Abstract
Cyclic GMP (cGMP)-mediated pathways regulate inflammatory responses in immune and CNS cells. Recently, cGMP phosphodiesterase inhibitors such as sildenafil, commonly used to treat sexual dysfunction in humans including multiple sclerosis (MS) patients, have been reported to be neuroprotective in animal models of stroke, Alzheimer’s disease, and focal brain lesion. In this work, we have examined if sildenafil ameliorates myelin oligodendrocyte glycoprotein peptide (MOG35–55)-induced experimental autoimmune encephalomyelitis (EAE), a mouse model of MS. We show for the first time that treatment with sildenafil after disease onset markedly reduces the clinical signs of EAE by preventing axonal loss and promoting remyelination. Furthermore, sildenafil decreases CD3+ leukocyte infiltration and microglial/macrophage activation in the spinal cord, while increasing forkhead box transcription factor 3-expressing T regulatory cells (Foxp3 Tregs). However, sildenafil treatment did not significantly affect MOG35–55-stimulated proliferation or release of Th1/Th2 cytokines in splenocytes but decreased ICAM-1 in spinal cord infiltrated cells. The presence of reactive astrocytes forming scar-like structures around infiltrates was enhanced by sildenafil suggesting a possible mechanism for restriction of leukocyte spread into healthy parenchyma. These results highlight novel actions of sildenafil that may contribute to its beneficial effects in EAE and suggest that treatment with this widely used and well-tolerated drug may be a useful therapeutic intervention to ameliorate MS neuropathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Experimental autoimmune encephalomyelitis (EAE) has been extensively used as an animal model of multiple sclerosis (MS) since it shares with the human autoimmune disease the presence of inflammatory infiltrates in the CNS parenchyma, demyelination and axonal loss predominantly in the spinal cord, and paralysis [13]. Like MS, EAE seems to be initiated by myelin antigen-specific CD4+ T-lymphocyte infiltration into the CNS. CD4+ cells together with infiltrated macrophages, dendritic cells, and resident microglia constitute the ultimate effector cells of neuroinflammation, progression of demyelination, and axonal damage [7, 13]. In contrast, accumulating evidence indicates that local astroglial activation is neuroprotective. As in locally triggered innate immune responses caused by trauma or stroke, reactive astrocytes in EAE have been reported to form scar-like barriers that restrict leukocyte infiltration into the CNS parenchyma [33, 35]. Additionally, astrocytes are known to release anti-inflammatory cytokines, ROS scavengers, and growth factors that could restrict local inflammation and promote nerve recovery [18]. Interestingly, recent evidence indicates that demyelination and oligodendrocyte degeneration follows inflammation-induced astrocyte dysfunction [30]. High levels of inflammatory mediators (cytokines, chemokines, and NO) are secreted by infiltrating immune cells and resident CNS cells and characterize the inflammatory environment during disease [28]. Up-regulation of NO synthase type 2 (NOS2) has been reported in MS and EAE, and NO and peroxynitrite, the highly reactive product of NO reaction with superoxide, have been implicated in tissue damage [28]. However, NOS2-deficient mice develop more severe EAE [38], suggesting that NO may be neuroprotective. A major target for NO in many cell types including CNS cells and lymphocytes is NO-sensitive guanylyl cyclase (GC) [12]. It is well established that NO via cGMP regulates important neuronal functions such as synaptic plasticity processes involved in memory formation; however, very little is known about the function of this pathway in glial cells or the contribution of cGMP to NO effects during neuroinflammation. In both neurons and astrocytes, the NO-cGMP pathway has been reported to activate antiapoptotic mechanisms [32]. Furthermore, we have recently shown that in astrocytes this signaling pathway is involved in the rearrangement of actin and GFAP filaments that results in astrocyte stellation and enhanced motility suggesting a role in the regulation of the reactive phenotype [3]. In agreement with this, other authors have demonstrated that the same pathway is involved in up-regulation of GFAP, a marker of astrogliosis [4]. Immune and glial cells also produce cGMP by stimulation of natriuretic peptide (NP) receptors [26]. Atrial natriuretic peptide (ANP) has been shown to affect innate and adaptive immune responses and to reduce production of proinflammatory mediators by macrophages and microglia [21, 34]. Neuroprotective actions of NPs have been reported in animal models of cerebral ischemia [37] and traumatic brain injury [16].
