Abstract
The dentate gyrus (DG) plays a pivotal role in the functional and anatomical organization of the hippocampus and is involved in learning and memory formation. However, the impact of structural DG abnormalities, i.e., granule cell dispersion (GCD), for hippocampal seizure susceptibility and its association with distinct lesion patterns in epileptic disorders, such as mesial temporal sclerosis (MTS) remains enigmatic and a large spectrum of pathological changes has been recognized. Here, we propose a clinico-pathological classification of DG pathology based on the examination of 96 surgically resected hippocampal specimens obtained from patients with chronic temporal lobe epilepsy (TLE). We observed three different histological patterns. (1) A normal granule cell layer was identified in 11 patients (no-GCP; 18.7%). (2) Substantial granule cell loss was evident in 36 patients (referred to as granule cell pathology (GCP) Type 1; 37.5%). (3) Architectural abnormalities were observed in 49 specimens, including one or more of the following features: granule cell dispersion, ectopic neurons or clusters of neurons in the molecular layer, or bi-lamination (GCP Type 2; 51%). Cell loss was always encountered in this latter cohort. Seventy-eight patients of our present series suffered from MTS (81.3%). Intriguingly, all MTS patients displayed a compromised DG, 31 (40%) with significant cell loss (Type 1) and 47 (60%) with GCD (Type 2). In 18 patients without MTS (18.7%), seven displayed focally restricted DG abnormalities, either cell loss (n = 5) or GCD (n = 2). Clinical histories revealed a significant association between DG pathology patterns and higher age at epilepsy surgery (p = 0.008), longer epilepsy duration (p = 0.004), but also with learning dysfunction (p < 0.05). There was no correlation with the extent of pyramidal cell loss in adjacent hippocampal segments nor with postsurgical seizure relief. The association with long-term seizure histories and cognitive dysfunction is remarkable and may point to a compromised regenerative capacity of the DG in this cohort of TLE patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesial temporal sclerosis (MTS) is the most recognized finding in drug resistant, chronic temporal lobe epilepsies [4]. Tailored resection strategies including selective amygdala-hippocampectomy are established treatment modalities and offer a favorable outcome with up to 80% postoperative seizure freedom within the first 2 years [4, 27, 48]. Clinical studies assume mesial temporal lobe epilepsy, however, as a heterogeneous entity with different etiologies and clinical histories [2, 49]. Besides segmental neuronal loss within hippocampal subfields, granule cell dispersion is observed in approximately 40% of sclerotic hippocampi [6, 25]. Different pathological patterns including a bilaminar dentate gyrus, granule cell loss or spreading of single and/or clusters of granule cells into the molecular layer are often described but yet not systematically examined [13, 24, 46]. As a consequence, there is no information available to which extent these structural abnormalities contribute to hippocampal seizure susceptibility or mnestic dysfunction.
Studies addressing the molecular pathomechanism of granule cell dispersion (GCD) point to a compromised reelin signaling pathway. An inverse correlation was described between the degree of GCD and reelin mRNA expression in epilepsy patients with hippocampal sclerosis. While compact layer organization could be associated with abundant reelin mRNA expression, TLE patients with pronounced GCD showed significantly reduced reelin mRNA levels [21] as well as increased promoter methylation of the reelin gene [29]. Reelin is synthesized and secreted by Cajal-Retzius cells, which are among the earliest neurons to be generated [14]. Biochemical and functional studies using either organotypic slice cultures or knock-out mice confirmed the relevance of reelin signaling for the formation of a densely packed granule cell layer [51].
