IDH1 gene in about 10 % of grade II–IV gliomas [173]. This SNP occurs independently of both IDH1/2 mutations and WHO grade, and although it does not substitute the glycine residue on codon 105, it nevertheless is associated with worse response to adjuvant therapy in both gliomas and AML [171, 173]. As of yet, there is no satisfying explanation for this remarkable finding.
Future directions
As we have seen, the sheer volume of data that has been generated by multiple laboratories on IDH1/2 mutations over the last few years is simply incredible. The field has now matured to the stage where rapid, descriptive papers on mutation frequencies and outcomes have been done, and mechanistic/experimental papers are becoming more frequent. Predictions as to the next major advances in this subfield are inherently uncertain, but some areas of interest include how IDH1/2 mutations promote gliomagenesis, what experimental models can be developed, why the mutations affect prognosis, and how to target IDH1/2 mutations in novel therapies.
Although we do not yet know exactly why IDH1/2 mutations are found in gliomas, there are a few clues in the literature. For example, if a glioma does not have an IDH1/2 mutation at its clinical outset, it never acquires one [95]. Likewise, 2-HG levels do not change as a lower-grade glioma progresses to GBM [76]. Mutations predate and are tightly associated with TP53 mutations in astrocytomas and 1p/19q codeletion in oligodendrogliomas [51, 84, 92, 118, 190]. Even some IDH1/2-mutant mixed oligoastrocytomas can have a TP53 mutation in the astrocytic region and 1p/19q codeletion in the oligodendroglial area [95]. Perhaps the mutation and its 2-HG metabolite are therefore not sufficiently oncogenic on their own, but instead act as selection agents favoring specific additional genetic alterations. And if a glioma manages to arise without a mutation, there is no selection pressure to do so. We also know that certain germline SNPs on 8q24.21 and 11q23 increase the risk of IDH1/2-mutant gliomas [67, 141]. What those SNPs are doing to promote IDH1/2 mutations is unknown, but will certainly be intensely investigated in coming years. Perhaps the SNPs and/or IDH1/2 mutations are indirectly causing gliomagenesis via congenital disruption of the normal stem cell microenvironment, in turn increasing the odds of neoplasia akin to what was hypothesized for carcinogenesis by James DeGregori [35]. Certainly, the effects of 2-HG on DNA and histone methylation will prove significant in gliomagenesis.
But 2-HG, however beneficial it might be to oncogenesis, clearly can have toxic side effects under certain conditions. Perhaps the favorable prognostic effects of IDH1/2 mutations are not fully realized unless the cells are abruptly stressed, thereby unmasking metabolic defects for which the tumor had gradually evolved compensatory mechanisms. Recalling the controversy mentioned earlier—whether IDH1/2 mutations are prognostically important at the grade II level—it is important to remember that most grade II gliomas are not irradiated upfront if it can be avoided, whereas radiotherapy is standard at the III–IV level. And while some have suggested that IDH1/2 mutations reduce cellular invasiveness and/or proliferation [18, 105], others have found no such correlations [52, 56, 135, 136, 191]. IDH1/2 prognostic significance may therefore be due more to heightened adjuvant therapy sensitivity as opposed to intrinsic defects in growth or invasiveness. After all, radiotherapy-containing regimens affect not just tumor cells, but also create a hostile microenvironment with necrosis, low pH, low oxygen, low glucose, and increased local concentrations of metabolic wastes. This might help account for the more equivocal prognostic importance of IDH1/2 in AMLs, as such an unpleasant microenvironment cannot be created in the circulation without killing the patient. Furthermore, glial cells could be particularly sensitive to 2-HG side effects, hence their strong predilection for one of the weakest 2-HG producers, R132H IDH1. In comparison, AMLs, by virtue of their being in the bloodstream, might have an easier time getting rid of excess 2-HG and thus not be subjected to the same selection pressure for a weak mutation. And recall that these mutations only produce d-2-HG and not l-2-HG, even though the latter is a more potent inhibitor of α-KG-dependent enzymes [30, 87, 184]. Taken together, it seems that the “therapeutic window” for 2-HG to promote oncogenesis is fairly narrow, and what is a beneficial mutation for the untreated tumor can quickly be rendered detrimental during adjuvant therapy.
Regarding its use as a biomarker, while the presence of an IDH1/2 mutation strongly indicates at least a grade II infiltrative glioma, its absence in an obviously low-grade setting does not automatically equate to a non-infiltrative grade I tumor, especially in children. Other mutations, including those in H3F3A and BRAF V600E, could be drivers of less common infiltrative subsets. Nevertheless, the next WHO classification will likely address IDH1/2 and emphasize its importance in the pathologic workup of gliomas. One group even suggested that a molecular panel including IDH1/2, MGMT, 1p/19q, and TP53 does a better job of prognostic stratification in grade II gliomas than histologic subtyping [104]. And once radiologic measurement of elevated 2-HG is optimized and standardized for routine use, it will have a tremendous impact on the workup and management of brain tumor patients, both in generating a more accurate preoperative differential as well as in discriminating genuine recurrences from therapy-induced changes.