The cGMP signal can be terminated by the action of several members of the large family of cyclic nucleotide phosphodiesterase (PDE) [8]. cGMP-selective PDE5, well known for its important actions in the cardiovascular system, is also expressed in neural and immune cells [8]. PDE5 inhibitors, such as sildenafil, which are widely used for treatment of erectile dysfunction in humans, have been shown to improve cognition in unimpaired and impaired rodents [27, 29], to increase neurogenesis and enhance functional recovery after stroke [40] and to regulate glial inflammatory responses and decrease neuronal cell death after cortical cryoinjury [25]. In this work, we show that sildenafil administration after disease onset rapidly ameliorates MOG35–55-induced EAE in mice by preventing axonal loss and enhancing remyelination. Sildenafil regulation of inflammatory cell infiltration and glial reactivity may underlay these beneficial effects. Results suggest that this drug may be beneficial in MS.
Materials and methods
Animals and treatments
Two-month-old female C57BL/6 mice (Charles River) were housed in the animal care facility of the Universitat Autonoma de Barcelona (UAB) under constant temperature and provided food and water ad libitum. EAE was induced by immunization with MOG35–55 peptide (Scientific Technical Service, Universitat Pompeu Fabra, Barcelona, Spain). On day 0, mice (n = 34) were injected subcutaneously (s.c.) into the hind flanks with an emulsion of 100 μl MOG35–55 (3 mg/ml) and 100 μl CFA (Sigma) supplemented with 4 mg/ml Mycobacterium tuberculosis H37RA (Difco). Additionally, animals received an intraperitoneal injection of 500 ng pertussis toxin (Sigma), which was repeated 2 days after immunization. As controls, a group of mice were immunized with BSA (n = 6). Animals were treated with sildenafil (10 mg/kg, s.c.; extracted from Pfizer Viagra tablets according to [11]) or vehicle (water) once a day starting at 18 days post-immunization (dpi). Mice were clinically scored for EAE daily according to the following criteria: 0 no signs of disease; 0.5 partial loss of tail tonus; 1 loss of tail tonus; 2 moderate hind limb paraparesis; 2.5 severe hind limb paraparesis; 3 partial hind limb paralysis; 3.5 hind limb paralysis; 4 tetraplegy, and 5 death. Mice were sacrificed under isofluorane anesthesia at 21 of 26 dpi (3 or 8 days post-initiation of treatment (dpt) respectively), and spinal cords and spleens were removed. Experiments were approved by the UAB Animal and Human Experimentation Ethics Committee.
Histological methods
The lumbar-thoracic region of the spinal cords was fixed in paraformaldehyde and embedded in paraffin for histological analysis. Longitudinal sections (8 μm) were stained with hematoxylin and eosin (H&E) or Luxol Fast Blue (LFB) for evidence of infiltrates, and demyelination and histological scores were evaluated blindly according to the following criteria. For cell infiltration (H&E): 0 no inflammation; 1 cellular infiltrates only around blood vessels and meninges; 2 moderate cellular infiltrates in parenchyma, less than 50% of the white matter (WM); 3 severe infiltrates in parenchyma, deep and/or more than 50% of the WM. For demyelination (LFB): 0 no demyelination; 0.5 little demyelination only around infiltrates; 1 superficial demyelination, which involves less than 25% of the WM; 1.5 superficial demyelination which involves more than 25% but less than 50% of the WM; 2 deep demyelination and/or demyelination that involves more than 50% of the WM; 3 diffuse and widespread demyelination. Axonal density was evaluated by Bielschowsky’s silver impregnation.
