Abstract
Sporadic tauopathies are characterized by differential cellular and topographical predominance of phospho-tau immunoreactivity and biochemical distinction of the tau protein. Established entities include progressive supranuclear palsy, corticobasal degeneration, Pick’s disease, and argyrophilic grain disease. During a community-based longitudinal study on aging, we detected tau pathologies not compatible with these categories. We immunostained for different phospho-tau epitopes, 4R and 3R tau isoforms, α-synuclein, amyloid-β, and phospho-TDP-43, analyzed the MAPT and ApoE genes, and performed western blotting for the tau protein. The mean age of patients (4 women, 3 men) was 83.8 years. Clinical presentations combined dementia with psychiatric symptoms and/or parkinsonism. In addition to neurofibrillary tangles and diffuse neuronal cytoplasmic tau immunoreactivity, the neuropathology was characterized by peculiar cytopathologies (diffuse granular immunopositivity of astrocytic processes and patchy accumulation of thin threads) in a distinctive distribution (frontal and temporal cortices, hippocampus, amygdala, basal ganglia, locus coeruleus, and substantia nigra). Argyrophilic grains were detected in four patients. Few to moderate densities of neuritic plaques but widespread phospho-TDP-43 pathology was observed in five patients. There was variability in the H1/H2 and ApoE alleles and biochemical features of tau protein. We propose these cases as complex tauopathy with a characteristic constellation: some features of primary tauopathies and Alzheimer’s disease mixed with additional cytopathologies including a distinctive astrogliopathy, in a characteristic distribution of lesions. These complex tauopathies in the elderly deserve specific diagnostic and eventually therapeutic considerations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to progressive loss of neurons, the major brain pathology of neurodegenerative diseases is extracellular or intracellular deposition of various proteins. The microtubule-associated protein tau is one of these and characterizes a group of disorders named tauopathies. They are further subclassified according to the cellular and topographical distribution of inclusions together with the biochemical signature of the tau protein. A diagnosis of Alzheimer’s disease (AD) is based on the density of neuritic plaques composed of amyloid-β (Aβ) and the stage of neurofibrillary degeneration (neuronal tau pathology) according to Braak and Braak [3, 5]. While tau pathology in AD features both 3R and 4R isoforms of tau and three major bands in western blot (at 60, 64, and 69 kDa), Pick’s disease is a 3R tauopathy (60, 64 kDa) with inclusions nearly exclusively in neurons, in contrast to progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) that are 4R tauopathies (64, 69 kDa). Both PSP and CBD involve subcortical regions and exhibit oligodendroglial and astroglial tau immunoreactivity; indeed, the morphology of astrocytic tau pathology is one distinguishing feature (tufted astrocyte vs. astrocytic plaque, respectively). In the elderly, argyrophilic grain disease (AGD) is also observed mainly accompanying other pathological alterations. In community-based cohorts, AD pathology is thought to represent the highest proportion. Although this may depend on the age of the examined population [27], Pick’s disease, PSP and CBD are only rarely thought to appear above 80 years of age. In addition to novel forms of tauopathies (summarized in Ref. [31]), variants of PSP and CBD are increasingly recognized [12, 33, 38, 41, 49, 54, 55], suggesting that a spectrum of neurodegeneration instead of disease entities may be a better concept to understand clinicopathological variability.
A mixture of pathological alterations including deposition of different proteins (tau, TDP-43, α-synuclein) in the brains of elderly demented individuals is also frequently reported. This suggests complex pathogenetic scenarios in neurodegenerative diseases [29] and supports the need of a molecular approach to neuropsychiatry [10]. TDP-43 pathology is usually restricted to the medial temporal region, hippocampus, and amygdala, and is mainly recognized together with other neurodegenerative disorders, although a recent study indicates that it may be present in elderly patients without disease [18]. α-Synucleinopathy as concomitant pathology usually manifests as a Lewy-body disorder following stages proposed by Braak et al. [6, 51] or is restricted to the amygdala.
In the present study, we characterize seven cases showing features of tauopathies and AD, which by themselves did not allow to reasonably classify them with established entities. The morphological constellation features a spectrum of pathological alterations with common characteristics and distinct cytopathologies, thus we propose the term complex tauopathy to distinguish these disorders. Parts of this study were presented as a conference abstract [30].
Materials and methods
Selection of cases
Cases were selected from two sources: The Vienna Trans-Danube Aging (VITA) study (five cases) and routine diagnostic practice (two cases). Since 2000, the VITA study follows longitudinally a community-based cohort of every inhabitant of the Vienna area on the left shore of the river Danube born between May 1925 and June 1926 [16]. By use of the official voting registry, 1,505 subjects were contacted and 697 participated in the clinical study. Brains of 169 patients (who died at ages 77–85) were evaluated systematically in the past decade.
Clinical examination
One participant (patient no. 1) underwent detailed neurological and neuropsychological testing on two occasions, including Mini Mental State (MMS), Aachen Aphasia Test, Unified Parkinson Disease Rating Scale (UPDRS), Short Geriatric Depression Scale, Hamilton Scale, and Clinical Dementia Rating (CDR). For further individuals, clinical records were available.
Neuropathology
Formalin fixed, paraffin-embedded tissue blocks (2.5 × 2.0 cm) were evaluated. Tissue blocks comprised frontal, cingular, temporal, parietal, occipital cortex and white matter, anterior and posterior portion of hippocampus, caudate nucleus, accumbens nucleus, putamen, globus pallidus, thalamus, mesencephalon, pons, medulla oblongata, cerebellar anterior vermis and cerebellar hemispherium and dentate nucleus. In addition to hematoxylin and eosin, Luxol fast red, Bielschowsky and Gallyas stainings, the following monoclonal antibodies were used for immunohistochemistry: anti-tau AT8 (pS202, 1:200) AT100 (pS212, 1:200), AT180 (pT231, 1:200), AT270 (pT181, 1:200), HT7 (tau 169–163, 1:100; all from Pierce Biotechnology, Rockford, IL, USA), anti-4R tau (RD4, 1:200, Upstate, Charlottesville, VA, USA), anti-3R tau (RD3, 1:2,000, Upstate), anti-phospho-TDP-43 (pS409/410, 1:2,000, Cosmo Bio, Tokyo, Japan), anti-TDP-43 (1:2,000, Abnova, Taipei, Taiwan), anti-ubiquitin (1:50,000, Millipore, Temecula, CA, USA), anti-α-synuclein (1:10,000, clone 4D6, Signet, Dedham, MA, USA), anti-Aβ (1:50, clone 6F/3D, Dako, Glostrup, Denmark). The DAKO EnVision© detection kit, peroxidase/DAB, rabbit/mouse (Dako) was used for visualization of antibody reactions. Except for the anterior vermis, all tissue blocks were immunostained for AT8, α-synuclein, and phospho-TDP-43. Frontal, parietal, temporal, occipital cortices, hippocampus, basal ganglia and cerebellum were immunostained for anti-Aβ. Further anti-Tau antibodies were applied in the frontal cortex, hippocampus, basal ganglia, and amygdala. Neuropathological alterations (astrogliosis, neuronal loss, and degree of various protein depositions) were semiquantitatively (none, mild, moderate, and severe) evaluated in all examined anatomical regions.