But gliomas apparently have strategies for evolving out of the IDH1/2 mutations, including monoallelic gene expression of only the wild-type allele [172], deletion of the mutant allele [95, 138], or even deletion of the wild-type allele so as to reduce 2-HG production [66, 68]. This suggests that, for any clinical trials aimed at targeted therapeutics or more accurate prognoses, degrees of mutant expression and functionality are probably more important than just the presence of a mutation [108]. For example, glutaminase inhibition is more effective in IDH1-mutant gliomas [151], but only works if the mutation is active. The same would hold for any strategy involving nanotechnology or other small molecules that specifically bind mutant enzyme, such as the R132H IDH1 inhibitor AGX-891 [108].
Generating a robust transgenic mouse model of IDH1/2-mutant tumors will be important for the field to really advance. As discussed earlier, Tak Mak’s laboratory has nicely demonstrated some of the difficulties in making this happen [146, 147], so for now the emphasis will be on xenografts like the one developed by Luchman et al. [112]. Pairing a conditional knock-in IDH1 mutation with some other biologically appropriate oncogene or tumor suppressor might eventually work.
Clearly, there is still a great deal to be done on IDH1/2 in gliomas and other cancers. Our diagnostic and prognostic power has already been strengthened, but realization of the Human Genome Project’s ultimate goal—development of personalized, bona fide cures for currently incurable diseases—will require even more hard work and tenacity.
References
Abbas S, Lugthart S, Kavelaars FG et al (2010) Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: prevalence and prognostic value. Blood 116(12):2122–2126
Aghili M, Zahedi F, Rafiee E (2009) Hydroxyglutaric aciduria and malignant brain tumor: a case report and literature review. J Neurooncol 91:233–236
Ahmad K, Henikoff S (2002) The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol Cell 9(6):1191–1200
Ahmadi R, Stockhammer F, Becker N et al (2012) No prognostic value of IDH1 mutations in a series of 100 WHO grade II astrocytomas. J Neurooncol 109(1):15–22
Akalin A, Garrett-Bakelman FE, Kormaksson M et al (2012) Base-pair resolution DNA methylation sequencing reveals profoundly divergent epigenetic landscapes in acute myeloid leukemia. PLoS Genet 8(6):e1002781
Amary MF, Bacsi K, Maggiani F et al (2011) IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol 224(3):334–343
Amary MF, Damato S, Halai D et al (2011) Ollier disease and Maffucci syndrome are caused by somatic mosaic mutations of IDH1 and IDH2. Nat Genet 43(12):1262–1265
Andronesi OC, Kim GS, Gerstner E et al (2012) Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med 4(116):116ra4
Atai NA, Renkema-Mills NA, Bosman J et al (2011) Differential activity of NADPH-producing dehydrogenases renders rodents unsuitable models to study IDH1R132 mutation effects in human glioblastoma. J Histochem Cytochem 59(5):489–503
Atkinson SP, Hoare SF, Glasspool RM, Keith WN (2005) Lack of telomerase gene expression in alternative lengthening of telomere cells is associated with chromatin remodeling of the hTR and hTERT gene promoters. Cancer Res 65(17):7585–7590
Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A (2008) Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol 116(6):597–602
Balss J, Pusch S, Beck AC et al (2012) Enzymatic assay for quantitative analysis of (D)-2-hydroxyglutarate. Acta Neuropathol 124(6):883–891
Bettegowda C, Agrawal N, Jiao Y et al (2011) Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science 333(6048):1453–1455
Bleeker FE, Atai NA, Lamba S et al (2010) The prognostic IDH1 (R132) mutation is associated with reduced NADP + dependent IDH activity in glioblastoma. Acta Neuropathol 119(4):487–494
Bleeker FE, Lamba S, Leenstra S et al (2009) IDH1 mutations at residue p.R132 (IDH1(R132)) occur frequently in high-grade gliomas but not in other solid tumors. Hum Mutat 30(1):7–11
Boisselier B, Gallego Perez-Larraya J, Rossetto M et al (2012) Detection of IDH1 mutation in the plasma of patients with glioma. Neurology 79(16):1693–1698
Borger DR, Tanabe KK, Fan KC et al (2012) Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 17(1):72–79
Bralten LB, Kloosterhof NK, Balvers R et al (2011) IDH1 R132H decreases proliferation of glioma cell lines in vitro and in vivo. Ann Neurol 69(3):455–463
Brauburger K, Burckhardt G, Burckhardt BC (2011) The sodium-dependent di- and tricarboxylate transporter, NaCT, is not responsible for the uptake of d-, l-2-hydroxyglutarate and 3-hydroxyglutarate into neurons. J Inherit Metab Dis 34(2):477–482