The hippocampus serves a major role in declarative conscious memory, i.e., semantic memory for facts and concepts, episodic memory and spatial memory [43]. Neuropsychological lesion studies and functional imaging in humans, as well as in experimental animal models, correlated memory functions with distinct anatomical subregions and activation of specific hippocampal circuitries, in particular the dentate gyrus [28, 40]. Ample evidence points to dentate granule cell neurogenesis as cellular correlate of learning and behavior in rodents [30, 41]. Similar mechanisms are likely to play a role also in humans [37]. Besides any associated pathology affecting the pyramidal cell layers of the hippocampus as well as axonal reorganization of the mossy fiber system, severe granule cell loss, in particular affecting the DG internal limb, associates most significantly with low memory scores in TLE patients [37]. However, the cause of granule cell loss in TLE patients remains to be specified. Whereas excitotoxic or apoptotic granule cell death is less reasonable due to their protective molecular environment [1]; decreased neurogenesis in the adult hippocampus may be envisaged. Indeed, a recent study identified a decline of neurogenesis around the age of 35 years, irrespective of the presence or absence of granule cell dispersion [19]. Functional consequences of decreased neurogenesis in the aged and/or chronic epileptic brain thus remain an important issue to be addressed. Herein, we qualitatively characterized different patterns of dentate gyrus pathology in a series of 96 TLE patients. Surgical specimens without evidence for a compromised granule cell layer (no-GCP, n = 11) were used to define the normal architecture. Notwithstanding, these specimens may harbor subtle histopathological alterations. To the best of our knowledge, there is no better “control” group available with respect to tissue processing, the young age of patients under study, and neuropsychological data on memory performance. Quantitative measurements were performed in regions of interest to characterize specific histopathological findings and to allow a correlation analysis with clinical data.
Methods
Subjects
A consecutive series of hippocampal specimens were obtained in the framework of a multi-center study including three German centers for epilepsy surgery (University Medical Schools of Berlin, Bonn, and Erlangen). However, only those tissue samples were included, in which the entire hippocampus and dentate gyrus was anatomically well preserved. Thus, surgical specimens were available from 96 patients [52 men (54.2%), 44 women (45.8%); mean age 38.2 ± 13.5 years; with 51 (53.1%) left- and 45 (46.9%) with right-sided resections].
All patients underwent presurgical evaluation including Video-EEG monitoring, high-resolution MR imaging and neuropsychological testing [9, 37]. Temporal lobe epilepsy refractory to medication with a mesio-temporal focus was diagnosed in all cases. Surgical strategy comprised tailored resections of anterior temporal lobe including the hippocampus, standard anterior temporal lobectomy with amygdalo-hippocampectomy, polar temporal resection plus hippocampectomy and selective amygdalo-hippocampectomy [10]. Informed consent was given by all the patients included in our study for further scientific investigations.
Postsurgical data were obtained from the patients’ interviews during postoperative outpatient visits. All clinical data were entered into epilepsy data banks at the respective centers. Postoperative seizure control was documented using Engel’s classification and available in 94 patients after a 6-month period, in 79 patients after 12 months and in 53 patients 24 months after operation.
Memory performance
Memory testing was performed in all patients. However, different test scales were applied at the three submitting Epilepsy centers. For our comparative approach, we only used data obtained from intracarotid amobarbital testing (IAT), which was available in 26 patients. This WADA testing method was described elsewhere [37]. In brief, intracarotid injection of 150 mg amobarbital was employed via transfemoral catheterization to anesthetize either the left or the right cerebral hemisphere (at 2 consecutive days). Three parallel test versions with equally difficult items for memory testing were applied (5 images, 5 words, 3 objects). In order to obtain comparable standards, normal IAT memory scores were obtained from a large series of none-affected left and right hemispheres and transformed into z-scores (mean = 0 and standard deviation = 1). The following calculation was used: (a) if the resected hippocampus was taken from the left hemisphere: z(IAT memory) = (total memory score − Meanleft)/SDleft; and (b) if the resected hippocampus was taken from the right hemisphere: z(IAT memory) = (total memory score − Meanright)/SDright [37].
Tissue preparation
For histopathological examination, hippocampal slices cut perpendicular to the anterior–posterior axis were used. Only tissue samples from the mid hippocampal body were chosen for this study [15]. All tissues were fixed overnight in 4% formalin and routinely processed into liquid paraffin. Respective tissue blocks were submitted to the Department of Neuropathology in Erlangen and further processed. All specimens were cut at 4 μm with a microtome (Microm, Heidelberg) stretched in water at 40°C and mounted on slides coated with silane (Langenbrinck; Emmendingen, Germany). The slides were air-dried in an incubator at 37°C overnight, deparaffined in descending alcohol concentrations and stained with hematoxylin and eosin (HE). Hippocampal pyramidal neurons and granule cells of the dentate gyrus were specifically detected using immunohistochemistry for the neuronal core antigen NeuN (A60, Chemicon, Temecula, USA, dilution 1:1000, pre-treated with microwave) and an automated staining apparatus using the streptavidin–biotin method (Ventana; Strasbourg, France) and 3,3′-diaminobenzidine as chromogen as well as hematoxylin counterstaining.