Double immunolabeling was performed using monoclonal anti-tau AT8 (1:100), anti-phosphorylated neurofilament SMI-31 (1:2000, Covance, Berkeley, CA), polyclonal anti-GFAP (1:1500, Dako) and polyclonal anti-TDP-43 (1:100, ProteinTech Group, Chicago, IL, USA). The fluorescence-labeled secondary antibodies were Alexa Fluor (AF) 555 donkey anti-mouse IgG (1:200, Molecular Probes, Inc., Eugene, OR, USA), AF 488 goat anti-rabbit (1:200, Molecular Probes, Inc.), Zenon AF 488 Mouse IgG1 (Molecular Probes, Inc.). The following combinations were applied: SMI-31 (AF 555)/tau (Zenon 488), and tau (AF 555)/GFAP (AF 488), and TDP-43 (AF 555)/tau (AF 488). We evaluated double immunofluorescent labeling with a Zeiss LSM 510 confocal laser microscope.
Preparing the brain samples for western blot
For comparative immunoblotting studies we used frozen hippocampus tissue samples from patients 2, 3, 4, 5, and 6, as well as one neuropathologically confirmed AD patient (84 years old). Insoluble tau fraction was extracted as described [24] with some modifications. Briefly, 20% cortical gray matter homogenates were prepared using a buffer containing 50 mM Tris (pH 7.4), 0.8 M NaCl, and 10 mM EGTA. Homogenates were centrifuged at 100,000g for 25 min and the resulting pellets were homogenized in 1 mL of buffer containing 10 mM Tris (pH 7.4), 0.85 M NaCl, 1 mM EDTA, 20 mM NaF and 10% sucrose. Following centrifugation for 25 min at 20,000g, sarkosyl was added to supernatants to a final concentration of 1%. After incubation at RT for 2 h, the supernatants were centrifuged at 100,000g for 45 min. The sarkosyl-insoluble pellets were divided into two parts to the end of electron microscopic and western blot investigations. The pellet was resuspended in nanopure water or Laemmli buffer for immunogold labeling and immunoblotting, respectively. The electrophoretic samples were heated for 10 min at 100°C. The samples had been frozen at −20°C.
Immunoblotting for tau
Samples with balanced protein contents were run on precast 4–12% gradient gels (NuPAGE, Novex Bis–Tris Mini Gel, Invitrogen, Carlsbad, CA, USA). Proteins were transferred onto nitrocellulose membranes (0.2 μm pore size, iBlot Dry Blotting System, Invitrogen) and blocked with 5% nonfat dry milk powder in TBS (50 mM TrisHCl, 150 mM NaCl, pH 7.6, 90 min, RT). Afterwards, the membranes were incubated using the following primary monoclonal antibodies: anti-tau AT8 (1:500), AT100 (1:200), AT180 (1:500), AT270 (1:1,000) (all from Pierce Biotechnology, Rockford, IL, USA), and a polyclonal anti-human tau (1:200, DakoCytomation, Glostrup, Denmark) in 3% nonfat dry milk powder in TBS-T (50 mM TrisHCl, 150 mM NaCl, pH 7.6, 0.05% Tween 20) (overnight at 4°C). After incubation with a goat anti-mouse or anti-rabbit IgG horseradish peroxidase (HRP)-conjugated antibody (Sigma-Aldrich, St. Louis, MO, USA) at a final dilution of 1:2,000 (in 3% nonfat dry milk powder in TBS-T, 90 min, RT), immunological detection was performed using Immobilon Western Chemiluminescent HRP Substrate according to the manufacturer’s instructions (Millipore, Temecula, MA, USA). The blots were exposed to Agfa X-ray film blue sensitive (Agfa, Mortsel, Belgium) or Amersham Hyperfilm ECL (GE Healthcare, Fairfield, CT, USA) using exposure times between 5 and 30 s.
Genetic analysis
Eleven MAPT exons (exons 1–5, 7, and 9–13), including exon/intron boundaries, were amplified from genomic DNA using the following PCR conditions: 95°C for 15 min; 10 cycles of 94°C for 30 s, 60°C for 30 s (decrease 1°C each cycle), and 72°C for 90 s; 25 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 90 s; 72°C for 6 min. Primer sequences are available upon request. All primer pairs were designed with alternate forward MTR and reverse M13 tails to facilitate dye-primer sequencing of both DNA strands. Each exon was individually analyzed by direct sequencing of each PCR product on an automated DNA sequencer (Long-Read Tower, Visible Genetics) using the dye-primer cycle sequencing chemistry (GE Healthcare). The H1/H2 haplotype was determined as follows: (a) tau exon 3 was amplified using forward 5′-GCTGCTTTCTGGCATATGG and reverse 5′-CCCTCACTTCTGTCACAGGTC primers. The resulting PCR products were digested with the restriction enzyme BanII, run on a 2% agarose gel and visualized under UV illumination. (b) The 238 bp deletion between exons 9 and 10 was analyzed by PCR-amplification using forward 5′-TTTCCACTGTTTCCAGAGTTCCTG and reverse 5′-CCCTGCGGCTGTTCTCAAG primers. The resulting PCR products were loaded onto a 2% agarose gel and visualized under UV illumination. Apolipoprotein E (ApoE) alleles were determined using the commercially available Inno-LiPA ApoE amplification kit (Innogenetics, Belgium) according to the manufacturer’s instructions.
Results
The seven cases were identified as complex tauopathies based on the morphological and anatomical spectrum of tau pathology as detailed below. Five of these cases originated from the VITA study, indicating that 3% (5 out of 169 deceased) of individuals in a community-based cohort, irrespective whether the study participant had any neuropsychiatric symptom, shows neuropathological features of a complex tauopathy. Given the fact that approximately 24% of patients who underwent clinical follow-up in the VITA-study were clinically diagnosed with possible or probable AD, the frequency of the clinical manifestations of this disorder may be around 10% (5/[169 × 0.24]) in demented patients.