Brehmer S, Pusch S, Schmieder K, von Deimling A, Hartmann C (2011) Mutational analysis of D2HGDH and L2HGDH in brain tumours without IDH1 or IDH2 mutations. Neuropathol Appl Neurobiol 37(3):330–332
Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL (2010) Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol 119(4):509–511
Capper D, Reuss D, Schittenhelm J et al (2011) Mutation-specific IDH1 antibody differentiates oligodendrogliomas and oligoastrocytomas from other brain tumors with oligodendroglioma-like morphology. Acta Neuropathol 121(2):241–252
Capper D, Simon M, Langhans CD et al (2011) 2-Hydroxyglutarate concentration in serum from patients with gliomas does not correlate with IDH1/2 mutation status or tumor size. Int J Cancer 131(3):766–768
Capper D, Weissert S, Balss J et al (2009) Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol 20(1):245–254
Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A (2009) Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol 118:599–601
Carrillo JA, Lai A, Nghiemphu PL et al (2012) Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR Am J Neuroradiol 33(7):1349–1355
Chalmers RA, Lawson AM, Watts RW et al (1980) d-2-hydroxyglutaric aciduria: case report and biochemical studies. J Inherit Metab Dis 3(1):11–15
Choi C, Ganji SK, DeBerardinis RJ et al (2012) 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med 18(4):624–629
Chou WC, Lei WC, Ko BS et al (2011) The prognostic impact and stability of isocitrate dehydrogenase 2 mutation in adult patients with acute myeloid leukemia. Leukemia 25(2):246–253
Chowdhury R, Yeoh KK, Tian YM et al (2011) The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep 12(5):463–469
Christensen BC, Smith AA, Zheng S et al (2011) DNA methylation, isocitrate dehydrogenase mutation, and survival in glioma. J Natl Cancer Inst 103(2):143–153
Clark K, Voronovich Z, Horbinski C (2013) How molecular testing can help (and hurt) in the workup of gliomas. Am J Clin Pathol 139(3):275–288
Clark KH, Villano JL, Nikiforova MN, Hamilton RL, Horbinski C (2013) 1p/19q testing has no significance in the workup of glioblastomas. Neuropathol Appl Neurobiol. doi:10.1111/nan.12031
Dang L, White DW, Gross S et al (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462(7274):739–744
DeGregori J (2011) Evolved tumor suppression: why are we so good at not getting cancer? Cancer Res 71(11):3739–3744
Desestret V, Ciccarino P, Ducray F et al (2011) Prognostic stratification of gliomatosis cerebri by IDH1 R132H and INA expression. J Neurooncol 105(2):219–224
Diehn M, Cho RW, Lobo NA et al (2009) Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458(7239):780–783
Dubbink HJ, Taal W, van Marion R et al (2009) IDH1 mutations in low-grade astrocytomas predict survival but not response to temozolomide. Neurology 73(21):1792–1795
Ducray F, El Hallani S, Idbaih A (2009) Diagnostic and prognostic markers in gliomas. Curr Opin Oncol 21(6):537–542
Duncan CG, Barwick BG, Jin G et al (2012) A heterozygous IDH1R132H/WT mutation induces genome-wide alterations in DNA methylation. Genome Res 22(12):2339–2355
Dunham MA, Neumann AA, Fasching CL, Reddel RR (2000) Telomere maintenance by recombination in human cells. Nat Genet 26(4):447–450
Duran M, Kamerling JP, Bakker HD, van Gennip AH, Wadman SK (1980) l-2-Hydroxyglutaric aciduria: an inborn error of metabolism? J Inherit Metab Dis 3(4):109–112
Elkhaled A, Jalbert LE, Phillips JJ et al (2012) Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas. Sci Transl Med 4(116):116ra5
Ellezam B, Theeler BJ, Walbert T et al (2012) Low rate of R132H IDH1 mutation in infratentorial and spinal cord grade II and III diffuse gliomas. Acta Neuropathol 124(3):449–451
Fathi AT, Sadrzadeh H, Borger DR et al (2012) Prospective serial evaluation of 2-hydroxyglutarate, during treatment of newly diagnosed acute myeloid leukemia, to assess disease activity and therapeutic response. Blood 120(23):4649–4652
Felsberg J, Wolter M, Seul H et al (2010) Rapid and sensitive assessment of the IDH1 and IDH2 mutation status in cerebral gliomas based on DNA pyrosequencing. Acta Neuropathol 119(4):501–507
Figueroa ME, Abdel-Wahab O, Lu C et al (2010) Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18(6):553–567
Filipp FV, Scott DA, Ronai ZA, Osterman AL, Smith JW (2012) Reverse TCA cycle flux through isocitrate dehydrogenases 1 and 2 is required for lipogenesis in hypoxic melanoma cells. Pigment Cell Melanoma Res 25(3):375–383
Gaal J, Burnichon N, Korpershoek E et al (2010) Isocitrate dehydrogenase mutations are rare in pheochromocytomas and paragangliomas. J Clin Endocrinol Metab 95(3):1274–1278