Neuropathological investigation
All tissue specimens were microscopically examined by experienced neuropathologists (IB, RB, MH). Quantitative analysis was performed in one representative section obtained from each specimen following either H&E staining or NeuN immunohistochemistry. All quantitative measurements were performed with a microcomputer imaging system (ColorView II CCD camera, AnalySIS imaging software, Stuttgart, Germany) equipped to a BX51 microscope (Olympus, Japan).
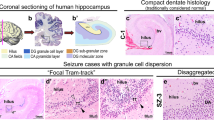
In the present series, normal architecture of the human dentate gyrus was determined in 11 surgical specimens without evidence for microscopic abnormalities of the hippocampal formation as previously described [4, 37]. The mean age at surgery was 37 ± 13.1 years, 4 were male, 7 female, 5 operated on the right side, 6 on the left side; mean age at epilepsy onset was 24.6 years, mean duration was 14.7 years. The dentate gyrus was always subdivided into three localizations representing one-third of the whole dentate gyrus, respectively: the mesial part of dentate gyrus, so called internal limb, the external limb opposite to hippocampal CA2 sector and the middle part of the dentate gyrus between internal and external limbs [37]. Respective images were digitally captured on the computer screen using AnalySIS software. All measurements were repeated ten times at a given area of the dentate gyrus. Distances were calculated at respective objective magnification using a drawing bar (according to the manufacturer’s protocols). Cell numbers were calculated per given area (NeuN-immunoreactive profiles of neuronal nuclei were manually tagged from the granule cell layer or molecular cell layer on the computer screen; numbers/mm² were calculated using Excel worksheet). Semi-quantitative analysis was also applied for the remaining 85 surgical specimens, studying the three different portions of the DG granule cell layer separately (internal and external limbs, as well as mid-portion) with respect to thickness and continuity of the granule cell layer as well as presence of aberrant granule cells within the molecular layer. The following architectural patterns were regarded as conspicuous and further examined (reference values referred to no-GCP cohort, see Table 1): (1) thinning of dentate granule cell layer (116.2 ± 16 μm in no-GCP) (2) cell free gaps (not present in no-GCP) (3) granule cell dispersion with spreading of granule cells into the molecular layer (8 ± 2 cell layers in no-GCP, tightly packed) (4) single ectopic granule cells within the molecular layer (132.5 ± 42 cells/mm2 in no-GCP) (5) clusters of ectopic granule cells within the molecular layer (not present in no-GCP), and (6) presence of a bilaminar granule cell layer (not present in no-GCP). Due to presumable artifacts, measurements were only performed in regions without curvatures of the dentate gyrus. Measurements included the thickness of the entire granule cell layer (in μm), number of cell layers in vertical direction, and distances between granule cell nuclei in horizontal and vertical directions (in μm). Mean values below or above the second standard deviation of the mean obtained from our no-GCP cohort should be regarded as pathological. Thickness measurements of the granule cell layer were not randomly placed. They rather included only those regions of interest in which the respective architectural disturbance was recognized after visual inspection. Number of cell layers and core-to-core distances were always measured at the same regions of interest (same captured images were used).
MTS was defined according to the new classification system [4], which separates five categories including classic (MTS type 1a) and severe MTS (MTS type 1b) as well as atypical forms with isolated neuronal loss within hippocampal sector CA1 (MTS type 2) or within the hilar region (MTS type 3). The group “no MTS” includes hippocampal specimens without histopathological evidence for segmental neuronal loss.
Statistical analysis
For statistical evaluation, SPSS for Windows, Version 16.0 was used. Student’s t test and variance analysis with one-way ANOVA and Bonferroni post-hoc was performed; p < 0.05 was defined as level of significance.
Results
Anatomical characterization of the dentate gyrus granule cell layer
Out of 96 patients included in this study, 11 presented without histopathological abnormalities within the dentate gyrus granule cell layer (no-GCP; 11.5%) and served for the determination of normal values. In these specimens, the granule cell layer had an average thickness of 116.2 ± 16.0 μm (Fig. 1a), corresponding to 8 ± 2 vertical granule cell rows, densely packed granule cells with similar horizontal (14.6 ± 1.3 μm) and vertical distances (14.2 ± 1.2 μm) between granule cells (Fig. 1b) and sharp boundaries towards the basal polymorphic and upper molecular layers. The number of ectopically placed NeuN immunoreactive neurons in these non-affected specimens was 123.5 ± 25 per mm2. Histopathological analysis of adjacent cortical tissue in this cohort of patients revealed gangliogliomas (n = 2), dysembryoplastic neuroepithelial tumors (n = 2), cavernoma (n = 1), a focal cortical dysplasia Type I (n = 1), or no histopathological lesion (n = 5).