Clinical and general autopsy findings
Major clinical, neuropathological, and genetic findings are summarized in Table 1.
Patient 1
At the age of 75, this gentleman underwent the basic clinical evaluation of the VITA study. He had no complaints; however, the MMS was 26/30 at this time. There was impairment in recent memory, naming and comprehension, while psychomotor activity was in the normal range. In Pocket smell test only one of three smells were identified. His wife noticed social inactivity in the last years and clear loss of recalling familiar faces and names. The CDR summary score was 0.5, and criteria for Alzheimer-type dementia was met according to DSM-IV. The Hamilton and Geriatric depression scales did not indicate depression. The neurological state at the first admission excluded focal signs, and there was a lack of Parkinson syndrome (UPDRS score: 2). Cranial MRI revealed mild diffuse atrophy with some accentuation in the temporal lobes. Six months later, further impairment of episodic memory and fluency of speech was documented. MMS remained at 26 points; however, it must be noted that he received cholinesterase-inhibitor treatment. His father had died at the age of 65 years without neuropsychiatric symptoms, while his mother had died at the age of 81 after forgetfulness was noticed. The patient refused to participate in further follow-up and died 6 years later at the age of 82 after perforation of a duodenal ulcus.
Patients 2, 3, 4, 5, 6 and 7
Patients 2, 4, and 5 were reported to have died after a dementing illness without further specifications. Parkinson syndrome was indicated for patient 5. Patient 6 was admitted to a psychiatry department after confusion, paranoidity and refusal to eat. Cognitive decline was mentioned for the last 3 years before admission. Neurological examination revealed rigidity and tremor in the left hand, furthermore urinary incontinence. A depressive mood, anxiety attacks with aggressivity and prominent decline of recent memory were also described. Patient 7 was admitted to a psychiatry department at the age of 77, because of progressive dementia and gait disorder in the preceding 12 months. Neurological examination revealed rigidity, broad-based ataxic gait and urinary incontinence. Neuroradiological evaluation was not available. Causes of death were bronchopneumonia for patients 2, 4, 6, and 7, hypertrophy and dilatation of the heart for patient 3, and sepsis for patient 5.
Neuropathology of cases with complex tauopathy
Macroscopy
Six brains showed mild to moderate atrophy accentuated in the frontal and temporal lobes, including moderate to severe atrophy of the hippocampus (Suppl. Fig. 1; Table 1). The substantia nigra appeared depigmented in two cases. Territorial infarcts were not detected; however, subcortical lacunar or cortical microinfarctions were observed in patients no. 1 (putamen and frontal cortex), 4 (caudate nucleus), and 7 (pons base, prefrontal cortex). In patient 7, moderately enlarged ventricles were also observed.
Classical histopathological features
In addition to the cortical and subcortical microinfarcts that showed gliosis and few macrophages, the classical histopathology revealed neuronal loss, reactive astrogliosis, amyloid plaques with argyrophilic neurites, and amyloid angiopathy (Table 1). Neuronal loss and astrogliosis predominated in the amygdala (severe, Suppl. Fig. 2a), followed by the frontal and temporal cortices, caudate nucleus (all moderate), and the hippocampus (mild to moderate in CA1 and subiculum). Hippocampal sclerosis was noted in two cases (patients 2 and 6; Suppl. Fig. 2b). Basal cholinergic nuclei showed variable alterations, but were relatively less involved than in cases with Alzheimer-type dementia (Suppl. Fig. 2c), while the substantia nigra (Suppl. Fig. 2d) and the ventral tegmental area showed mild (patients 1, 3, 5, 6, 7) to moderate (patients 2 and 4) neuronal loss. Bielschowsky silver staining, performed in the regions proposed by CERAD criteria, demonstrated only few plaques (patients 2, 3, 4, 5) or moderate (patients 1, 6, 7) density of neuritic plaques, thus only possible or probable AD could be diagnosed. Ballooned neurons were observed in five cases, however in a variable anatomical distribution (Table 1).
Immunohistochemistry for phospho-tau
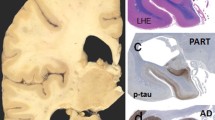
Tau pathology consisted of neurofibrillary tangles, diffuse cytoplasmic neuronal immunoreactivity (Gallyas negative), grains in some cases, and thin threads and neurites with a characteristic patchy accentuation, not overlapping with amyloid-β deposition, and with focal dot-like ubiquitin immunoreactivity (Figs. 1a–c, 2a). These threads were different from the short neuropil threads that are diffusely distributed in the cortex in AD, and were reminiscent of those in CBD, although clearly less extensive, and showed focal Gallyas positivity (see Suppl. Figs. 3 and 4). Typical tufted astrocytes, defined as star-like tufts of densely packed, tau immunoreactive and Gallyas positive fibers in the proximal segments of astrocytic processes; and astrocytic plaques, defined as tau-immunoreactive and Gallyas positive annular cluster of short stubby processes representing the distal segment of astrocytic processes (Figs. 1d, 3), were seen only occasionally. In contrast to these well-known features, in our cases astrocytic tau immunoreactivity was characterized by diffuse granular tau immunopositivity along astrocytic processes and in some associated with perinuclear accentuation (Figs. 1e–l, 2b, 3). The majority of these processes were Gallyas-negative (Fig. 1m, p) and were reminiscent of those described in AGD [2], however in a much wider distribution expanding beyond the stages of associated AGD (Table 2). Some showed focal Gallyas positivity (Fig. 1n, q), while only occasional ones resembled tufted astrocytes (Fig. 1o, r). For practical purposes, in AT8 immunostaining, this astrogliopathy can be distinguished from the patchy accentuation of threads/neurites based on the presence of an astroglial nucleus with a perinuclear enhancement of immunopositivity in the centre of a meshwork of tau immunoreactive processes (see also Suppl. Fig. 4). Only few to moderate number of oligodendroglial coiled bodies were seen, usually in the frontal, cingular, temporal lobe, and hippocampal white matter, and capsula interna as well as in the basal ganglia and only in some cases in the anterior commissure or corpus callosum (Table 2). Thread pathology in the white matter was observed in regions where coiled bodies were noted.