Glas M, Bahr O, Felsberg J et al (2011) NOA-05 phase 2 trial of procarbazine and lomustine therapy in gliomatosis cerebri. Ann Neurol 70(3):445–453
Gorovets D, Kannan K, Shen R et al (2012) IDH mutation and neuroglial developmental features define clinically distinct subclasses of lower grade diffuse astrocytic glioma. Clin Cancer Res 18(9):2490–2501
Goze C, Bezzina C, Goze E et al (2012) 1P19Q loss but not IDH1 mutations influences WHO grade II gliomas spontaneous growth. J Neurooncol 108(1):69–75
Gravendeel LA, Kloosterhof NK, Bralten LB et al (2010) Segregation of non-p.R132H mutations in IDH1 in distinct molecular subtypes of glioma. Hum Mutat 31(3):E1186–E1199
Gravendeel LA, Kouwenhoven MC, Gevaert O et al (2009) Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res 69(23):9065–9072
Green CL, Evans CM, Hills RK, Burnett AK, Linch DC, Gale RE (2010) The prognostic significance of IDH1 mutations in younger adult patients with acute myeloid leukemia is dependent on FLT3/ITD status. Blood 116(15):2779–2782
Gross S (2010) Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med 207:339–344
Gupta R, Flanagan S, Li CC et al (2013) Expanding the spectrum of IDH1 mutations in gliomas. Mod Pathol. doi:10.1038/modpathol.2012.210
Hartmann C, Hentschel B, Wick W et al (2010) Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 120(6):707–718
Hartmann C, Meyer J, Balss J et al (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 118(4):469–474
Hegi ME (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Horbinski C (2012) Something old and something new about molecular diagnostics in gliomas. In: Hunt JL (ed) Surgical pathology clinics: molecular oncology. Elsevier, Philadelphia, pp 919–939
Horbinski C, Kelly L, Nikiforov YE, Durso MB, Nikiforova MN (2010) Detection of IDH1 and IDH2 mutations by fluorescence melting curve analysis as a diagnostic tool for brain biopsies. J Mol Diagn 12(4):487–492
Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN (2009) Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol 68:1319–1325
Horbinski C, Kofler J, Yeaney G et al (2011) Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol 21(5):564–574
Houillier C, Wang X, Kaloshi G et al (2010) IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology 75(17):1560–1566
Ichimura K (2009) IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol 11:341–347
Jenkins RB, Xiao Y, Sicotte H et al (2012) A low-frequency variant at 8q24.21 is strongly associated with risk of oligodendroglial tumors and astrocytomas with IDH1 or IDH2 mutation. Nat Genet 44(10):1122–1125
Jin G, Reitman ZJ, Duncan CG et al (2013) Disruption of wild-type IDH1 suppresses D-2-hydroxyglutarate production in IDH1-mutated gliomas. Cancer Res 73(2):496–501
Jin G, Reitman ZJ, Spasojevic I et al (2011) 2-hydroxyglutarate production, but not dominant negative function, is conferred by glioma-derived NADP-dependent isocitrate dehydrogenase mutations. PLoS One 6(2):e16812
Jo SH, Lee SH, Chun HS et al (2002) Cellular defense against UVB-induced phototoxicity by cytosolic NADP(+)-dependent isocitrate dehydrogenase. Biochem Biophys Res Commun 292(2):542–549
Jo SH, Son MK, Koh HJ et al (2001) Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP + -dependent isocitrate dehydrogenase. J Biol Chem 276(19):16168–16176
Jones DT, Mulholland SA, Pearson DM et al (2011) Adult grade II diffuse astrocytomas are genetically distinct from and more aggressive than their paediatric counterparts. Acta Neuropathol 121(6):753–761
Joseph NM, Phillips J, Dahiya S et al (2012) Diagnostic implications of IDH1-R132H and OLIG2 expression patterns in rare and challenging glioblastoma variants. Mod Pathol 26(3):315–326
Juratli TA, Kirsch M, Geiger K et al (2012) The prognostic value of IDH mutations and MGMT promoter status in secondary high-grade gliomas. J Neurooncol 110(3):325–333
Juratli TA, Kirsch M, Robel K et al (2012) IDH mutations as an early and consistent marker in low-grade astrocytomas WHO grade II and their consecutive secondary high-grade gliomas. J Neurooncol 108(3):403–410
Juratli TA, Peitzsch M, Geiger K, Schackert G, Eisenhofer G, Krex D (2013) Accumulation of 2-hydroxyglutarate is not a biomarker for malignant progression in IDH-mutated low-grade gliomas. Neuro Oncol [Epub ahead of print]
Kang MR, Kim MS, Oh JE et al (2009) Mutational analysis of IDH1 codon 132 in glioblastomas and other common cancers. Int J Cancer 125(2):353–355
Kannan K, Inagaki A, Silber J et al (2012) Whole-exome sequencing identifies ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget 3(10):1194–1203
Khuong-Quang DA, Buczkowicz P, Rakopoulos P et al (2012) K27 M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 124(3):439–447
