Abstract
Stress affects microglial function and viability during adulthood and early postnatal life; however, it is unknown whether stress to the pregnant dam might alter offspring microglia. The effects of prenatal stress on microglial development and distribution in the postnatal brain were studied using Wistar rats. Prenatal stress consisting of 20 min of forced swimming occurred on embryonic days 10–20. On postnatal days 1 and 10, stressed and control pups were killed. Microglia were identified using Griffonia simplicifolia lectin and quantified in the whole encephalon. In addition, plasma corticosterone was measured in dams at embryonic day 20, and in pups on postnatal days 1 and 10. At postnatal day 1, there was an increase in number of ramified microglia in the parietal, entorhinal and frontal cortices, septum, basal ganglia, thalamus, medulla oblongata and internal capsule in the stressed pups as compared to controls, but also there was a reduction of amoeboid microglia and the total number of microglia in the corpus callosum. By postnatal day 10, there were no differences in the morphologic type or the distribution of microglia between the prenatal stress and control groups, except in the corpus callosum; where prenatal stress decreased the number of ramified microglia. The stress procedure was effective in producing plasma rise in corticosterone levels of pregnant rats at embryonic day 20 when compared to same age controls. Prenatal stress reduced the number of immature microglia and promoted an accelerated microglial differentiation into a ramified form. These findings may be related to an increase in plasma corticosterone in the pregnant dam.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The origin of microglia is one of the most controversial issues on glial research. Several hypotheses have been proposed in relation to the origin of microglia; microglia have been considered to be of mesodermal, neuroectodermal (similar to astrocytes and oligodendrocytes) or monocytic origin [10, 28]. Consensus opinion currently holds that microglia derive from the embryonic mesoderm [10]; specifically, microglia are thought to derive from peripheral blood monocytes that infiltrate the central nervous system via local blood vessels during embryonic and early postnatal life [32, 42, 51]. In the rat, microglia are first seen between embryonic days 12–14 [51]. During early phases of neural development, microglia are amoeboid. As development progresses, microglia acquire a mature ramified form [28, 32]. At birth, amoeboid microglia concentrate in three colonies in the rat forebrain: the corpus callosum, internal capsule and ventral part of the external capsule [16, 30, 33]. From those reservoirs, amoeboid microglia migrate to all remaining neuroanatomic sites during the later embryonic stages and the first 3 postnatal weeks [11]. In the early postnatal rat, ramified microglia with short stout processes are the predominant form in the neocortex and hippocampus, and there is a reduced number of amoeboid microglia [12, 55]. In those regions, amoeboid microglia are found mainly in the deeper neocortical layers immediately over the corpus callosum [55] and in the ontogenetically newer hippocampal areas, such as the dentate gyrus and CA1 region [12].
Microglia express both high and low-affinity glucocorticoid receptors [50] as well as receptors for the corticotrophin-releasing hormone (CRH) [53]. The possibility exists that stress might alter the normal functioning and development of microglia. In vitro, glucocorticoids exert inhibitory effects on microglial function by acting on the type II or low-affinity glucocorticoid receptor, while glucocorticoids bound to type I receptors, or high-affinity mineralocorticoid receptor, stimulate microglial-inducible nitric oxide synthase and acid phosphatase activities. When present, both enzymes reflect appropriate functioning of microglial cells [50]. High levels of glucocorticoids, acting on type II receptors, shrink microglial soma [50], diminish cell proliferation [17, 50] and migration [58], and increase accumulation of undigested materials inside microglial lysosomes and vacuoles [50]. Like glucocorticoids, the CRH adversely affects microglial function; in vitro, CRH reduces 20% microglial cell viability and induces apoptotic cell death [40].
Experiments in vivo also indicate that stress hormones present during early life alter microglial development and also affect other neurodevelopmental processes, such as neurogenesis, synaptogenesis, astrocyte morphology and development of the blood–brain barrier [6, 19, 22, 36]. In the newborn rat, cortisone administration reduces the number of amoeboid microglia by 50% in the corpus callosum during the first postnatal week [31, 56]. It has been suggested that administration of the synthetic glucocorticoid dexamethasone on postnatal days 1, 3 and 5 (which diminished 40–60% of the number of amoeboid microglia in the corpus callosum of 1-week-old rat pups) reduces microglial cell proliferation, accelerates maturation and increases microglial cell death [26, 56].
Both in vitro and in vivo experiments indicate that stress hormones may affect microglial function and viability; however, it is still unknown whether stress to the dam during the period when the first microglial progenitors infiltrate into the fetal central nervous system affects microglial development and distribution in the postnatal brain. Therefore, this study aimed to investigate the effect of prenatal stress on microglial development and distribution in the whole neonatal rat brain. It was considered that prenatal stress would alter microglial distribution in the whole brain concomitantly to the microglial changes in the main glial reservoir, the corpus callosum, and it was thought that the change would occur during early postnatal life as reported previously [26, 31, 56].