Tau immunoreactive neurites with patchy accentuation and astrocytic profiles. Tau immunoreactive neurites show a patchy accentuation (a frontal cortex, case 7) that does not show overlap with amyloid-β deposition (b), but some show fine dot-like ubiquitin immunopositivity (c). Tufted astrocytes in the caudate nucleus in a representative case of progressive supranuclear palsy (d, left side), astrocytic plaque in the parietal cortex in a representative case of corticobasal degeneration (d, right side), and a spectrum of various astrocytic tau immunopositivities in case 1 (e, f, temporal cortex), 2 (g, temporal cortex), 3 (h, frontal cortex), 4 (i, frontal cortex), 5 (j, caudate nucleus), 6 (k, frontal cortex), and 7 (l, temporal cortex). Tau immunoreactive astrocytes (m–o) in the present tauopathy usually lack Gallyas positivity (p adjacent to m) or show only focal argyrophilia (q adjacent to n). Gallyas positive tufted astrocytes were rare (r adjacent to o). Bar in a represents 75 μm for a–c, e; 50 μm for d; 25 μm for f–l and 10 μm for m–r. Asterisk in a–c indicates the same small vessel
Differential tau immunoreactivities (upper panel) with their contrast images (lower panel) of a typical tufted astrocyte (in progressive supranuclear palsy, PSP), diffuse granular immunoreactivity of astrocytic processes with occasional perinuclear accentuation in the present complex tauopathy, and an astrocytic plaque (in corticobasal degeneration, CBD)
Graphic representation of the mean score of astrocytic and neuronal tau immunoreactivity (diffuse granular cytoplasmic immunopositivity and neurofibrillary tangles) in seven cases. Arrowheads on the top indicate those regions where a patchy accentuation of threads was noted. The degree of various protein depositions was semiquantitatively (0: none, 1: mild, 2: moderate, 3: severe) evaluated, and mean scores are given in the graphic for the examined anatomical regions
The anatomical distribution of tau immunoreactivity was uniform except some variation mainly in subcortical areas. Frontal, temporal, cingular cortices, hippocampus, and amygdala showed most neuronal, astroglial, and thread-like tau immunoreactivity, followed by the caudate and accumbens nuclei, the putamen and variably in brainstem nuclei including mainly the substantia nigra, ventral tegmental area, and locus coeruleus (for details see Figs. 4, 5, 6). In the hippocampus/entorhinal cortex neuronal tau pathology (neurofibrillary tangles and cytoplasmic immunoreactivity) involved all subregions including the granular layer of the dentate gyrus, CA1-2-3-4 as well as the entorhinal cortex in six brains (except patient no. 4 where CA2/3/4 lacked neuronal positivity), while threads were noted in the entorhinal cortex, CA1/subiculum border, outer molecular layer of the dentate gyrus in all; full extent of CA1, subiculum as well as CA 2/3 and CA4 in five (except nos. 3 and 4) patients.
Using anti-tau antibodies directed against different phospho-epitopes or specific for 4R or 3R tau isoforms, a spectrum of immunostaining patterns was demonstrated. While neurofibrillary tangles and neuronal cytoplasmic tau immunoreactivity were generally detected by AT270, AT8, AT100, AT180, and HT7 (tangles), fine neuritic profiles and bush-like fine astrocytic immunopositivity were visible mainly with AT8 and AT180, and less with AT100 and AT270, but not with the HT7 antibody (Suppl. Fig. 5). 4R tau immunoreactivity (Suppl. Fig. 4) was seen in the astroglial lesions, oligodendroglial coiled bodies, white matter threads, and in patchy accumulation of fine threads in the cortex, neuronal cytoplasmic diffuse immunoreactivity and neurofibrillary tangles, while 3R immunopositivity was confined to the neurofibrillary tangles in the hippocampus and entorhinal cortex as well as basal nucleus of Meynert. Neurofibrillary degeneration was staged II (patients 2, 4, 5), III (patients 3, 7), IV (patient 1), and V (patient 6) according to Braak and Braak (Table 1).
Immunohistochemistry for α-synuclein, amyloid-β, and TDP-43
Immunostaining for α-synuclein revealed Lewy type pathology only in a single case (patient 1); this was restricted to the substantia nigra (Fig. 7a). Glial cytoplasmic inclusions were not observed. Different forms of amyloid-β immunoreactive plaques compatible with the morphology detected in AD were observed in all; in addition, mild degree of cerebral amyloid angiopathy (not involving capillaries) was seen in five cases (Fig. 7b). TDP-43 and phospho-TDP-43 immunoreactive structures were observed in five patients in the form of neuronal cytoplasmic inclusions and neuritic profiles (scattered also in the white matter), while glial cytoplasmic inclusions were detected only in a single case; neuronal nuclear inclusions were lacking in all patients. Anti-phospho-TDP-43 demonstrated to a wider extent pathological immunodeposits in comparison with anti-TDP-43. The anatomical distribution was restricted to the hippocampus, entorhinal, frontal, and temporal cortical areas only in two cases (patients 6 and 7), but was widespread, involving the caudate nucleus, putamen, nucleus accumbens, globus pallidus, thalamus, septal nuclei, and brainstem nuclei, including the substantia nigra, dorsal raphe and the inferior olive in three cases (patients 2, 4, and 5) (Fig. 7c–h). In the hippocampus CA1 and subiculum were involved in all, CA2/3 in two, granular layer of dentate gyrus in three cases, while CA4 in none. Immunoreactivity in the cerebral white matter was observed only in a single case (patient 4). In general, the patterns observed here did not fit clearly into proposed subtypes [9, 34, 43]. According to double immunolabeling for TDP-43 and phospho-tau (AT8), TDP-43 immunoreactivity usually appeared in regions where tau pathology was also observed, including rare co-localization in inclusions; however, there were also areas where TDP-43 immunopositivity predominated (i.e. septal area) (Suppl. Fig. 6).
Immunostaining for α-synuclein, amyloid-β and phospho-TDP-43. α-Synuclein immunoreactive Lewy-bodies in case 1 (a, arrows). Note the lack of immunoreactivity in the locus coeruleus (a, right lower inset). Mild cerebral amyloid angiopathy (b, amyloid-β immunoreactivity; case 3, occipital cortex). Hippocampus CA1 exhibiting abundant small threads and few neuronal cytoplasmic inclusions immunoreactive for phospho-TDP-43 (c, case 6). Entorhinal cortex (d), amygdala (e) and nucleus accumbens (f) with neuritic and neuronal cytoplasmic phospho-TDP-43 deposits (case 2). Skein-like inclusions (arrows) in the substantia nigra (g) and rounded-neuronal cytoplasmic phospho-TDP-43 immunoreactive structures in the septal area (h, here area subcallosa) in case 4. Bar in a represents 60 μm for a, 90 μm for b and 20 μm for c–h
Immunoblotting for tau
At first, we evaluated the reproducibility of the phospho-tau banding patterns with different antibodies (AT8, AT100, AT180, AT270 monoclonal, and human anti-tau polyclonal antibodies). We found that all of the examined antibodies resulted in identical banding patterns at 74, 69, 64 and 60 kDa in the definite AD case sample and was used henceforth as a reference in our comparative western blot studies to analyze the phospho-tau patterns in cases 2, 3, 4, 5 and 6 (Fig. 8a, b). In patient 6 the immunoblotting pattern developed by AT100, AT180 and AT270 antibodies showed all bands characteristic for AD (Fig. 8a). Similarly, the AT270 antibody recognized the AD-specific bands at 74, 69, 64 and 60 kDa in patients 2 and 3 as well (Fig. 8c). However, the AT8 antibody failed to recognize the 64 kDa band in case 6 (Fig. 8a).