Kil IS, Huh TL, Lee YS, Lee YM, Park JW (2006) Regulation of replicative senescence by NADP + -dependent isocitrate dehydrogenase. Free Radic Biol Med 40(1):110–119
Kil IS, Kim SY, Lee SJ, Park JW (2007) Small interfering RNA-mediated silencing of mitochondrial NADP+-dependent isocitrate dehydrogenase enhances the sensitivity of HeLa cells toward tumor necrosis factor-alpha and anticancer drugs. Free Radic Biol Med 43(8):1197–1207
Kil IS, Lee JH, Shin AH, Park JW (2004) Glycation-induced inactivation of NADP(+)-dependent isocitrate dehydrogenase: implications for diabetes and aging. Free Radic Biol Med 37(11):1765–1778
Kim J, Kim JI, Jang HS, Park JW, Park KM (2011) Protective role of cytosolic NADP(+)-dependent isocitrate dehydrogenase, IDH1, in ischemic pre-conditioned kidney in mice. Free Radic Res 45(7):759–766
Kim YH, Nobusawa S, Mittelbronn M et al (2010) Molecular classification of low-grade diffuse gliomas. Am J Pathol 177(6):2708–2714
Kim YH, Pierscianek D, Mittelbronn M et al (2011) TET2 promoter methylation in low-grade diffuse gliomas lacking IDH1/2 mutations. J Clin Pathol 64(10):850–852
Kipp BR, Voss JS, Kerr SE et al (2012) Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol 43(10):1552–1558
Koivunen P, Lee S, Duncan CG et al (2012) Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 483(7390):484–488
Korshunov A, Meyer J, Capper D et al (2009) Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol 118(3):401–405
Koszarska M, Bors A, Feczko A et al (2012) Type and location of isocitrate dehydrogenase mutations influence clinical characteristics and disease outcome of acute myeloid leukemia. Leuk Lymphoma [Epub ahead of print]
Kranendijk M, Struys EA, van Schaftingen E et al (2010) IDH2 mutations in patients with d-2-hydroxyglutaric aciduria. Science 330(6002):336
Krell D, Assoku M, Galloway M, Mulholland P, Tomlinson I, Bardella C (2011) Screen for IDH1, IDH2, IDH3, D2HGDH and L2HGDH mutations in glioblastoma. PLoS One 6(5):e19868
Labussiere M, Idbaih A, Wang XW et al (2010) All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology 74(23):1886–1890
Laffaire J, Everhard S, Idbaih A et al (2011) Methylation profiling identifies 2 groups of gliomas according to their tumorigenesis. Neuro Oncol 13(1):84–98
Lai A, Kharbanda S, Pope WB et al (2012) Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol 29(34):4482–4490
Lass U, Numann A, von Eckardstein K et al (2012) Clonal analysis in recurrent astrocytic, oligoastrocytic and oligodendroglial tumors implicates IDH1- mutation as common tumor initiating event. PLoS One 7(7):e41298
Latini A, da Silva CG, Ferreira GC et al (2005) Mitochondrial energy metabolism is markedly impaired by d-2-hydroxyglutaric acid in rat tissues. Mol Genet Metab 86(1–2):188–199
Latini A, Scussiato K, Rosa RB et al (2003) d-2-hydroxyglutaric acid induces oxidative stress in cerebral cortex of young rats. Eur J Neurosci 17(10):2017–2022
Lazovic J, Soto H, Piccioni D et al (2012) Detection of 2-hydroxyglutaric acid in vivo by proton magnetic resonance spectroscopy in U87 glioma cells overexpressing isocitrate dehydrogenase-1 mutation. Neuro Oncol 14(12):1465–1472
Lee JH, Kim SY, Kil IS, Park JW (2007) Regulation of ionizing radiation-induced apoptosis by mitochondrial NADP+dependent isocitrate dehydrogenase. J Biol Chem 282(18):13385–13394
Lee SM, Huh TL, Park JW (2001) Inactivation of NADP(+)-dependent isocitrate dehydrogenase by reactive oxygen species. Biochimie 83(11–12):1057–1065
Lee SM, Koh HJ, Park DC, Song BJ, Huh TL, Park JW (2002) Cytosolic NADP(+)-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic Biol Med 32(11):1185–1196
Lee SM, Park SY, Shin SW, Kil IS, Yang ES, Park JW (2009) Silencing of cytosolic NADP(+)-dependent isocitrate dehydrogenase by small interfering RNA enhances the sensitivity of HeLa cells toward staurosporine. Free Radic Res 43(2):165–173
Leonardi R, Subramanian C, Jackowski S, Rock CO (2012) Cancer-associated isocitrate dehydrogenase mutations inactivate NADPH-dependent reductive carboxylation. J Biol Chem 287(18):14615–14620
Leu S, von Felten S, Frank S et al (2013) IDH/MGMT-driven molecular classification of low-grade glioma is a strong predictor for long-term survival. Neuro Oncol [Epub ahead of print]
Li S, Chou AP, Chen W et al (2013) Overexpression of isocitrate dehydrogenase mutant proteins renders glioma cells more sensitive to radiation. Neuro Oncol 15(1):57–68
Liu XY, Gerges N, Korshunov A et al (2012) Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol 124(5):615–625
Lopez GY, Reitman ZJ, Solomon D et al (2010) IDH1(R132) mutation identified in one human melanoma metastasis, but not correlated with metastases to the brain. Biochem Biophys Res Commun 398(3):585–587