Materials and methods
Animals
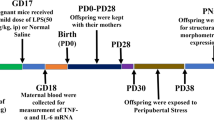
Twelve pregnant Wistar rats (200 g), 10 weeks of age at the beginning of the experiment, and their pups (ten per litter) were used; extra pups were randomly removed after delivery to obtain a litter size equal to ten pups at postnatal day 1. Dams were born and reared in our vivarium, at the Instituto de Investigaciones Biomédicas of the Universidad Nacional Autónoma de México, and were maintained on a 12-hour light/dark cycle (lights on at 0700 hours) with constant temperature (20–22°C) and humidity (45–55%). Commercial rat chow and tap water were available ad libitum to dams throughout the experiment. Two weeks before the beginning of gestation, all animals were handled daily during 15 min and afterward were exposed to the swimming tank filled with 23°C water during 5 min to avoid adverse effects (e.g., sudden death) of abrupt prolonged forced swimming [7]. The 23°C water temperature is deemed neutral for the rat and consequently is commonly used in other non-stressful swimming procedures such as the Morris water maze (e.g., [49]). After the habituation to experimental handling, groups of two to three dams were caged with an adult sexually experienced male rat for 5 days. Embryonic day 0 (E0) occurred when the daily vaginal smear contained spermatozoids with typical cornified estrus cells [34], therefore, oocyte fertilization should have occurred during the day following the pairing with the male rat [48]. Beginning on E0, pregnant rats were caged in groups of three until day E20, when each rat was caged individually and delivery occurred on E22. The day pups were born was considered as postnatal day 0 (P0). Each pregnant rat was assigned at random to one of two groups: (I) forced swimming stress group or (II) control group. The experiments were approved by the local committee on animal research. The experiments were done in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research of the Institute for Laboratory Animal Research, USA [39] and were designed to reduce to the minimum the number of animals used.
Materials
A transparent plastic water tank (60 × 44 × 30 cm) with thermostat was used for the prenatal stress procedure (forced swimming). The water was maintained at a level of 22–24 cm so that rats could not rest their hind limbs on the bottom.
For microglial histochemistry, the lectin from Griffonia simplicifolia conjugated to horseradish peroxidase (GSAI-B4-HRP) (Sigma, L5391, USA) and the peroxidase substrate diaminobenzidine kit (Vector Labs, SK4100, USA) were used.
For corticosterone quantification by radioimmunoassay, the Coat-A-Count rat corticosterone kit (Diagnostic Products Corporation, PITKRC-2, USA) and a gamma counter (COBRA II, Packard Co., USA) were used.
Quantitative analysis of stress effects on microglial development and distribution was done using the software ImageJ version 1.32j [43] and the plug-in cell counter [15], provided freely by the National Institutes of Health, USA. The statistical analyses were carried on the Statistical Package for the Social Sciences (SPSS) software, version 10.0 for Windows.
Prenatal stress
Prenatal stress (in utero stress) was carried on from E10 to E20, beginning 2 days ahead of the first appearance of microglial cells in the rat central nervous system during the day when neurulation occurs [25]. Prenatal stress consisted of 20 min of daily forced swimming in 32°C water, a temperature that is known to be stressful for the rat [1, 9, 18]. The stress procedure used, with the concomitant control of session duration and water temperature, retards the stress–response adaptation as observed under chronic exposure to other physical stressors such as immobilization or predator presence [2, 14]. To avoid water pollution with the urine and fecal boli of preceding rats, the tank was re-filled with clean water once the swimming session of each subject was over. At the end of the swimming session, the pregnant rats were dried gently with a cotton towel for 1 min and placed back in their home cages (40 × 22 × 20 cm) until the next swimming session (24 h later). Controls were kept in the same environment as stressed rats, but without any stress manipulation; the only handling of controls occurred every third day to change bed material.
Microglial histochemistry
Characterization of the microglial cells is difficult, as these cells share several antigens with different cell types (e.g., macrophages, endothelial cells, lymphocytes) [20]; however, microglial cells bind lectins avidly during the early developmental stages [13, 16, 46, 47, 54–56]. Therefore, to study stress effects on microglial development and distribution in the rat brain, free floating sections were stained with the lectin G. simplicifolia, which binds to the α-d-galactosyl residues of membranous glycoproteins [21] in the rodent microglia [47]. The lectin derived from G. simplicifolia has been shown to be a reliable marker and an accurate method for the identification of microglial cells in rodent nervous tissues, though in addition to microglia, it stains blood vessel endothelium [46, 47]. Given the morphological differences between microglial and endothelial cells, identification and quantification of microglia did not represent a problem in the present study as noted also by others using the same method [16, 46, 47, 54–56].
Microglial differentiation occurs predominantly during the early postnatal period. In the newborn rat microglia concentrate in the glial reservoirs (in the corpus callosum and internal capsule) and during the second postnatal week microglia migrate to the whole encephalon and differentiate into the mature ramified form [11, 16, 30, 33]. Therefore, to study stress effects on microglial postnatal development, three to five pups of each group (prenatal stress and control) on postnatal days 1 and 10 (total, 18 pups) were anesthetized with intraperitoneal pentobarbital sodium (0.063 g/kg body weight) and perfused transcardially with normal saline solution followed by 4% paraformaldehyde in 0.1 M saline-phosphate buffer (PBS). One to two pups per litter were used at each age (pups were obtained from six different litters). The brains were removed and post-fixed in 4% paraformaldehyde in 0.1 M PBS during 4 days at 4°C, followed by cryoprotection in 30% sucrose solution for 4 days. The whole brain was cut serially in a freezing microtome into 40 μm thick coronal sections. Sections were preincubated in 1% H2O2 for 10 min before the application of the lectin to block endogenous peroxidase activity. After a brief washing with 1 mM CaCl2 and MgCl2 in 1% Triton-PBS, sections were incubated in G. simplicifolia lectin (10 μg/mL) with 1 mM CaCl2 and MgCl2 diluted in 1% Triton-PBS [47] for 48 h at 4°C. The sections were then reacted with the peroxidase substrate diaminobenzidine kit, collected on slides, dehydrated and coverslipped.