Immunostaining of longitudinal lumbar-thoracic sections was performed as previously described [25]. Antigen retrieval was done by the appropriate standard protocol (citrate, EDTA or protease type XIV; Sigma). Sections were incubated with the primary antibodies: polyclonal rabbit purified anti-glial fibrillary acidic protein (GFAP; Dako, Cat. no. Z0334; 1:900) for astrocytes; polyclonal rabbit purified anti-Iba1 (WAKO, Cat. no. 19-19741; 1:250) for macrophages/microglia; polyclonal rabbit purified anti-CD3 (Dako, Cat. no. A0452; 1:100) for T lymphocytes; monoclonal anti-Foxp3 (a gift from Dr. G. Roncador, Monoclonal Antibodies Core Unit, Spanish National Cancer Research Center (CNIO), Madrid [1]) for Tregs; monoclonal purified anti-ICAM-1 (Pharmingen, Cat. no. 554967; 1:100); monoclonal purified anti-SMI-32 (Covance, Cat. no. SMI-32R; 1:300) for axonal damage. Appropriate secondary antibodies conjugated to biotin (Vector, 1:200) were used and detected using streptavidin/horseradish peroxidase (Vector, 1:300) and the peroxidase substrate DAB Kit (Vector). Control sections were incubated in the absence of primary antibodies. Sections were counterstained with 0.25% cresyl Echt Violet (Sigma) or hematoxylin before mounting. Images were acquired using Nikon Digital camera DXM 1200F and Nikon Act-1 software.
Staining quantifications
Four to eight areas (1 mm2) randomly chosen along the length of each of 3–4 different longitudinal spinal cord sections separated at least 300 μm were analyzed by two independent investigators blinded to treatment and clinical score. The analysis software Scion Image (NIH) was used to quantify LFB, Bielschowsky, SMI-32, ICAM-1, Iba1 and GFAP staining. Quantification of CD3- and Foxp3-positive cells was performed manually in infiltrates (4–8 infiltrates per section).
Splenocyte responses
Splenocytes were isolated from the spleens of animals killed at 21 dpi. Cells were flushed out from the spleens gently using a syringe plunger with PBS and centrifuged (220×g, 4°C). The pellet was resuspended and left 5 min at 37°C in 5 ml of RBC lysis buffer (Sigma, 17 mM Tris, 140 mM NH4Cl) to remove erythrocytes. After further centrifugation, cells were washed once again with PBS, centrifuged and resuspended in 10 ml of complete medium (RPMI + l-glutamine supplemented with 15% FCS (Ingelheim diagnostic, Barcelona, Spain), pen/strep antibiotics, 1 mM sodium piruvate and 50 μM β-mercaptoethanol). Cells were seeded at a density of 2–4 × 106 cells/ml in 96- or 24-well plates and exposed to MOG35–55 (10 μg/ml, 72 h). Proliferation was measured in 96-well plates using EZ4U kit (Biomedica Gruppe, Vienne, France). Cytokine release was determined in the media of 24-well plates with BD CBA mouse Th1/Th2 cytokine kit.
Statistical analysis
Differences in clinical scores, LFB, Bielschowsky, histological scores, CD3, and cytokines were analyzed by two-way ANOVA followed by Bonferroni’s post-hoc test. Student’s t test was used for two group comparisons (ICAM, Foxp3, Iba-1, GFAP, and SMI32). Results shown are mean ± SEM of the indicated number of animals.
Results and discussion
Sildenafil administration after disease onset ameliorates clinical symptoms and neuropathology in a chronic model of EAE
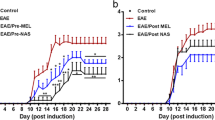
C57BL/6 mice immunized with MOG35–55 developed EAE clinical symptoms after 7 days and at 18 dpi, when the incidence of clinical EAE was 90–91% and the average score around 2; animals (16–17 per group) were injected s.c. with sildenafil (10 mg/kg) or vehicle (water) daily and were killed 3 (21 dpi) or 8 (26 dpi) days later for central and peripheral immune response analysis. As shown in Fig. 1 and Table 1, disability in vehicle-treated mice continued to increase, whereas mice treated with sildenafil showed a lower cumulative score and a rapid recovery that was already significant after 4 days of treatment. During 5–8 days of treatment, the clinical score stabilized around 1, and interestingly more than half of the animals presented virtually full recovery (score 0, n = 2; score 0.5, n = 7). In a different experiment, animals given 10 doses of 5 mg/kg sildenafil every other day starting at 20 dpi showed a smaller but significant improvement in clinical score by 43 dpi (controls: 2.2 ± 0.2, n = 10; sildenafil-treated: 1.6 ± 0.2, n = 7), indicating that the effect depends on the dose. A group of mice (n = 6) immunized in parallel with BSA did not present clinical symptoms of EAE (not shown).