Losman JA, Looper R, Koivunen P et al (2013) (R)-2-Hydroxyglutarate Is Sufficient to Promote Leukemogenesis and Its Effects Are Reversible. Science [Epub ahead of print]
Loussouarn D, Le Loupp AG, Frenel JS et al (2012) Comparison of immunohistochemistry, DNA sequencing and allele-specific PCR for the detection of IDH1 mutations in gliomas. Int J Oncol 40(6):2058–2062
Lovejoy CA, Li W, Reisenweber S et al (2012) Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet 8(7):e1002772
Lu C, Ward PS, Kapoor GS et al (2012) IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483(7390):474–478
Luchman HA, Stechishin OD, Dang NH et al (2012) An in vivo patient-derived model of endogenous IDH1-mutant glioma. Neuro Oncol 14(2):184–191
Malmstrom A, Gronberg BH, Marosi C et al (2012) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 13(9):916–926 (Epub 2012 Aug 8)
Marcucci G, Maharry K, Wu YZ et al (2010) IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol 28(14):2348–2355
McDonald KL, McDonnell J, Muntoni A et al (2010) Presence of alternative lengthening of telomeres mechanism in patients with glioblastoma identifies a less aggressive tumor type with longer survival. J Neuropathol Exp Neurol 69(7):729–736
Metallo CM, Gameiro PA, Bell EL et al (2011) Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481(7381):380–384
Metellus P, Colin C, Taieb D et al (2011) IDH mutation status impact on in vivo hypoxia biomarkers expression: new insights from a clinical, nuclear imaging and immunohistochemical study in 33 glioma patients. J Neurooncol 105(3):591–600
Metellus P, Coulibaly B, Colin C et al (2010) Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol 120(6):719–729
Meyer J, Pusch S, Balss J et al (2010) PCR- and restriction endonuclease-based detection of IDH1 mutations. Brain Pathol 20(2):298–300
Mukasa A, Takayanagi S, Saito K et al (2011) The significance of IDH mutations varies with tumor histology, grade, and genetics in Japanese glioma patients. Cancer Sci 2:1349–7006
Mulholland S, Pearson DM, Hamoudi RA et al (2012) MGMT CpG island is invariably methylated in adult astrocytic and oligodendroglial tumors with IDH1 or IDH2 mutations. Int J Cancer 131(5):1104–1113
Mullen AR, Wheaton WW, Jin ES et al (2011) Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481(7381):385–388
Muller T, Gessi M, Waha A et al (2012) Nuclear exclusion of TET1 is associated with loss of 5-hydroxymethylcytosine in IDH1 wild-type gliomas. Am J Pathol 181(2):675–683
Nguyen DN, Heaphy CM, de Wilde RF et al (2012) Molecular and morphologic correlates of the alternative lengthening of telomeres phenotype in high grade astrocytomas. Brain Pathol 29:1750–3639
Nobusawa S, Watanabe T, Kleihues P, Ohgaki H (2009) IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res 15(19):6002–6007
Noushmehr H, Weisenberger DJ, Diefes K et al (2010) Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17(5):510–522
Okita Y, Narita Y, Miyakita Y et al (2012) IDH1/2 mutation is a prognostic marker for survival and predicts response to chemotherapy for grade II gliomas concomitantly treated with radiation therapy. Int J Oncol. doi:10.3892/ijo.2012.1564
Olar A, Raghunathan A, Albarracin CT et al (2012) Absence of IDH1-R132H mutation predicts rapid progression of nonenhancing diffuse glioma in older adults. Ann Diagn Pathol 16(3):161–170
Pang B, Durso MB, Hamilton RL, Nikiforova MN (2013) A novel cold-PCR/FMCA assay enhances the detection of low-abundance IDH1 mutations in gliomas. Diagn Mol Pathol 22(1):28–34
Parsons DW, Jones S, Zhang X et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321(5897):1807–1812
Patel JP, Gonen M, Figueroa ME et al (2012) Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 366(12):1079–1089
Patnaik MM, Hanson CA, Hodnefield JM et al (2011) Differential prognostic effect of IDH1 versus IDH2 mutations in myelodysplastic syndromes: a mayo clinic study of 277 patients. Leukemia 26(1):101–105
Perez C, Martinez-Calle N, Martin-Subero JI et al (2012) TET2 mutations are associated with specific 5-methylcytosine and 5-hydroxymethylcytosine profiles in patients with chronic myelomonocytic leukemia. PLoS One 7(2):e31605
Pietrak B, Zhao H, Qi H et al (2011) A tale of two subunits: how the neomorphic R132H IDH1 mutation enhances production of alpha HG. Biochemistry 50(21):4804–4812
Pollack IF, Hamilton RL, Sobol RW et al (2011) IDH1 mutations are common in malignant gliomas arising in adolescents: a report from the Children’s Oncology Group. Childs Nerv Syst 27(1):87–94
Preusser M, Hoeftberger R, Woehrer A et al (2012) Prognostic value of Ki67 index in anaplastic oligodendroglial tumours—a translational study of the European organization for research and treatment of cancer brain tumor group. Histopathology 60(6):885–894