Quantification of stress effects on microglial development and distribution
Microglial development was studied in the following central nervous system regions: visual, auditory, somatosensory and motor cortices, cingulate gyrus, insular, frontal, orbitofrontal and entorhinal cortices, hippocampus, septum, basal ganglia, amygdala (corticomedial nucleus), thalamus (dorsomedial nucleus), hypothalamus (paraventricular nucleus), olfactory bulb, cerebellum, midbrain, pons, medulla oblongata, corpus callosum, internal capsule and cerebellar white matter. Localization of each structure in the rat brain was based on the plates from Altman and Bayer [3] for 1-day-old pups and plates from Paxinos and Watson [41] for 10-day-old pups. Slices to be photographed were randomly selected between all the slices containing each region of interest. One photomicrograph per region per pup was obtained with a Zeiss microscope (model 1702, Carl Zeiss, Mexico) and a digital camera (model DSC-S85, Sony, Mexico). Photomicrographs were obtained using the 25× objective; a total area of 0.185 mm2 (0.50 mm × 0.37 mm), equivalent to the visual field of the microscope, was photographed for each of the central nervous system structures studied. Photomicrographs were saved as TIFF files without any manipulation and quantitatively analyzed with the plug-in cell counter [15] of the software ImageJ, version 1.32j [43].
Lectin-immunoreactive cells were classified into four morphological types based on their cell shape and configuration of their cytoplasmic processes, according to a modified version of the classification done by Wu et al. [54]. These comprised amoeboid cells (type 1), amoeboid cells bearing pseudopodia (type 2), cells with round soma bearing short, stout processes (type 3), and cells with a flattened elongated soma, thin and long processes (type 4) considered as the last transition stage to the mature resting microglia (Fig. 1). The numbers of each type of lectin-immunoreactive cells and the total number of microglial cells were obtained for each brain region studied; thereafter, mean numbers of each lectin-immunoreactive cell type per region were used to construct distribution maps in the prenatal stress and control groups on postnatal days 1 and 10, using plates 20 and 84 from Paxinos and Watson [41] as a template. The sagittal map was reconstructed from the quantified coronal slices. In both distribution maps (coronal and sagittal), each dot represents one lectin-immunoreactive cell per 0.185 mm2.
Microglial cell types. Both photomicrographs and drawings show the classification of microglial cell types used in the present study based on the classification by Wu et al. [56]. a Photomicrograph of the control corpus callosum at postnatal day 1 showing representative type 1 amoeboid microglial cells (arrow) and type 2 amoeboid microglia (arrowhead). The inset in (a) shows a higher magnification of type 2 microglia. b Photomicrograph of the 10-day-old control midbrain showing representative type 3 microglia (arrow). The inset in (b) shows a higher magnification of the marked type 3 microglia. c Photomicrograph of the control somatosensory cortex at postnatal day 10 showing representative type 4 microglia (arrow). The inset in (c) shows a higher magnification of the marked type 4 microglia. a-c Lectin-immunoreactive blood vessels are observed in addition to microglia. Scale bar 50 μm applies to the three photomicrographs
Plasma corticosterone quantification
Plasma corticosterone was quantified to assess the effectiveness of the stress procedure to generate a prolonged stress response in both dams and pups and also to ascertain the possible role of glucocorticoids as mediators of the stress effects on microglial development and distribution. On E20, pregnant rats from control and stress groups were killed by rapid decapitation and blood was collected to quantify plasma corticosterone. Pregnant rats from the stress group were killed 5 min after the end of the stress procedure (forced swimming). Six pups per group (control and prenatal stress) at postnatal days 1 and 10 (total, 24 pups) were also killed and their blood was obtained. Only two pups per litter were used for the plasma corticosterone quantification. Given the low quantity of trunk blood obtained from 1-day-old and 10-day old pups, blood from the same condition and age rats was pooled for each of the three ages studied (pups were obtained from six different litters, some were of the same litter as those used for the microglial quantification). To prevent the effects of corticosterone circadian rhythm, all rats were decapitated at 1200 hours. Blood was collected in ice-cooled tubes containing a clot activator (Terumo, Venoject, Europe), the tubes were centrifuged at 6000 rpm for 15 min and the plasma was stored until further processing at −20°C. Plasma corticosterone concentration was quantified by radioimmunoassay using the 125I rat corticosterone as a marker, following the manufacturer’s instructions (Diagnostic Products Corporation, PITKRC-2, USA). Briefly, 50 μL plasma was incubated with 125I rat corticosterone in plain polypropylene tubes during 2 h at room temperature, followed by rapid decantation and removal of moisture. Controls included uncoated polypropylene tubes and nonspecific binding tubes (total counts). Tube radioactivity was counted during 1 min in a Gamma counter; the kit used was able to detect as little as 5.7 ng/mL of plasma corticosterone concentration. Corticosterone antiserum of the Coat-A-Count rat corticosterone kit is highly specific to rat corticosterone with low cross-reactivity to 11-deoxycorticosterone (<2.9%), progesterone (<0.9%), cortisol (<0.4%), aldosterone (<0.3%), testosterone (<0.05%), 18-hydroxy deoxycorticosterone (<0.02%) and 17α-hydroxy progesterone (<0.05%). The intra-assay CVs for the rat corticosterone quantification were about 6.8%, while inter-assay CVs were about 8.5%.
Statistical analysis
The data fulfilled the normality and variance homogeneity requirements for performing statistical parametric analysis. Thus, the statistical test used to compare the differences between the prenatal stress group and the control group in microglial development was a two-way ANOVA test (experimental condition x age) [8, 23, 29]. To show specific differences in microglial development and distribution between the prenatal stress group and the control group at each age, orthogonal contrast codes (C) were performed. The orthogonal contrast codes were used instead of traditional ANOVA post hoc tests as a mean to lessen the probability of statistical error type I associated with such multiple comparisons (experimental condition x age per each nervous region) [8]. Contrast coefficients of −1 and 1 were assigned to the control and prenatal stress groups, respectively; for each test of the coefficient contrast, the alpha level was set at 0.05. Because pooled plasma samples were used, no statistical test was performed on the corticosterone data.