Sildenafil ameliorates clinical symptoms associated with EAE. C57BL/6 mice immunized with MOG35–55 developed clinical symptoms of EAE after 1 week. Sildenafil administration (10 mg/kg, s.c.) daily starting 18 dpi significantly decreased disease severity after 4 days when compared to vehicle-treated animals. Values are mean ± SEM (n = 12–13). Statistical significance: *p < 0.05; ***p < 0.001
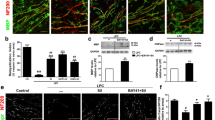
To investigate if clinical improvement was accompanied by decreased neuropathology, we examined demyelination and axonal loss in longitudinal sections of the lumbar-thoracic region of spinal cords by LFB and Bielschowsky’s silver staining in comparison to BSA-immunized mice that presented no pathology (Supplemental Fig. 1). As shown in Fig. 2a, the decrease in LFB staining intensity and the corresponding demyelination score were similar in vehicle-treated MOG-immunized mice at both 21 and 26 dpi. However, in 21 dpi animals treated with sildenafil for the last 3 days, demyelination was already less evident and by 26 dpi, after 8 days of sildenafil treatment, there was a significant increase in LFB staining and a decrease in demyelination score suggesting that sildenafil promotes remyelination. In contrast, quantification of Bielschowsky’s staining showed that axonal loss increased from 21 to 26 dpi in vehicle-treated animals but not in sildenafil-treated animals (Fig. 2b), indicating that sildenafil prevents further axon degeneration. In agreement with this, staining of non-phosphorylated neurofilaments with anti-SMI-32, a marker of axonal damage, was significantly lower in 8-day sildenafil-treated animals compared to vehicle-treated controls (Fig. 2c). Taken together, these results indicate that the functional recovery produced by sildenafil treatment in MOG-immunized animals results from axonal protection and remyelination.
Sildenafil ameliorates neuropathology associated with EAE. Longitudinal lumbar-thoracic spinal cord sections from MOG-immunized mice treated or not with sildenafil for 3 (n = 4) or 8 days (n = 12–13) starting at 18 dpi were stained to evaluate a demyelination (LFB), b axonal loss (Bielschowsky), and c axonal damage (SMI-32) (bars 50 μm in a, b; 20 μm in c). Demyelination scores and staining intensities [arbitrary units (a.u.)/mm2] were quantified as described in “Materials and methods”. Note that immunization with MOG causes severe demyelination and axonal damage at 26 dpi that is significantly reduced by 8 day treatment with sildenafil. Values are mean ± SEM. Statistically significant difference versus vehicle-treated animals: *p < 0.05; ***p < 0.001; or versus 3 dpt (21 dpi): ### p < 0.001
Sildenafil treatment decreases inflammatory cell infiltration and ICAM-1 expression while increasing the proportion of Foxp3+ Tregs in spinal cord of EAE mice
Autoreactive T cells infiltrating the CNS are the initiator and early effector cells in EAE development, but infiltrated macrophages, dendritic cells, and resident microglia constitute the ultimate effector cells that amplify neuroinflammation and tissue damage. Thus, we next examined the effect of sildenafil on cellular infiltration in the spinal cord. As estimated by H&E staining at 21 dpi, administration of sildenafil for the previous 3 days dramatically decreased the severity of cell infiltration, being the infiltrates smaller and largely confined to the submeningeal area (Fig. 3a). Quantification of CD3+ T cells in infiltrates showed that sildenafil significantly reduced the proportion of this cell population at 3 dpt (Fig. 3b). At 26 dpi, vehicle-treated animals presented significantly lower infiltration score (H&E) and number of CD3+ T cells per infiltrate respect to 21 dpi, and levels were no different to those determined in animals treated with sildenafil for 3 or 8 days (Fig. 3a, b). However, activated macrophages/microglia in WM (strongly Iba1+ globoid cells) were significantly reduced by sildenafil at both 3 and 8 dpt (Fig. 4a, b). These results agree with a recent report showing that inflammatory cell infiltration in MOG-immunized mice decreases after peak disease despite generalized axonal loss and demyelination and persistent clinical disability [15] and suggest that decreased macrophage/microglial activation may bear a more direct relation to the axon protective effect of sildenafil than decreased T cell infiltration. Additionally, a contribution of a direct effect of sildenafil in axons and oligodendrocytes potentiating cGMP-mediated neuroprotective pathways cannot be ruled out [2, 14, 22].