Preusser M, Wohrer A, Stary S, Hoftberger R, Streubel B, Hainfellner JA (2011) Value and limitations of immunohistochemistry and gene sequencing for detection of the IDH1-R132H mutation in diffuse glioma biopsy specimens. J Neuropathol Exp Neurol 70(8):715–723
Pusch S, Sahm F, Meyer J, Mittelbronn M, Hartmann C, von Deimling A (2011) Glioma IDH1 mutation patterns off the beaten track. Neuropathol Appl Neurobiol 37(4):428–430
Qi ST, Yu L, Lu YT et al (2011) IDH mutations occur frequently in Chinese glioma patients and predict longer survival but not response to concomitant chemoradiotherapy in anaplastic gliomas. Oncol Rep 26(6):1479–1485
Reitman ZJ, Jin G, Karoly ED et al (2011) Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci USA 108(8):3270–3275
Rice T, Zheng S, Decker PA et al (2013) Inherited variant on chromosome 11q23 increases susceptibility to IDH-mutated but not IDH-normal gliomas regardless of grade or histology. Neuro Oncol [Epub ahead of print]
Royds JA, Al Nadaf S, Wiles AK et al (2011) The CDKN2A G500 allele is more frequent in GBM patients with no defined telomere maintenance mechanism tumors and is associated with poorer survival. PLoS One 6(10):e26737
Sahm F, Capper D, Jeibmann A et al (2012) Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch Neurol 69(4):523–526
Sahm F, Koelsche C, Meyer J et al (2012) CIC and FUBP1 mutations in oligodendrogliomas, oligoastrocytomas and astrocytomas. Acta Neuropathol 123(6):853–860
Sanson M, Marie Y, Paris S et al (2009) Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 27(25):4150–4154
Sasaki M, Knobbe CB, Itsumi M et al (2012) d-2-hydroxyglutarate produced by mutant IDH1 perturbs collagen maturation and basement membrane function. Genes Dev 26(18):2038–2049
Sasaki M, Knobbe CB, Munger JC et al (2012) IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature 488(7413):656–659
Schwartzentruber J, Korshunov A, Liu XY et al (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482(7384):226–231
Seiz M, Tuettenberg J, Meyer J et al (2010) Detection of IDH1 mutations in gliomatosis cerebri, but only in tumors with additional solid component: evidence for molecular subtypes. Acta Neuropathol 120(2):261–267
Sellner L, Capper D, Meyer J et al (2010) Increased levels of 2-hydroxyglutarate in AML patients with IDH1-R132H and IDH2-R140Q mutations. Eur J Haematol 85(5):457–459
Seltzer MJ, Bennett BD, Joshi AD et al (2010) Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res 70(22):8981–8987
Shibata T, Kokubu A, Miyamoto M, Sasajima Y, Yamazaki N (2011) Mutant IDH1 confers an in vivo growth in a melanoma cell line with BRAF mutation. Am J Pathol 178(3):1395–1402
Sjoblom T, Jones S, Wood LD et al (2006) The consensus coding sequences of human breast and colorectal cancers. Science 314(5797):268–274
Songtao Q, Lei Y, Si G et al (2011) IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci 103(2):269–273
Steenweg ME, Jakobs C, Errami A et al (2010) An overview of L-2-hydroxyglutarate dehydrogenase gene (L2HGDH) variants: a genotype-phenotype study. Hum Mutat 31(4):380–390
Stockhammer F, Misch M, Helms HJ et al (2012) IDH1/2 mutations in WHO grade II astrocytomas associated with localization and seizure as the initial symptom. Seizure 21(3):194–197
Struys EA (2006) d-2-Hydroxyglutaric aciduria: unravelling the biochemical pathway and the genetic defect. J Inherit Metab Dis 29(1):21–29
Sturm D, Witt H, Hovestadt V et al (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22(4):425–437
Taal W, Dubbink HJ, Zonnenberg CB et al (2011) First-line temozolomide chemotherapy in progressive low-grade astrocytomas after radiotherapy: molecular characteristics in relation to response. Neuro Oncol 13(2):235–241
Tabatabai G, Hegi M, Stupp R, Weller M (2012) Clinical implications of molecular neuropathology and biomarkers for malignant glioma. Curr Neurol Neurosci Rep 12(3):302–307
Takano S, Kato Y, Yamamoto T et al (2012) Immunohistochemical detection of IDH1 mutation, p53, and internexin as prognostic factors of glial tumors. J Neurooncol 108(3):361–373
Takeuchi T, Watanabe Y, Takano-Shimizu T, Kondo S (2006) Roles of jumonji and jumonji family genes in chromatin regulation and development. Dev Dyn 235(9):2449–2459
Tefferi A, Jimma T, Sulai NH et al (2011) IDH mutations in primary myelofibrosis predict leukemic transformation and shortened survival: clinical evidence for leukemogenic collaboration with JAK2V617F. Leukemia 26(3):475–480
Thon N, Eigenbrod S, Kreth S et al (2012) IDH1 mutations in grade II astrocytomas are associated with unfavorable progression-free survival and prolonged postrecurrence survival. Cancer 118(2):452–460