Results
Development and distribution of microglia in the control brains
In the 1-day-old control pups, amoeboid cells were concentrated in the two major brain reservoirs, corpus callosum and internal capsule, and in the subcortical white matter of the cerebellum (Figs. 2a, 3a). Approximately, 50% of the amoeboid lectin-immunoreactive cells in the 1-day-old control pups were localized in the corpus callosum, 40% in the internal capsule and the remaining 10% in the white matter of the cerebellum and in subcortical regions such as the septum, olfactory bulb, midbrain, pons and medulla oblongata. As is shown in the distribution maps of the control group in Figs. 2a, 3a and 4, at postnatal day 1, the majority of lectin-immunoreactive cells found in both brain reservoirs were of type 1, the most primitive stage of microglial development, or type 2, amoeboid cells with pseudopodia. On postnatal day 1, there were only few lectin-immunoreactive cells with short stout processes (type 3) in either reservoir. On the other hand, all the cortical and subcortical regions studied showed predominance of type 3 lectin-reactive cells and a small number of type 4 microglia on postnatal day 1 (Figs. 2a, 3a, 4).
Microglial distribution in coronal maps at postnatal days 1 and 10. a Control group on postnatal day 1. b Prenatal stress group on postnatal day 1. c Control group on postnatal day 10. d Prenatal stress group on postnatal day 10. In the four distribution maps, type 1 microglial cells are represented as blue dots, type 2 as green dots, type 3 as yellow dots and type 4 as red dots. BG basal ganglia, CC corpus callosum, CG cingulate gyrus, H hypothalamus, IC internal capsule, ICx insular cortex, MCx motor cortex, S septum, SCx somatosensory cortex. Modified from Plate 20 of Paxinos and Watson [41]
Microglial distribution in sagittal maps on postnatal days 1 and 10. The drawings are sagittal reconstructions from coronal slices. a Control group on postnatal day 1. b Prenatal stress group on postnatal day 1. c Control group on postnatal day 10. d Prenatal stress group on postnatal day 10. In the four distribution maps, type 1 microglial cells are represented as blue dots, type 2 as green dots, type 3 as yellow dots and type 4 as red dots. A amygdala, BG basal ganglia, Cer cerebellar cortex, CC corpus callosum, ECx entorhinal cortex, FCx frontal cortex, Hip hippocampus, IC internal capsule, M midbrain, MCx motor cortex, MO medulla oblongata, OB olfactory bulb, OCx orbitofrontal cortex, P pons, SCx somatosensory cortex, T thalamus, VCx visual cortex, WMC white matter of the cerebellum. Modified from Plate 84 of Paxinos and Watson [41]
Microglial development in the control group. The graphs show the mean number of each microglial cell type in the control group on postnatal days 1 (P1, n = 5) and 10 (P10, n = 3) in all the brain structures studied. Type 3 lectin-immunoreactive cells appear as black bars, type 4 as white bars and total number of microglia as gray bars. Type 1 and type 2 lectin-immunoreactive cells are not represented in the graphs because of the low density of those microglial cell types in cortical and subcortical regions. Error bars represent standard error. *Significant differences as compared to P1 (P < 0.05). Abbreviations are as previously stated
By postnatal day 10, amoeboid microglia in the corpus callosum (P < 0.01) and internal capsule (P > 0.05) reservoirs were severely decreased (Fig. 4). At the same age (postnatal day 10), controls also presented a rise in the number of ramified lectin-immunoreactive cells (mainly type 3) in both reservoirs as compared to postnatal day 1 (Fig. 4). Significant differences were found in both, corpus callosum (P < 0.001) and internal capsule (P < 0.001). Several other brain regions that were studied presented increases in the total number of microglial cells per 0.185 mm2 area, due mainly to a rise in the number of ramified microglia (see Fig. 4). Brain regions with a higher number of total microglia on postnatal day 10 as compared to postnatal day 1 were the insular, visual, entorhinal and frontal cortices (P < 0.05), thalamus, septum, amygdala, basal ganglia and olfactory bulb (P < 0.05). The number of ramified lectin-immunoreactive cells per area increased significantly in the visual, insular, entorhinal and frontal cortices (P < 0.01), and also in the septum, basal ganglia, amygdala, olfactory bulb, medulla oblongata and cerebellum (P < 0.05) of 10-day-old control brains as compared to newborn controls.
Stress effects on microglial development and distribution
Prenatal stress modified the microglial development and distribution in the postnatal rat brain. In the corpus callosum, prenatal stress reduced the number of amoeboid microglia on postnatal day 1 compared to the same age control group (Figs. 5, 6). As shown in the distribution maps (Figs. 2a, b, 3a, b), the most primitive form of microglia (type 1) were decreased by more than 40% in the corpus callosum of the 1-day-old prenatally stressed pups with respect to the controls (P < 0.05), and the number of type 2 lectin-immunoreactive cells (amoeboid cells with pseudopodia) were reduced, approximately 25%, in the prenatal stress group as compared to the controls (P = 0.056). Prenatal stress also diminished the total number of microglia in the corpus callosum in 1-day-old pups as compared to controls (P < 0.05; Fig. 6). In the internal capsule reservoir, prenatal stress appeared to accelerate microglial development in 1-day-old pups as compared to same age controls, without affecting total number of microglia (Fig. 5c, d). The distribution maps (Figs. 2a, b, 3a, b) and Fig. 6 show that on postnatal day 1, prenatal stress had increased the number of ramified lectin-immunoreactive cells (mainly type 3 microglia) in the internal capsule by more than 200% when compared to controls (P < 0.05). In a similar manner, prenatally stressed pups also showed an increase in the total number of microglia in some neuroanatomic regions on postnatal day 1 as compared to the control group (Figs. 5, 7). Brain regions with increased number of total microglia per 0.185 mm2 area in the prenatal stress group were the entorhinal and parietal cortices (P < 0.005), the septum, amygdala and thalamus (P < 0.05; Figs. 2a, b, 3a, b, 7). In those regions, the increase in the total number of microglia was due to an increase in the mean number of ramified lectin-immunoreactive cells (both type 3 and type 4 microglia) (Fig. 7). Globally, prenatal stress increased significantly the number of type 4 microglia per 0.185 mm2 whole brain area (P < 0.01), and slightly decreased type 1 and type 2 microglia density (P < 0.05), but did not increase the total number of microglia in the whole neonatal brain (Table 1).