Sildenafil decreases inflammatory cell infiltration and ICAM-1 expression and increases Foxp3+ cells in spinal cords of EAE mice. Spinal cord sections from MOG-immunized mice treated or not with sildenafil for 3 (n = 4) or 8 days (n = 12–13) starting at 18 dpi were stained to evaluate a general cell infiltration (H&E), b T lymphocytes (anti-CD3), c Tregs (anti-Foxp3), and d ICAM-1. Arrowheads points to infiltrated cells and arrow points to blood vessel (bars 50 μm in a; 20 μm in b–d). Cell infiltration score, percentage of CD3+ and Foxp3+ cells in infiltrates and ICAM-1 staining were quantified as indicated in “Materials and methods”. Sildenafil significantly decreased inflammatory cell infiltration and ICAM-1 in infiltrates and increased Foxp3+ cells after 3 days of treatment. Inflammatory cell infiltration decreased significantly from 21 to 26 dpi in vehicle-treated animals, and 8-day sildenafil treatment did not reduce it further. Values are mean ± SEM. Statistically significant difference versus vehicle-treated animals: *p < 0.05; **p < 0.01; ***p < 0.001; or versus 3 dpt (21 dpi): ### p < 0.001
Sildenafil decreases macrophage/microglia activation in the spinal cord of EAE mice. a Spinal cord sections from MOG-immunized mice treated or not with sildenafil for 3 (n = 4) or 8 days (n = 12–13) were immunostained for Iba1 to evaluate macrophage/microglia activation (bar 50 μm); b quantification of Iba1 staining intensity in WM. Treatment with sildenafil significant reduced macrophage/microglia activation at both treatment times. Inserts magnification of GM areas showing that sildenafil promotes a resting morphology in microglia (bar 20 μm). Values are mean ± SEM. Statistical significance: *p < 0.05
To investigate if regulation of the peripheral immune response might be involved in sildenafil reduction of inflammation in the spinal cord, we examined the immune response of splenocytes upon ex vivo stimulation with MOG35–55. No significant differences were found in the proliferative response or the release of INF-γ, TNF-α, IL-2 or IL-6 in splenocytes from vehicle- and sildenafil-treated animals 3 dpt (Fig. 5), thus ruling out that sildenafil compromises the ability of T cells to be activated.
Sildenafil treatment does not affect in vitro responses to MOG35–55 in splenocytes from EAE mice. Splenocytes isolated from vehicle- or sildenafil-treated MOG-immunized mice (21 dpi) were incubated with or without MOG35–55 (10 μg/ml) for 72 h, and the proliferative response (a) and the release of cytokines (b) were measured. Values are mean ± SEM (n = 4). Statistically significant differences versus control splenocytes: # p < 0.05; ## p < 0.01; ### p < 0.001
ICAM-1, a type-1 membrane-bound glycoprotein expressed in most leukocyte subtypes, endothelial cells, and CNS glial cells, is involved in leukocyte entry, lymphocyte activation, and other immune responses and plays a central role in the development of MS and EAE [6, 17]. Thus, we examined if sildenafil was affecting ICAM-1 expression in spinal cords of EAE mice at 21 dpi when significant reduction in cell infiltration was observed (Fig. 3b). In control mice, ICAM-1-immunoreactivity was observed in blood vessels and in numerous cells within and around infiltrates (Fig. 3d). In contrast, in sildenafil-treated animals, it was almost absent in infiltrates but was still observed in blood vessels. Studies on ICAM-1 null mice have evidenced the critical role of ICAM-1 expression in T cells for the modulation of effector T cell responses [6]. Interestingly, NO via cGMP was reported to reduce T cell adhesion to human brain microvessels in vitro, but NO did not modulate adhesion molecule expression in the endothelial cells, suggesting a direct action on the T cells [39]. Thus, down-regulation by sildenafil of ICAM-1 expression in T cells could be involved in decreasing T cell infiltration and/or activation in the spinal cord of EAE mice. Additionally, by increasing cGMP in macrophages sildenafil could attenuate TNF-α release and TNF-α-induced expression of chemokines and adhesion molecules in endothelial cells [34].