Immunoblotting for tau. a The immunoblotting patterns recognized by monclonals AT8, AT100, AT180, AT270 and a polyclonal anti-tau antibodies in case 6 and case 4. b AT270 positive bands of patient 2, 3, 4, 5 and 6 can be seen next to an AD sample. c A graphic summary of western blot results. The shading of squares indicates the labeling intensity. Note the AD-like immunopositive pattern with characteristic strong bands at 69 and 60 kDa recognized with all antibodies in case 6. A similar pattern was identified by AT270 in case 2 and 3. A typical 64, 60 kDa tau doublet can be seen in case 4, and a 69, 64 kDa tau doublet is the most prominent feature of case 5. The immunoblots of AD samples marked with asterisks are not shown in a and b
Interestingly, in patient 4 none of the applied anti-tau antibodies labeled the 74 kDa band, while all of the monoclonal antibodies recognized bands at 55 kDa. Patient 5 was distinct from AD by the absence of a 74 kDa isoform, and from patient 4 by the absence of 55 kDa band(s) as well (Fig. 8b). Notably, immunoblotting patterns developed by the same antibodies showed differences in their intensity (Fig. 8a–c). While the AT270 antibody showed intense bands at 69 kDa in case 3, and at 69 and 60 kDa in cases 2 and 6, the predominance of the 64–60 kDa tau doublet was characteristic in case 4. The latter observation was confirmed by AT100, AT180 and the polyclonal anti-human tau antibodies in patient 4. In addition, a different pattern was detected by AT270 in case 5, where the 69, 64 kDa tau doublet was the most characteristic feature (Fig. 8c).
In summary, according to the “bar code” classification of tauopathies [47], patients 2, 3, and 6 resembled type I (i.e. AD), patient 5 type II (i.e. PSP), and patient 4 type III (i.e. Pick). However, some deviations were noted, exemplified by the strong 55 kDa band in case 4 and the 60 kDa band in case 5.
Genetic analysis of MAPT and ApoE
Five patients were analyzed for mutations in the coding exons and exon–intron boundaries 1–5, 7, and 9–13 of the tau gene (MAPT). We could not detect any pathogenic changes in MAPT, but in one patient (patient 3) a previously described polymorphism N255N was observed. Two patients (3 and 5) showed an H1/H2 haplotype, all others had an H1/H1 haplotype. Three individuals (2, 4 and 6) carried ε3/3 ApoE alleles, and single ones ε4/3 (case 3) or ε2/3 (case 5) (see Table 1).
Discussion
Here, we describe seven patients with a complex tauopathy. In addition to neurofibrillary tangles and diffuse cytoplasmic neuronal immunoreactivity, distinctive features comprise (1) the presence of diffuse granular tau immunoreactivity in astrocytic processes, distinct from tufted astrocytes or astrocytic plaques (Figs. 1, 3); (2) threads mainly in gray matter structures showing a patchy accentuation (Figs. 1; Suppl. 3 and 4); and (3) involvement of the frontal and temporal cortices, hippocampus, dentate gyrus, amygdala, caudate and accumbens nuclei, putamen, as well as the locus coeruleus and substantia nigra. Variability between the cases include presence of argyrophilic grains in 4 out of 7 cases; different stages of neurofibrillary degeneration; TDP-43 proteinopathy and amyloid angiopathy (Aβ) in 5 out 7; few to moderate density of neuritic plaques; Lewy bodies in a single case; as well as differences in MAPT H1/H2 and ApoE alleles.
While some of these neuropathological alterations are reminiscent of other tauopathies, this constellation of features does not allow to classify these disorders according to entities defined by diagnostic criteria [9, 13, 14, 21, 22, 36]; a comparison is summarized in Table 3. AGD is characterized by argyrophilic grains that are mainly restricted to limbic regions and associate with bush-like astrocytes in later stages of the disease [4, 40, 50]. In 2003, Maurage et al. [35] reported two cases (both with H1/H1 tau genotype), which they named diffuse AGD. AD-related changes, either neurofibrillary tangles or Aβ deposition, were lacking, in contrast to our patients who showed neurofibrillary tangles in the hippocampus. Since we had only the hippocampus for biochemical examination that showed AD-related changes, our observation of 60, 64, and 69 kDa bands would not exclude the possibility of detecting a similar disorder. Indeed, using isoform-specific antibodies we noted predominance of 4R tau immunoreactivity in extrahippocampal regions; however, typical grains were not seen in all cortical and subcortical regions. In addition, only four of our cases exhibited grains; moreover, the anatomical distribution of the astrogliopathy was also distinctive.
The anatomical involvement of the striatum, subthalamic nucleus, substantia nigra, locus coeruleus could suggest PSP. Nevertheless, globose NFTs were not prominent, and characteristic tufted astrocytes were rarely detected. Interestingly, in a study attempting to delineate early astrocytic changes of oxidation in PSP, Santpere and Ferrer [44] reported five patients (aged 66–79 years) with combined tauopathy, where typical tufted astrocytes were lacking, but fine tau immunoreactive networks, resembling those seen in our cases, were detected. On one hand, this could argue that these are early signs of a disease entity (i.e. PSP), on the other hand our patients died after several years of dementing illness, thus most likely the neuropathological alterations are representatives of ongoing or even later stage disease processes. PSP is increasingly recognized to be a spectrum of disorders, from typical PSP syndrome through parkinsonism, corticobasal syndrome, pure akinesia with freezing, to dementia [12, 55]. Tau pathology varies between these clinical syndromes from cortical or brainstem predominance, to classical forms (pallido-nigro-luysial degeneration) [12]. Nevertheless, the spectrum of alterations (i.e. lack of, or very few, tufted astrocytes, only few coiled bodies, preservation of the globus pallidus and relatively minor involvement of the dentate nucleus, cerebellar white matter, and pontine nuclei) argues against PSP in our cases that also differ from those reported as early-stage PSP lesions [42, 54].