Prenatal stress effects on microglial development on postnatal day 1. a Photomicrograph of the control corpus callosum on postnatal day 1. b Corpus callosum of the prenatal stress group. c Photomicrograph of the control internal capsule. d Prenatal stress group internal capsule. e Photomicrograph of the control thalamus. f Thalamus in the 1-day-old prenatally stressed pups. a-f Lectin-immunoreactive blood vessels are observed in addition to stained microglia. Scale bar 50 μm applies to the six photomicrographs
Microglial distribution in brain reservoirs in the control and prenatal stress groups on postnatal day 1. The graph shows the mean number of each microglial cell type and the total number of microglia in both brain reservoirs studied, corpus callosum and internal capsule, in the control (black bars) and prenatal stress (white bars) groups. Error bars represent standard error. *Significant differences as compared to the control group (P < 0.05)
Microglial distribution in the whole brain in the control and prenatal stress groups on postnatal day 1. The graphs shows the mean number of type 3, type 4, and the total number of microglia in the neocortex and other brain regions studied in the control (Ctrl) and prenatal stress (PrS) groups. Type 3 microglia appear as black bars, type 4 lectin-immunoreactive cells as white bars and total microglia as gray bars. Type 1 and type 2 lectin-immunoreactive cells are not represented in the graphs because of the low density of those microglial cell types in cortical and subcortical regions. Error bars represent standard error. *Significant differences as compared to same age controls (P < 0.05). Abbreviations are as previously stated
On postnatal day 10, both groups showed similar mean number, distribution and developmental stages of microglia across all brain regions studied (P < 0.05; Figs. 2c, d, 3c, d; Table 1). In the brain reservoirs, amoeboid microglia had differentiated into ramified microglia and the mean numbers of each cell type showed no significant difference between prenatally stressed and control pups, except in the corpus callosum, where prenatally stressed pups showed 50% less number of type 3 microglia as compared to same age controls (P < 0.05) and no significant statistical reduction in the total number of microglia (P > 0.05). The mean number of type 3 microglia in the corpus callosum of 10-day-old prenatally stressed pups was 31.66 ± 11.49 versus 61.09 ± 6 in controls. Meanwhile, total number of microglia in the corpus callosum of prenatally stressed pups was 74.27 ± 12.26 in comparison with 110.69 ± 15.53 in the control group.
Plasma corticosterone levels
Stress during gestation increased plasma corticosterone levels by more than 400% in dams 2 days prior to delivery. At E20, pregnant rats subjected to stress had a plasma corticosterone concentration of 1,552 ng/mL; meanwhile, control pregnant rats presented 332 ng/mL corticosterone concentrations. In pups, stress also produced an increase in plasma corticosterone concentration; however, both groups presented lower net values than that observed in pregnant adult rats due to the stress hyporesponsive period [45]. On postnatal day 1, the corticosterone concentration in prenatally stressed pups was 76 ng/mL and in controls, 46 ng/mL. By postnatal day 10, both groups, prenatal stress and control, had low levels of corticosterone in plasma (8 ng/mL in controls and 11 ng/mL in the prenatal stress group).
Discussion
This study investigated the effects of stress, during the period when the first monocytes infiltrate into the central nervous system, on microglial development and distribution in the postnatal rat brain. In utero stress reduced the number of amoeboid lectin-immunoreactive cells and total microglia in the corpus callosum reservoir in 1-day-old rat pups resulting in a decreased number of ramified microglia in the same structure on postnatal day 10. Concomitantly, prenatal stress accelerated microglial development in the second brain reservoir, the internal capsule, at 1 day of age, by slightly decreasing type 1 amoeboid microglia and considerably increasing type 3 ramified lectin-immunoreactive cells without affecting the total microglia population. In other neuroanatomic regions, such as the entorhinal and parietal cortices, septum, amygdala and thalamus, prenatal stress also increased the total number of microglia, mainly by increasing the numbers of ramified microglia (both type 3 and type 4). Prenatal stress increased type 4 ramified microglia in the whole neonatal rat brain without affecting the total number of microglia in the brain taken as a whole. Those parallel changes in microglial cell types in the corpus callosum and the other nervous regions studied could be related to accelerated microglial development associated with stress, suggesting that lectin-immunoreactive cells from the corpus callosum of the prenatally stressed pups might have spread to other brain regions earlier than in the control group. This is the first time that prenatal stress has been reported to modify microglial development and distribution in the early postnatal rat brain.