The role of Foxp3+ Tregs in suppressing autoreactive T cells is well established. Additionally, Foxp3+ Tregs have been shown to play a critical role in protection and recovery from EAE [24]. Since sildenafil afforded both protection and recovery, we investigated if it affected the population of Foxp3+ cells. As shown in Fig. 3c, at 21 dpi, Foxp3+ cells in spinal cord infiltrates of sildenafil-treated mice were notably increased compared to vehicle-treated controls. Thus, up-regulation of Tregs may be another factor contributing to the beneficial effects of sildenafil in recovery from EAE. This result is in clear contrast with a recent report on splenocytes showing that NO inhibits expression of Foxp3 in myelin basic protein-primed T cells via cGMP [5]. This discrepancy may be reconciled in light of the observation that development of Foxp3+ Tregs in response to antigen stimulation is antagonized by Th1/Th2 lineage differentiation activities [36]. This may occur in splenocytes from immunized mice but not in the spinal cord of animals treated with sildenafil where T cell infiltration is greatly reduced.
Sildenafil treatment regulates reactive gliosis
In addition to heavily Iba1-stained globoid macrophages/microglia within and around infiltrates, MOG-immunized mice presented activated ramified microglia (strongly Iba1-stained) throughout the spinal cord, more evident at 21 than at 26 dpi. In contrast, animals treated with sildenafil for 3 or 8 days showed a decreased intensity of Iba1 staining in activated microglia and increased numbers of cells with long and thin ramifications typical of resting microglia (Fig. 4a, inserts), suggesting that decreased microglial activation may also contribute to the neuroprotective effect of sildenafil. In vitro studies have shown that cGMP-mediated pathways reduce expression and release of inflammatory mediators from both macrophages and microglia [21, 23, 34]. Furthermore, we have recently shown that treatment with cGMP-PDE inhibitors decreases recruitment and activation of macrophages/microglia around a cortical cryoinjury in rodents [25]. Since microglia does not seem to express NO-sensitive GC [26], it is tempting to speculate that increases in cGMP occur in response to NPs. Interestingly, a recent study showed that administration of brain natriuretic peptide downregulates microglial activation in murine models of traumatic brain injury and intracerebral hemorrhage [16].
Increasing evidence indicates that astrogliosis can play a crucial role in the pathogenesis and resolution of demyelinating disease. Astrocytes can promote and perpetuate immune-mediated demyelination by priming autoreactive T cells and expressing cytokines, chemokines, and costimulatory and adhesion molecules [9]. However, astrocytes also promote anti-inflammatory responses and form perivascular barriers that restrict the influx of leukocytes into CNS parenchyma [18, 31]. We have previously shown that increasing cGMP in astrocytes regulates cytoskeleton dynamics and accelerates migration in a scratch wound assay in vitro [3]. In addition, we have shown that treatment with PDE5 inhibitors enhances astrogliosis around a cortical cryolesion suggesting that cGMP-mediated pathways can accelerate glial scar formation [25]. Thus, we examined the effect of sildenafil on GFAP immunoreactivity in the spinal cord of EAE mice. Different effects were observed in WM and gray matter (GM). Severely reactive astrocytes with long overlapping processes (anisomorphic gliosis) were observed throughout WM but particularly in areas of heavy inflammatory cell infiltration (Fig. 6a). Although no significant differences in GFAP overall staining intensity were observed between vehicle- and sildenafil-treated animals, a stronger tendency to form scar-like structures around confined infiltrates could be generally observed in the latter (Fig. 6a), suggesting a role in controlling the spreading of infiltration. In the GM moderate astrogliosis was widespread in vehicle-treated mice, and in contrast to WM, sildenafil had a biphasic effect, significantly decreasing GFAP immunoreactivity after 3 days but increasing it after 8 days of treatment (Fig. 6b). At this treatment time, activated astrocytes were evenly distributed in GM and presented a more stellate shape, features typical of isomorphic gliosis. Numerous evidences indicate that these activated astrocytes exert suppressive effects on inflammatory cells and release antioxidants and growth factors that protect neurons and oligodendrocytes [18]. Thus, it will be of interest to investigate how the different effects of sildenafil on astroglial reactivity in WM and GM observed at the different treatment times relate to the reduced inflammation, axon protection, and remyelination the drug produces in EAE mice.