The presence of threads and astrocytic tau immunoreactivity could raise the suspicion of CBD, another 4R tauopathy [13, 15, 22], which also shows several atypical forms [38, 41, 49] emphasizing the difficulties of morphological classification. However, ballooned neurons were not a consistent feature and not detected in usual areas as in CBD, the white matter tau pathology was also less, and typical astrocytic plaques were lacking or could be identified only in a few astrocytic lesions. In spite of the predominance of 60 and 64 kDa bands in the hippocampus sample of some of our cases, 3R immunopositive spherical neuronal cytoplasmic inclusions (i.e. Pick bodies [9]) were lacking in all cases.
The spectrum of rare unclassifiable tauopathies [31] is increasing but none are comparable with the cases reported here. Further tauopathy forms can be excluded based on the lack of abundant neurofibrillary tangle pathology restricted to the medial temporal lobe (neurofibrillary tangle only dementia), or because of the less prominent tangle formation in subcortical and brainstem structures (postencephalitic parkinsonism). It is tempting to speculate that several disease processes are initiated in these brains described here; each of them may be able to lower the threshold for the detectability of clinical symptoms. Indeed, the concept that the presence of one pathology may lower the brain reserve capacity for further ones was raised in community-based studies where older persons without dementia were also shown to have pathological alterations [46]. In the present cases, the neuropathological alterations may represent a spectrum of tau pathologies where none of the components reach the level to be defined as an entity but together as constellation they are plausible neuropathological substrates for a dementing illness.
It is of note that we observed a spectrum of astrocytic tau immunoreactive profiles (Figs. 1, 3): most frequent was the diffuse granular tau immunoreactivity in astrocytic processes that was Gallyas negative, less frequent was one that was focally Gallyas positive, and finally there were occasional tufted astrocytes (Gallyas positive). This “maturation” of astrocytic immunoreactivity is reminiscent of the Gallyas staining pattern of neuronal diffuse cytoplasmic tau immunoreactivity (Gallyas negative) and neurofibrillary tangles (Gallyas positive). This could suggest that we observed “pre-tufted-astrocytes” or “pre-astrocytic-plaques” analogously to the concept of pretangles and tangles [2, 26]. However, since we do not have evidence that these lesions evolve to the stage of becoming a tufted astrocyte or astrocytic plaque, the above-used simple description appears more appropriate to designate this type of immunoreactivity. It must be noted that some tau immunoreactive structures that are difficult to classify may be observed in elderly individuals. However, those are usually restricted to some anatomical regions (e.g. periventricular or amygdala) and do not appear to have a clinical correlate, while in our cases the distribution and amount of these structures are more extensive and seen in demented individuals.
Calculation of the frequency of complex tauopathies in our community-based study suggests that around 10% of demented elderly patients may manifest a complex tauopathy with AD-related changes not fulfilling criteria of definite AD. Community-based cohorts show a different spectrum of neuropathological diagnoses when compared to clinical cohorts [45]. Moreover, the spectrum of alterations changes with age; patients show less severe AD-related pathology (or discrepancy between amyloid plaque load and neurofibrillary degeneration [39]) but more vascular and mixed forms [7]. Atypical cases or “other” diagnoses vary considerably between studies (summarized in Ref. [27]) but are thought to be more prevalent in clinical cohorts [45]. Highly variable combinations of lesion types are also emphasized in the elderly [27]. However, those studies usually focus on AD-related, Lewy-type, or vascular pathology and provide less details on the presence and spectrum of other types of tau immunoreactivities; thus our observations supplement these by suggesting that, in addition to less severe AD-related pathology and vascular pathology, a spectrum of tau immunoreactivities other than any established tauopathy entity may be encountered. The hierarchical distribution of tau deposition in the hippocampus in aging was suggested to be related to neuroanatomical connections [32]. In our cases, some deviation from this process was seen, including involvement of the granular layer, suggesting that additional local processes may also contribute to the induction of pathological tau accumulation.
The question why this peculiar tauopathy was not reported previously may be considered. We screened brains from more than 170 elderly individuals with the same systematic approach using a large panel of immunostains in many anatomical areas. This number of elderly patients is rarely achieved in diagnostic series examined with extensive immunostaining that has become available only in the past 15 years or later. Moreover, the characteristic tau immunoreactivities seen in our cases may be overlooked by silver stainings. According to widely used protocols that focus on the hippocampus and temporal, occipital cortices, our cases could have been classified as possible/probable AD, occasionally with grains. Moreover, the atrophy pattern of the brains examined was not suggestive to screen for PSP or CBD.
We did not have the possibility to compare several anatomical regions by immunoblotting, and examined only the hippocampus, which in all cases showed neurofibrillary tangles at light microscopic level. Immunogold electron microscopy of the sarkosyl-insoluble pellets prepared for western blot analysis confirmed that all cases contain tau filaments (data not shown). Immunoblotting of all samples showed bands resembling those of AD in most, and did not reveal a common and distinct tau signature with additional new bands [20, 47]. However, all antibodies, mapping specific phospho-epitopes on tau, confirmed that the western blotting band pattern of patient 4 is reminiscent of, but not equal to, the doublet characterized by prominent bands at 60 and 64 kDa (type III pattern), while in case 5 a PSP-like tau doublet at 64, 69 kDa (type II pattern) was the most notable hallmark [48, 53, 56]. Although there may be variability between subregions in the homogenates used from the hippocampus/entorhinal cortex, together with a wide spectrum of phospho-epitope detectability by different phospho-tau antibodies in our samples, our results suggest that these disorders show biochemical features of tauopathies. Variability in H1/H2 alleles may also influence the expression of tau-isoforms [8]. Nevertheless, our comprehensive morphological and immunoblotting observations demonstrate deviations from AD and other tauopathy entities, suggesting that these complex tauopathies are distinctive. Furthermore, our immunoblotting experiments suggest that molecular biological examinations without immunohistochemical correlate may not be sufficient to classify a disorder. Although in two patients the immunoblot was reminiscent of types II or III; however, they were not identical with these types. This supports the immunohistochemical observations that these disorders are characterized by a mixture of pathological tau forms.