Other studies that attempted to depict the effects exerted by pharmacological stress on microglial development have used procedures and timing different from ours [26, 31, 56]. Ling [31] and Wu et al. [56] administered a single subcutaneous dose of cortisone acetate on postnatal day 1, while Kaur et al. [26] and Wu et al. [56] injected subcutaneously dexamethasone to rat pups on postnatal days 1, 3 and 5. Those studies were limited to the corpus callosum, where the number of amoeboid microglia was reduced by 40–60% [26, 31, 56]. Our findings were different. We quantified microglia in both corpus callosum and internal capsule in one-day-old pups, and found the number of amoeboid microglia to be reduced by only 15–40%. A possible explanation for the discrepancy between our study and those of others might be the different developmental timing in which stress was exerted (prenatal versus postnatal) and the different stress procedures used (physical versus pharmacological). Moreover, the lesser decrease in the corpus callosum amoeboid microglia found in our study could also be explained by the sustained proliferation of microglial cells inside the postnatal rat brain and by the continued infiltration of monocytes to the brain parenchyma as previously shown by Dalmau et al. [13], Perry and Gordon [42] and Tseng et al. [51].
Kaur et al. [26] and Wu et al. [56] attributed the glucocorticoid-induced reduction in the number of amoeboid microglia in the corpus callosum reservoir to reduced microglial cell proliferation, accelerated maturation or increased microglial cell death. However none of those hypotheses were tested directly by Kaur et al. [26] and Wu et al. [56]. In vitro experiments indicate that glucocorticoids acting on type II, low-affinity receptors, similar to the glucocorticoid-binding pattern during stress, may reduce microglial viability [50]. In our study, the absence of a global reduction in the number of microglial cells in the whole encephalon indicates that the amoeboid microglial reduction in the corpus callosum may not be a consequence of increased microglial cell death, but instead it appears that the corpus callosum amoeboid microglia had differentiated into the ramified form and had spread to their final positions in other brain regions, as denoted by an increase in ramified and total numbers of microglia in the parietal and entorhinal cortices, thalamus, amygdala and septum. However, the incidence of microglial cell death was not addressed directly in our study; therefore, more studies are needed to clarify the mechanism by which prenatal stress decreases amoeboid microglia in the corpus callosum reservoir.
Regarding the normal microglial development, in our 1-day-old control group, amoeboid microglia were concentrated in the corpus callosum, internal capsule and white matter of the cerebellum. Previously, both the corpus callosum and internal capsule have been identified as glial reservoirs in the postnatal rat brain [16, 30]. Similar to our findings in the neonatal rat, amoeboid microglia have been also described to concentrate in clumps in the central white matter of the cerebellum in the newborn mice and, beginning on postnatal day 3 amoeboid microglia spread to folial white matter [5]. Like the corpus callosum and internal capsule [16, 24, 30, 32, 54], the cerebellar white matter could thus represent an additional brain reservoir of amoeboid microglia, particularly relevant to the development of microglia in the cerebellar cortex, subcortical cerebellar nuclei and adjacent areas in the brainstem. Moreover, amoeboid microglia of the cerebellar white matter exert major effects on cerebellar cytoarchitecture arrangement. Cerebellar amoeboid microglia are involved in phagocytosis of dying cells, as in other nervous regions during early development [5], and are also known to trigger Purkinje cell death program by partially surrounding Purkinje cells and releasing reactive oxygen species in a manner similar to a respiratory burst [35].
In a further study, Wu et al. [55] reported data on the presence of ramified microglia in a few neocortical areas of the newborn and 4-day-old rat. From postnatal day 0 to 4, Wu et al. [55] found a change in the prevalence of each type of microglia in the rat neocortex. At postnatal day 0, the majority of microglial cells were ramified with round soma and short stout processes. By postnatal day 4, cortical microglia acquired their mature morphology (a flattened elongated soma with thin and long processes) [55]. We observed a similar change in the microglial morphology from postnatal day 1 to 10. In addition, in our 10-day-old control rats, ramified microglia increased by more than 500% in the brain reservoirs, and similarly in the neocortex, subcortical nuclei, cerebellar cortex and brainstem as compared to 1-day-old controls.
During early development of the central nervous system, microglia exert multiple protective, trophic and organizational functions that afford proper neural development [28]. In particular, amoeboid microglial cells form a protective phagocytic barrier, which is deemed to be necessary in the perinatal period when the blood–brain barrier is still immature [27]. Like adult activated microglia, amoeboid microglial cells may also differentiate into activated microglia (with a large soma bearing very short or no processes) after contacting an injured area along its migration pathway in the central nervous system and are capable of phagocytosis [44]. Moreover, microglia may also play an active role in triggering apoptotic Purkinje cell death in the developing cerebellum [35]. The trophic microglial functions include the release of neurotrophic factors, the promotion of angiogenesis and astrocyte proliferation [37] and in the developing white matter, amoeboid microglia also promote axonal growth and function as guides for developing axons [28]. The functions of microglia in neural environment maintenance extend until adulthood, when resting microglia processes display highly motile filopodia-like protrusions that constantly survey the neural microenvironment. By this means, microglia may effectively clear the brain parenchyma of accumulated metabolic products and deteriorated tissue debris [38]. This is the first time that prenatal stress is shown to modify microglial development and distribution, therefore it is unknown whether the observed stress effects on microglia at postnatal day 1 had any long-lasting consequences. Given that during the early postnatal period occur numerous brain developmental processes (e.g. neurogenesis, myelination, synaptogenesis, astrogliogenesis, neuronal cell death and blood–brain barrier maturation) [6, 19, 22, 25, 36, 52] it is possible that altered microglial development induced by in utero stress may affect other developmental processes either changing microenvironment molecular constitution or triggering earlier inflammatory changes secondary to the blood–brain barrier opening induced by prenatal stress [19, 22]. Although punctual, the altered microglial development might alter extensively the other neurodevelopmental processes ensuing perdurable structural changes; for example it is possible that the change in the distribution pattern of microglia in the prenatal stress group may render vulnerable some neuroanatomic regions due to the reduction of neurotrophic factors, such as the corpus callosum where there is a continuous axonal growth [28, 37].