Sildenafil modifies astrogliosis in the spinal cord of EAE mice. Spinal cord sections from MOG-immunized mice treated or not with sildenafil for 3 (n = 4) or 8 days (n = 12–13) were immunostained for GFAP to evaluate astroglial activation (bar 20 μm). Quantification of GFAP staining in the WM (a) showed no significant differences between treated and untreated animals, but scar-like structures around infiltrates were more prominent in sildenafil-treated animals. In the GM (b), sildenafil had a biphasic effect on astroglial activation, decreasing GFAP staining intensity at 3 dpt but increasing it at 8 dpt. Values are mean ± SEM. Statistical significance: *p < 0.05; ***p < 0.001
To our knowledge, this is the first report demonstrating efficacy of a PDE5 inhibitor in a mouse model of MS. Sexual dysfunction is a common symptom in MS patients, and treatment with PDE5 inhibitors including sildenafil [10] and tadalafil [19] are commonly used to treat these symptoms. However, to our knowledge, there have not been any published studies to determine if these treatments modify MS pathology although a presentation at the 2005 ECTRIMS meeting suggested radiological benefit in a small cohort of MS patients [20].
References
Alvaro TM, Lejeune MT, Salvado R et al (2005) Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res 11:1467–1473
Benjamins JA, Nedelkoska L (2007) Cyclic GMP-dependent pathways protect differentiated oligodendrocytes from multiple types of injury. Neurochem Res 32:321–329
Boran MS, Garcia A (2007) The cyclic GMP-protein kinase G pathway regulates cytoskeleton dynamics and motility in astrocytes. J Neurochem 102:216–230
Brahmachari S, Fung YK, Pahan K (2006) Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci 26:4930–4939
Brahmachari S, Pahan K (2010) Myelin basic protein priming reduces the expression of Foxp3 in T cells via nitric oxide. J Immunol 184:1799–1809
Bullard DC, Hu X, Schoeb TR, Collins RG, Beaudet AL, Barnum SR (2007) Intercellular adhesion molecule-1 expression is required on multiple cell types for the development of experimental autoimmune encephalomyelitis. J Immunol 178:851–857
Bynoe MS, Bonorino P, Viret C (2007) Control of experimental autoimmune encephalomyelitis by CD4+ suppressor T cells: peripheral versus in situ immunoregulation. J Neuroimmunol 191:61–69
Conti M, Beavo J (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76:481–511
Dong Y, Benveniste EN (2001) Immune function of astrocytes. Glia 36:180–190
Fowler CJ, Miller JR, Sharief MK, Hussain IF, Stecher VJ, Sweeney M (2005) A double blind, randomised study of sildenafil citrate for erectile dysfunction in men with multiple sclerosis. J Neurol Neurosurg Psychiatry 76:700–705
Francis SH, Sekhar KR, Rouse AB, Grimes KA, Corbin JD (2003) Single step isolation of sildenafil from commercially available Viagra tablets. Int J Impot Res 15:369–372
Friebe A, Koesling D (2009) The function of NO-sensitive guanylyl cyclase: what we can learn from genetic mouse models. Nitric Oxide 21:149–156
Friese MA, Montalban X, Willcox N, Bell JI, Martin R, Fugger L (2006) The value of animal models for drug development in multiple sclerosis. Brain 129:1940–1952
Garthwaite G, Goodwin DA, Garthwaite J (1999) Nitric oxide stimulates cGMP formation in rat optic nerve axons, providing a specific marker of axon viability. Eur J Neurosci 11:4367–4372
Herrero-Herranz E, Pardo LA, Gold R, Linker RA (2008) Pattern of axonal injury in murine myelin oligodendrocyte glycoprotein induced experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Neurobiol Dis 30:162–173
James ML, Wang H, Venkatraman T, Song P, Lascola CD, Laskowitz DT (2010) Brain natriuretic peptide improves long-term functional recovery after acute CNS injury in mice. J Neurotrauma 27:217–228
Lebedeva T, Dustin ML, Sykulev Y (2005) ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol 17:251–258
Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW (2004) Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem 89:1092–1100
Lombardi G, Macchiarella A, Del Popolo G (2010) Efficacy and safety of tadalafil for erectile dysfunction in patients with multiple sclerosis. J Sex Med 7:2192–2200
Manson SC, Burke G, Voets N, Palace J and Matthews PM (2005) Presented at the 21st congress of the European Committee for the Treatment and Research in Multiple Sclerosis, Poster no. 237