A TDP-43 proteinopathy may be present as concomitant pathology in a range of neurodegenerative diseases but also in elderly adults with and without mental illness [1, 17, 18, 23, 25, 28, 37, 52]. In these cases, TDP-43 immunoreactive inclusions or neurites are usually seen in the temporal cortex, amygdala and the hippocampus, and in some circumstances also in the inferior olive [11] or more widespread, in particular, in elderly subjects [18]. Interestingly, in few cases of CBD the distribution of TDP-43 pathology extended beyond the temporal cortex and involved the basal ganglia as well [52]. Moreover, annular clusters of thread-like processes resembling astrocytic plaques were seen [52]. Although Uryu et al. [52] failed to detect TDP-43 pathology in PSP, a recent study indicated immunoreactivity in limbic structures, although basal ganglia were not included in that cohort [57]. In our series, TDP-43 immunoreactivity similar to that reported in CBD [52] was not present, but we observed thin threads as shown in the subiculum of PSP [57] and mainly neuronal cytoplasmic inclusions and granular cytoplasmic immunoreactivity, in addition to dots in the neuropil resembling synaptic structures. In addition, phospho-TDP-43 pathology extended into other areas including the substantia nigra, accumbens nucleus and striatum as well as the septal area, not always corresponding to the involvement by tau pathology. On one hand, this implies prominent involvement of these regions with influence on the clinical presentation (i.e. involvement of the septal cholinergic pathway projecting to the hippocampus or involvement of the limbic or motor striatum); on the other hand, we do not know how specific this involvement is, as other studies did not evaluate these anatomical areas systematically; however, some areas (e.g. basal ganglia or brainstem) have been reported to show TDP-43 immunoreactive profiles in elderly controls [18].
Clinical symptoms observed in our patients do not have any specific feature. Clinical suspicion was usually Alzheimer-type dementia, but the frequent occurrence of psychiatric symptoms as well as parkinsonism was already indicative of a combined pathology. Interestingly, patients with AGD frequently show psychiatric symptoms, as well as urinary incontinence, as present in some of our patients [50]. We also observed atrophy of the hippocampus, a neuroradiological hallmark for AD, but hippocampal sclerosis only in a single patient. This emphasizes the need for further radio-pathological comparative studies in hippocampal atrophy. In one case (patient 7), we observed moderately enlarged ventricles together with a constellation of clinical symptoms reminiscent of normal pressure hydrocephalus that was not proven in vivo. Although neuropathological studies on normal pressure hydrocephalus are mainly based on biopsy samples reporting minimal change or AD-related alterations, it is of note that in a single case astrocytic tau immunoreactivity was also described [19].
In summary, neuropathological features do not suggest these complex cases to belong to an established morphological and/or biochemical tauopathy entity. We propose that it is the constellation of morphological features that makes these disorders unique. We define these cases by diffuse granular tau immunoreactivity in astrocytic processes in the frontal and temporal cortex, hippocampus, amygdala, and basal ganglia, together with patchy accentuation of thin threads and diffuse neuronal cytoplasmic immunoreactivity in the same regions but also the substantia nigra and locus coeruleus. These features are variably combined with AD-related neurofibrillary degeneration and argyrophilic grains and the frequent presence of TDP-43 proteinopathy that extends beyond the medial temporal region. This condition may be observed on a variable genetic background (i.e. MAPT haplotypes and APOE). We propose the term complex tauopathy to indicate that these characteristics distinguish these from well-established entities (“diagnostic boxes”), since they may have different etiologies and may require a differential approach for diagnostic and eventual therapeutic strategies (e.g. therapies interacting with Aβ metabolism may have less effect, and tau-aimed strategies may have more relevance).
References
Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, Iritani S, Onaya M, Akiyama H (2009) Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol 117:125–136
Botez G, Probst A, Ipsen S, Tolnay M (1999) Astrocytes expressing hyperphosphorylated tau protein without glial fibrillary tangles in argyrophilic grain disease. Acta Neuropathol 98:251–256
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404
Braak H, Braak E (1998) Argyrophilic grain disease: frequency of occurrence in different age categories and neuropathological diagnostic criteria. J Neural Transm 105:801–819
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, Xuereb JH, Paykel E, Mukaetova-Ladinska EB, Huppert FA, O’Sullivan A, Dening T (2009) Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge city over-75 s cohort (CC75C) study. J Alzheimers Dis 18:645–658
Caffrey TM, Joachim C, Paracchini S, Esiri MM, Wade-Martins R (2006) Haplotype-specific expression of exon 10 at the human MAPT locus. Hum Mol Genet 15:3529–3537
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL 3rd, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22
Cummings JL (2003) Toward a molecular neuropsychiatry of neurodegenerative diseases. Ann Neurol 54:147–154
Davidson Y, Amin H, Kelley T, Shi J, Tian J, Kumaran R, Lashley T, Lees AJ, DuPlessis D, Neary D, Snowden J, Akiyama H, Arai T, Hasegawa M, Bandopadhyay R, Sikkink S, Pickering-Brown S, Mann DM (2009) TDP-43 in ubiquitinated inclusions in the inferior olives in frontotemporal lobar degeneration and in other neurodegenerative diseases: a degenerative process distinct from normal ageing. Acta Neuropathol 118:359–369
Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA (2010) Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol 23:394–400
Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS, Tabaton M, Vonsattel JP, Wakabayashi K, Litvan I (2002) Office of rare diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61:935–946
Dickson DW, Rademakers R, Hutton ML (2007) Progressive supranuclear palsy: pathology and genetics. Brain Pathol 17:74–82
Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396
Fischer P, Jungwirth S, Zehetmayer S, Weissgram S, Hoenigschnabl S, Gelpi E, Krampla W, Tragl KH (2007) Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology 68:288–291
Fujishiro H, Uchikado H, Arai T, Hasegawa M, Akiyama H, Yokota O, Tsuchiya K, Togo T, Iseki E, Hirayasu Y (2009) Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta Neuropathol 117:151–158
Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, Moberg PJ, Moore EM, Van Deerlin VM, Lee VM, Arnold SE, Trojanowski JQ (2010) Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol 67:1238–1250
Hamilton R, Patel S, Lee EB, Jackson EM, Lopinto J, Arnold SE, Clark CM, Basil A, Shaw LM, Xie SX, Grady MS, Trojanowski JQ (2010) Lack of shunt response in suspected idiopathic normal pressure hydrocephalus with Alzheimer disease pathology. Ann Neurol 68:535–540