Accelerated microglial development could be related to binding of glucocorticoids to their intracellular low-affinity receptors, type II receptors [50]. We documented increased plasma corticosterone in our stressed pregnant dams during the last day of exposure to the stress procedure (forced swimming). In rodents, corticosterone crosses the placental barrier [57], enters the fetal circulation, crosses rapidly the immature fetal blood–brain barrier [4] and binds to its receptors in the developing microglia [50]. After increased glucocorticoid levels, microglia proliferation is reduced and microglial cell death also occurs as previously reported in in vivo experiments [26, 56] and also in vitro [17, 50].
In conclusion, prenatal stress reduced the number of immature microglia in the two main brain reservoirs of amoeboid microglia, corpus callosum and internal capsule, and promoted an accelerated microglial differentiation into a ramified form in the internal capsule and brain regions such as the entorhinal cortex, parietal lobe neocortex, thalamus and septum in the neonate Wistar rat model. The effects of prenatal stress were associated with a rise in plasma corticosterone in the pregnant rat, and visible effects on microglial development and differentiation are likely mediated via the microglial corticosterone receptors.
References
Abel EL (1993) Physiological correlates of the forced swim test in rats. Physiol Behav 54:309–317
Abel EL, Hannigan JH (1992) Effects of chronic forced swimming and exposure to alarm substance: physiological and behavioral consequences. Physiol Behav 52:781–785
Altman J, Bayer SA (1995) Atlas of prenatal rat brain development. CRC Press, Boca Ratón
Arya V, Demarco VG, Issar M, Hochhaus G (2006) Contrary to adult, neonatal rats show pronounced brain uptake of corticosteorids. Drug Met Disp 34:939–942
Ashwell K (1990) Microglia and cell death in the developing mouse cerebellum. Dev Brain Res 55:219–230
Barros VG, Duhalde-Vega M, Caltana L, Brusco A, Antonelli MC (2006) Astrocyte-neuron vulnerability to prenatal stress in adult rat brain. J Neurosci Res 83:787–800
Binik YW, Theriault G, Shustak B (1977) Sudden death in the laboratory rat: cardiac function, sensory and experimental factors in swimming deaths. Psychosom Med 39:82–92
Box GEP, Hunter JS, Hunter WG (2005) Statistics for experimenters. Design, innovation, and discovery. Wiley-Interscience, New Jersey
Bruner C, Vargas I (1994) The activity of rats in a swimming situation as a function of water temperature. Physiol Behav 55:21–28
Chan WY, Kohsaka S, Rezaie P (2007) The origin and cell lineage of microglia: new concepts. Brain Res Rev 53:344–354
Chugani DC, Kedersha NL, Rome LH (1991) Vault immunofluorescence in the brain: new insights regarding the origin of microglia. J Neurosci 11:256–268
Dalmau I, Finsen B, Zimmer J, González B, Castellano B (1998) Development of microglia in the postnatal rat hippocampus. Hippocampus 8:458–474
Dalmau I, Vela JM, González B, Finsen B, Castellano B (2003) Dynamics of microglia in the developing rat brain. J Comp Neurol 458:144–157
DalZotto S, Martí O, Armario A (2000) Influence of single or repeated experience of rats with forced swimming on behavioral and physiological responses to the stressor. Behav Brain Res 114:175–181
De Vos K (2004) Cell counter. In: Rasband WS. Image J. National Institutes of Health, Bethesda, Maryland, USA. http://rsb.info.nih.gov/ij/1997–2005
Earle KL, Mitrofanis J (1998) Development of glia and blood vessels in the internal capsule of rats. J Neurocytol 27:127–139
Ganter S, Northoff H, Mannel D, Gebicke-Harter PJ (1992) Growth control of cultured microglia. J Neurosci Res 33:218–230
Gavrilovic L, Dronjak S (2005) Activation of rat pituitary–adrenocortical and sympathoadrenomedullary system in response to different stressors. Neuro Endocrinol Lett 26:515–520
Gómez-González B, Escobar A (2009) Altered functional development of the blood–brain barrier after early life stress in the rat. Brain Res Bull 79:376–387
Guillemin GJ, Brew BJ (2004) Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leuk Biol 75:388–397
Hayes CE, Goldstein IJ (1974) An α-d-galactosyl-binding lectin from Bandeiraea simplicifolia seeds: isolation by affinity chromatography and characterization. J Biol Chem 249:1904–1914
Huang WL, Harper CG, Evans SF, Newnham JP, Dunlop SA (2001) Repeated prenatal corticosteroid administration delays astrocyte and capillary tight junction maturation in fetal sheep. Int J Dev Neurosci 19:487–493
Huck SW (2000) Fully repeated measures analyses of variance. In: Huck SW (ed) Reading statistics and research. Longman, New York, pp 467–500
Imamoto K, Leblond CP (1978) Radioautographic investigation of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells. J Comp Neurol 180:139–163
Jacobson M (1991) Developmental neurobiology. Plenum Press, New York
Kaur C, Wu CH, Wen CY, Ling EA (1994) The effects of subcutaneous injections of glucocorticoids on amoeboid microglia in postnatal rats. Arch Histol Cytol 57:449–459
Kaur C, Too HF, Ling EA (2004) Phagocytosis of Escherichia coli by amoeboid microglial cells in the developing brain. Acta Neuropathol (Berlin) 107:204–208
Kaur C, Dheen ST, Ling E-A (2007) From blood to brain: amoeboid microglial cell, a nascent macrophage and its functions in developing brain. Acta Pharmacol Sin 28:1087–1096