Moriyama N, Taniguchi M, Miyano K, Miyoshi M, Watanabe T (2006) ANP inhibits LPS-induced stimulation of rat microglial cells by suppressing NF-kappaB and AP-1 activations. Biochem Biophys Res Commun 350:322–328
Nakamizo T, Kawamata J, Yoshida K et al (2003) Phosphodiesterase inhibitors are neuroprotective to cultured spinal motor neurons. J Neurosci Res 71:485–495
Paris D, Town T, Parker TA et al (1999) Inhibition of Alzheimer’s beta-amyloid induced vasoactivity and proinflammatory response in microglia by a cGMP-dependent mechanism. Exp Neurol 157:211–221
Paust S, Cantor H (2005) Regulatory T cells and autoimmune disease. Immunol Rev 204:195–207
Pifarre P, Prado J, Giralt M, Molinero A, Hidalgo J, Garcia A (2010) Cyclic GMP phosphodiesterase inhibition alters the glial inflammatory response, reduces oxidative stress and cell death and increases angiogenesis following focal brain injury. J Neurochem 112:807–817
Prado J, Baltrons MA, Pifarre P, Garcia A (2010) Glial cells as sources and targets of natriuretic peptides. Neurochem Int 57:367–374
Puzzo D, Staniszewski A, Deng SX et al (2009) Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer’s disease mouse model. J Neurosci 29:8075–8086
Raivich G, Banati R (2004) Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res Brain Res Rev 46:261–281
Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J (2009) Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology 202:419–443
Sharma R, Fischer MT, Bauer J et al (2010) Inflammation induced by innate immunity in the central nervous system leads to primary astrocyte dysfunction followed by demyelination. Acta Neuropathol 120:223–236
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Takuma K, Lee E, Enomoto R, Mori K, Baba A, Matsuda T (2001) Ibudilast attenuates astrocyte apoptosis via cyclic GMP signalling pathway in an in vitro reperfusion model. Br J Pharmacol 133:841–848
Toft-Hansen H, Fuchtbauer L, Owens T (2010) Inhibition of reactive astrocytosis in established experimental autoimmune encephalomyelitis favors infiltration by myeloid cells over T cells and enhances severity of disease. Glia 59:166–176
Vollmar AM (2005) The role of atrial natriuretic peptide in the immune system. Peptides 26:1086–1094
Voskuhl RR, Peterson RS, Song B et al (2009) Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 29:11511–11522
Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX (2007) Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci USA 104:18169–18174
Wiggins AK, Shen PJ, Gundlach AL (2003) Atrial natriuretic peptide expression is increased in rat cerebral cortex following spreading depression: possible contribution to sd-induced neuroprotection. Neuroscience 118:715–726
Willenborg DO, Staykova M, Fordham S, O’Brien N, Linares D (2007) The contribution of nitric oxide and interferon gamma to the regulation of the neuro-inflammation in experimental autoimmune encephalomyelitis. J Neuroimmunol 191:16–25
Wong D, Prameya R, Wu V, Dorovini-Zis K, Vincent SR (2005) Nitric oxide reduces T lymphocyte adhesion to human brain microvessel endothelial cells via a cGMP-dependent pathway. Eur J Pharmacol 514:91–98
Zhang L, Zhang RL, Wang Y et al (2005) Functional recovery in aged and young rats after embolic stroke: treatment with a phosphodiesterase type 5 inhibitor. Stroke 36:847–852
Acknowledgments
This work was supported by grants SAF2007-64164 and SGR2005-939 to AG and SAF2008-00435 and RETICS (REEM, RD07/0060/0002) to JH. We thank Mar Castillo and David Lligé for technical support and Dr. Dolores Jaraquemada for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplemental Fig. 1
. BSA-immunized mice do not present demyelination or axonal loss. Spinal cord sections from BSA-immunized mice 26 dpi (n = 4), treated or not with sildenafil for 8 days, stained with LFB (a) or Bielschowsky (b) (JPEG 638 kb)
Rights and permissions
About this article
Cite this article
Pifarre, P., Prado, J., Baltrons, M.A. et al. Sildenafil (Viagra) ameliorates clinical symptoms and neuropathology in a mouse model of multiple sclerosis. Acta Neuropathol 121, 499–508 (2011). https://doi.org/10.1007/s00401-010-0795-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-010-0795-6