Tian X, Fang J (2007) Current perspectives on histone demethylases. Acta Biochim Biophys Sin (Shanghai) 39(2):81–88
Toedt G, Barbus S, Wolter M et al (2011) Molecular signatures classify astrocytic gliomas by IDH1 mutation status. Int J Cancer 128(5):1095–1103
Turcan S, Rohle D, Goenka A et al (2012) IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483(7390):479–483
van den Bent MJ, Brandes AA, Taphoorn MJ et al (2013) Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 31(3):344–350
van den Bent MJ, Hartmann C, Preusser M et al (2013) Interlaboratory comparison of IDH mutation detection. J Neurooncol [Epub ahead of print]
Vissers LE, Fano V, Martinelli D et al (2011) Whole-exome sequencing detects somatic mutations of IDH1 in metaphyseal chondromatosis with d-2-hydroxyglutaric aciduria (MC-HGA). Am J Med Genet A 155A(11):2609–2616
Wagner K, Damm F, Gohring G et al (2012) Impact of IDH1 R132 mutations and an IDH1 single nucleotide polymorphism in cytogenetically normal acute myeloid leukemia: SNP rs11554137 is an adverse prognostic factor. J Clin Oncol 28(14):2356–2364
Walker EJ, Zhang C, Castelo-Branco P et al (2012) Monoallelic expression determines oncogenic progression and outcome in benign and malignant brain tumors. Cancer Res 72(3):636–644
Wang XW, Boisselier B, Rossetto M et al (2012) Prognostic impact of the isocitrate dehydrogenase 1 single-nucleotide polymorphism rs11554137 in malignant gliomas. Cancer 119(4):806–813
Ward PS, Cross JR, Lu C et al (2012) Identification of additional IDH mutations associated with oncometabolite R(-)-2-hydroxyglutarate production. Oncogene 31(19):2491–2498
Ward PS, Lu C, Cross JR et al (2013) The potential for isocitrate dehydrogenase mutations to produce 2-hydroxyglutarate depends on allele specificity and subcellular compartmentalization. J Biol Chem 288(6):3804–3815
Ward PS, Patel J, Wise DR et al (2010) The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17(3):225–234
Watanabe T, Nobusawa S, Kleihues P, Ohgaki H (2009) IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol 174:1149–1153
Watanabe T, Vital A, Nobusawa S, Kleihues P, Ohgaki H (2009) Selective acquisition of IDH1 R132C mutations in astrocytomas associated with Li-Fraumeni syndrome. Acta Neuropathol 117:653–656
Weller M, Felsberg J, Hartmann C et al (2009) Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol 27(34):5743–5750
Wick W, Hartmann C, Engel C et al (2009) NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol 27(35):5874–5880
Williams SC, Karajannis MA, Chiriboga L, Golfinos JG, von Deimling A, Zagzag D (2011) R132H-mutation of isocitrate dehydrogenase-1 is not sufficient for HIF-1 alpha upregulation in adult glioma. Acta Neuropathol 121(2):279–281
Wise DR, Ward PS, Shay JE et al (2011) Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci USA 108(49):19611–19616
Wu G, Broniscer A, McEachron TA et al (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44(3):251–253
Xu W, Yang H, Liu Y et al (2010) Oncometabolite 2-Hydroxyglutarate Is a Competitive Inhibitor of alpha-Ketoglutarate-Dependent Dioxygenases. Cancer Cell 19(1):17–30
Yan H, Parsons DW, Jin G et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360(8):765–773
Yang B, Zhong C, Peng Y, Lai Z, Ding J (2010) Molecular mechanisms of “off-on switch” of activities of human IDH1 by tumor-associated mutation R132H. Cell Res 20(11):1188–1200
Yazici N, Sarialioglu F, Alkan O, Kayaselcuk F, Erol I (2009) Glutaric aciduria type II [corrected] and brain tumors: a case report and review of the literature. J Pediatr Hematol Oncol 31(11):865–869
Yen KE, Bittinger MA, Su SM, Fantin VR (2010) Cancer-associated IDH mutations: biomarker and therapeutic opportunities. Oncogene 29(49):6409–6417
Yip S, Butterfield YS, Morozova O et al (2012) Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol. 226(1):7–16
Zhao S, Lin Y, Xu W et al (2009) Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science 324(5924):261–265
Zhu J, Cui G, Chen M et al (2012) Expression of R132H Mutational IDH1 in Human U87 Glioblastoma Cells Affects the SREBP1a Pathway and Induces Cellular Proliferation. J Mol Neurosci. doi:10.1007/s12031-012-9890-6
Acknowledgments
CH was supported by National Cancer Institute K08 CA155764-01A1, National Institute of General Medical Sciences 2P20 RR020171, and the University of Kentucky College of Medicine Physician Scientist Program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Horbinski, C. What do we know about IDH1/2 mutations so far, and how do we use it?. Acta Neuropathol 125, 621–636 (2013). https://doi.org/10.1007/s00401-013-1106-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1106-9