Kristensen M, Hansen T (2004) Statistical analyses of repeated measures in physiological research: a tutorial. Adv Phsyiol Educ 28:2–14
Ling EA (1976) Some aspects of amoeboid microglia in the corpus callosum and neighboring regions of neonatal rats. J Anat 121:29–45
Ling EA (1982) Influence of cortisone on amoeboid microglia and microglial cells in the corpus callosum in postnatal rats. J Anat 134:705–717
Ling EA, Wong WC (1993) The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia 7:9–18
Ludkiewicz B, Domaradzka-Pytel B, Morys J (2001) Microglial and astroglial cells in the rat paraclaustral reservoir during postnatal development: an immunohistochemical study. Acta Neurobiol Exp 61:35–43
Marcondes FK, Bianchi FJ, Tanno AP (2002) Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62:609–614
Marín-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M (2004) Microglia promote the death of developing Purkinje cells. Neuron 41:535–547
Mirescu C, Peters JD, Gould E (2004) Early life experience alters response of adult neurogenesis to stress. Nat Neurosci 7:841–846
Nakajima K, Kohsaka S (1993) Functional roles of microglia in the brain. Neurosci Res 17:187–203
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318
NRC (National Research Council) (2003) Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academic Press, Washington DC
Ock J, Lee H, Kim S et al (2006) Induction of microglial apoptosis by corticotropin-releasing hormone. J Neurochem 98:962–972
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press–Elsevier, San Diego
Perry VH, Gordon S (1988) Macrophages and microglia in the nervous system. Trends Neurosci 11:273–277
Rasband WS (2004) Image J. National Institutes of Health, Bethesda, Maryland, USA. http://rsb.info.nih.gov/ij/1997–2005
Sánchez-López AM, Cuadros MA, Calvente R, Tassi M, Marín-Teva JL, Navascués J (2005) Activation of immature microglia in response to stab wound in embryonic quail retina. J Comp Neurol 492:20–33
Sapolsky RM, Meaney MJ (1986) Maturation of the adrenal stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res Rev 11:65–76
Streit WJ (1990) An improved staining method for rat microglial cells using the lectin from Griffonia simplicifolia (GSA I-B4). J Histochem Cytochem 38:1683–1686
Streit WJ, Kreutzberg GW (1987) Lectin binding by resting and reactive microglia. J Neurocytol 16:249–260
Suckow MA, Weisbroth SH, Franklin CL (2006) The laboratory rat. American College of Laboratory Animal Medicine Series. Academic Press, New York
Szyndler J, Piechal A, Blecharz-Klin K, Skórzewska A, Maciejak P, Walkowiak J, Turzyńska D, Bidziński A, Plaźnik A, Widy-Tyszkiewics E (2006) Effect of kindled seizures on rat behavior in water Morris maze test and amino acid concentrations in brain structures. Phramacol Rep 58:75–82
Tanaka J, Fujita H, Matsuda S, Toku K, Sakanaka M, Maeda N (1997) Glucocorticoid- and mineralocorticoid receptors in microglial cells: the two receptors mediate differential effects of corticosteroids. Glia 20:23–37
Tseng CY, Ling EA, Wong WC (1983) Light and electron microscopic and cytochemical identification of amoeboid microglial cells in the brain of prenatal rats. J Anat 136:837–849
Uno H, Lohmiller L, Thieme C et al (1990) Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Dev Brain Res 53:157–167
Wang W, Ji P, Riopelle RJ, Dow KE (2002) Functional expression of corticotropin-releasing hormone (CRH) receptor 1 in cultured rat microglia. J Neurochem 80:287–294
Wu CH, Wen CY, Shieh JY, Ling EA (1992) A quantitative and morphometric study of the transformation of amoeboid microglia into ramified microglia in the developing corpus callosum in rats. J Anat 181:423–430
Wu CH, Wen CY, Shieh JY, Ling EA (1993) A quantitative study of the differentiation of microglial cells in the developing cerebral cortex in rats. J Anat 182:403–413
Wu CH, Chien HF, Chang CY, Chen SH, Huang YS (2001) Response of amoeboid and differentiating ramified microglia to glucocorticoids in postnatal rats: a lectin histochemical and ultrastructural study. Neurosci Res 40:235–244
Zarrow MX, Phillpot JE, Denenberg VH (1970) Passage of 14C-4-corticosterone from the rat mother to the foetus and neonate. Nature 226:1058–1059
Zhou Y, Ling EA, Dheen ST (2007) Dexamethasone suppresses MCP-1 production via kinase phosphatase-1 dependent inhibition of JNK and p38 MAPK in activated microglia. J Neurochem 102:667–678
Acknowledgments
Supported by research grant PAPIIT IN219407 from DGAPA UNAM to A Escobar, and stipend 188838 from CONACyT to B Gómez-González. We thank Dr. Karen M. Weidenheim, M.D., Chief of the Division of Neuropathology, Montefiore Medical Center, for the correction of the English language and for her valuable comments on the final version of the manuscript. We thank Dr. Carolina Escobar for kindly providing the Coat-A-Count rat corticosterone kit, Dr. Carolina Escobar and Roberto Salgado for their valuable technical help and guidance with the radioimmunoassay technique, Jorge Ruiz for the guidance with the statistical tests, and Guadalupe Flores Cruz for the revision of an early version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez-González, B., Escobar, A. Prenatal stress alters microglial development and distribution in postnatal rat brain. Acta Neuropathol 119, 303–315 (2010). https://doi.org/10.1007/s00401-009-0590-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-009-0590-4