Hanger DP, Byers HL, Wray S, Leung KY, Saxton MJ, Seereeram A, Reynolds CH, Ward MA, Anderton BH (2007) Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J Biol Chem 282:23645–23654
Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I (1994) Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44:2015–2019
Hazrati LN, Bergeron C (2008) Neuropathology and genetics of corticobasal degeneration. Handb Clin Neurol 89:523–532
Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Kosaka K, Arai H (2007) Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res 1184:284–294
Horiguchi T, Uryu K, Giasson BI, Ischiropoulos H, LightFoot R, Bellmann C, Richter-Landsberg C, Lee VM, Trojanowski JQ (2003) Nitration of tau protein is linked to neurodegeneration in tauopathies. Am J Pathol 163:1021–1031
Hu WT, Josephs KA, Knopman DS, Boeve BF, Dickson DW, Petersen RC, Parisi JE (2008) Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol 116:215–220
Ikeda K, Akiyama H, Arai T, Nishimura T (1998) Glial tau pathology in neurodegenerative diseases: their nature and comparison with neuronal tangles. Neurobiol Aging 19:S85–S91
Jellinger KA, Attems J (2010) Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol 119:421–433
Kadokura A, Yamazaki T, Lemere CA, Takatama M, Okamoto K (2009) Regional distribution of TDP-43 inclusions in Alzheimer disease (AD) brains: their relation to AD common pathology. Neuropathology 29:566–573
Kovacs GG, Botond G, Budka H (2010) Protein coding of neurodegenerative dementias: the neuropathological basis of biomarker diagnostics. Acta Neuropathol 119:389–408
Kovacs GG, Botond G, Molnar K, Laszlo L, Ströbel T, Fischer P, Budka H (2010) Complex tauopathies in the elderly. Dementia Geriatr Cogn Dis 30(Suppl 1):33
Kovacs GG, Budka H (2010) Current concepts of neuropathological diagnostics in practice: neurodegenerative diseases. Clin Neuropathol 29:271–288
Lace G, Savva GM, Forster G, de Silva R, Brayne C, Matthews FE, Barclay JJ, Dakin L, Ince PG, Wharton SB (2009) Hippocampal tau pathology is related to neuroanatomical connections: an ageing population-based study. Brain 132:1324–1334
Ling H, O’Sullivan SS, Holton JL, Revesz T, Massey LA, Williams DR, Paviour DC, Lees AJ (2010) Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain 133:2045–2057
Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DM (2006) Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol 112:539–549
Maurage CA, Sergeant N, Schraen-Maschke S, Lebert F, Ruchoux MM, Sablonniere B, Pasquier F, Delacourte A (2003) Diffuse form of argyrophilic grain disease: a new variant of four-repeat tauopathy different from limbic argyrophilic grain disease. Acta Neuropathol 106:575–583
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, Arnold SE, Siderowf A, Grossman M, Leverenz JB, Woltjer R, Lopez OL, Hamilton R, Tsuang DW, Galasko D, Masliah E, Kaye J, Clark CM, Montine TJ, Lee VM, Trojanowski JQ (2007) Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol 114:221–229
Ohara S, Tsuyuzaki J, Oide T, Arai H, Higuchi S, Hasegawa M, Iwatsubo T (2002) A clinical and neuropathological study of an unusual case of sporadic tauopathy. A variant of corticobasal degeneration? Neurosci Lett 330:84–88
Prohovnik I, Perl DP, Davis KL, Libow L, Lesser G, Haroutunian V (2006) Dissociation of neuropathology from severity of dementia in late-onset Alzheimer disease. Neurology 66:49–55
Saito Y, Ruberu NN, Sawabe M, Arai T, Tanaka N, Kakuta Y, Yamanouchi H, Murayama S (2004) Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol 63:911–918
Sakai K, Piao YS, Kikugawa K, Ohara S, Hasegawa M, Takano H, Fukase M, Nishizawa M, Kakita A, Takahashi H (2006) Corticobasal degeneration with focal, massive tau accumulation in the subcortical white matter astrocytes. Acta Neuropathol 112:341–348
Sakai K, Yamada M (2011) Early-stage progressive supranuclear palsy with degenerative lesions confined to the subthalamic nucleus and substantia nigra. Neuropathology 31:77–81
Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VM (2006) Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol 169:1343–1352
Santpere G, Ferrer I (2009) Delineation of early changes in cases with progressive supranuclear palsy-like pathology. Astrocytes in striatum are primary targets of tau phosphorylation and GFAP oxidation. Brain Pathol 19:177–187
Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA (2009) The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis 18:691–701
Schneider JA, Arvanitakis Z, Bang W, Bennett DA (2007) Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69:2197–2204
Sergeant N, Delacourte A, Buee L (2005) Tau protein as a differential biomarker of tauopathies. Biochim Biophys Acta 1739:179–197
Sergeant N, Wattez A, Delacourte A (1999) Neurofibrillary degeneration in progressive supranuclear palsy and corticobasal degeneration: tau pathologies with exclusively “exon 10” isoforms. J Neurochem 72:1243–1249
Tan CF, Piao YS, Kakita A, Yamada M, Takano H, Tanaka M, Mano A, Makino K, Nishizawa M, Wakabayashi K, Takahashi H (2005) Frontotemporal dementia with co-occurrence of astrocytic plaques and tufted astrocytes, and severe degeneration of the cerebral white matter: a variant of corticobasal degeneration? Acta Neuropathol 109:329–338
Tolnay M, Clavaguera F (2004) Argyrophilic grain disease: a late-onset dementia with distinctive features among tauopathies. Neuropathology 24:269–283
Uchikado H, Lin WL, DeLucia MW, Dickson DW (2006) Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol 65:685–697
Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ, Neumann M (2008) Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 67:555–564
van Eersel J, Bi M, Ke YD, Hodges JR, Xuereb JH, Gregory GC, Halliday GM, Gotz J, Kril JJ, Ittner LM (2009) Phosphorylation of soluble tau differs in Pick’s disease and Alzheimer’s disease brains. J Neural Transm 116:1243–1251
Williams DR, Holton JL, Strand C, Pittman A, de Silva R, Lees AJ, Revesz T (2007) Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome. Brain 130:1566–1576
Williams DR, Lees AJ (2009) Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol 8:270–279
Wray S, Saxton M, Anderton BH, Hanger DP (2008) Direct analysis of tau from PSP brain identifies new phosphorylation sites and a major fragment of N-terminally cleaved tau containing four microtubule-binding repeats. J Neurochem 105:2343–2352
Yokota O, Davidson Y, Bigio EH, Ishizu H, Terada S, Arai T, Hasegawa M, Akiyama H, Sikkink S, Pickering-Brown S, Mann DM (2010) Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol 120:55–66
Acknowledgments
The technical assistance of Irene Leisser, Elisabeth Dirnberger, and Sabine Kaindl is highly appreciated. Lajos László’s, Kinga Molnár’s and Gergö Botond’s investigations were financially supported by the Hungarian Scientific Research Fund (OTKA-NK78012) and the European Social Fund (TÁMOP 4.2.1./B-09/1/KMR-2010-0003.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kovacs, G.G., Molnár, K., László, L. et al. A peculiar constellation of tau pathology defines a subset of dementia in the elderly. Acta Neuropathol 122, 205–222 (2011). https://doi.org/10.1007/s00401-011-0819-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-011-0819-x