Histopathological spectrum of dentate gyrus pathology in TLE patients. a Normal appearance of the human dentate gyrus (no-GCP) with densely packed granule cells and sharp borders to subgranular and molecular layer (10× magnification, bar 100 μm, refers also to c–h). b Higher magnification of a (scale bar 50 μm). Granule cell pathology Type 1 with significant granule cell loss indicated by thinning of dentate gyrus (c) or cell free gaps (d, asterisk). e–h Granule cell pathology Type 2 presenting with ectopic granule cells in the molecular layer, e.g., granule cell dispersion with spreading of granule cells into the molecular layer (e, arrow), single ectopic granule cells within the molecular layer (f, arrow), clusters of ectopic granule cells within the molecular layer (g, arrow) or with a bilaminar architecture (h, the arrow indicates the aberrant upper layer in the molecular layer, asterisk indicates a cell free gap between both layers). NeuN immunohistochemistry with hematoxylin counterstaining. GC granule cell layer, ML molecular layer, TLE temporal lobe epilepsy
Dentate gyrus pathology
In the remaining 85 patients, histopathologic patterns of the dentate gyrus granule cell layer were qualitatively described as thinned, containing cell free gaps, bilayered, dispersed, containing ectopic neurons or clusters of neurons in the molecular layer. It is important to note that such patterns were usually not observed throughout the entire granule cell layer but occurred locally restricted. A combination of different lesion patterns was also frequently encountered (see below). A quantitative analysis was applied in order to better characterize the specific histopathological appearance (values twofold above or below the respective standard error obtained from our no-GCP cohort were regarded as significant; see Table 1). In all specimens, ten high power fields were evaluated at the different anatomical divisions of the DG, i.e., internal, external limbs as well as its mid portion. Measures were not randomly placed and included regions of interest with most significant alterations following visual inspection.
Thinning of the dentate granule cell layer
The dentate granule cell layer was significantly thinned out in 78 patients (p < 0.001; Fig. 1c). Mean vertical width was only 73.2 ± 12.4 μm (corresponding to 4.5 ± 0.8 cell rows). Distances between granule cells were larger in horizontal (18.2 ± 2.2 μm) and vertical (18.6 ± 2.6 μm) direction (p < 0.001).
Cell free gaps
In contrast to thinning of the dentate granule cell layer, a focal disruption of the layer’s continuity occurred in 41 patients (Fig. 1d). It is important to note that similar gaps can be seen in the vicinity of blood vessels. Respective regions were, therefore, not included in this analysis. The mean horizontal width of a gap was 69.2 ± 23.0 μm.
Granule cell dispersion (GCD)
Granule cell dispersion was herein defined by spreading of granule cells into the molecular layer. The vertical as well as horizontal continuity of the granule cell layer was not disrupted but the upper boundary towards the molecular layer was blurred (Fig. 1e). Compared to no-GCP, the mean vertical width of the granule cell layer was enlarged (404.3 ± 107.2 μm), corresponding to 16.4 ± 2.8 rows of granule cells (p < 0.001). Furthermore, horizontal (22.1 ± 4.5 μm) and vertical (22.8 ± 3.2 μm) distances between granule cells were significantly increased (p < 0.001).
Ectopic granule cells within the molecular layer
In contrast to granule cell dispersion, the border between the granule cell and molecular layers remained detectable in specimens with ectopic displacement of granule cells in the molecular layer (Fig. 1f). It is of note that occasional granule cells occur also in the normal dentate gyrus molecular layer (mean 123.5 ± 25 per mm2). Statistical analysis revealed, however, a significant increase in affected regions (447.5 ± 117.5 per mm2).
Clusters of ectopic granule cells within the molecular layer
Clusters of ectopic granule cells in the molecular layer were detected in 18 patients. In respective specimens, noduli were visible distinctly separated from the granule cell layer by a cell free space (mean width 88.2 ± 23.8 μm; Fig. 1g). The mean number of ectopic granule cells within a cluster was 5.0 ± 2.2 in vertical and 5.1 ± 2.1 in horizontal direction. The underlying dentate gyrus was significantly thinned with a mean width of 68.2 ± 27.0 μm, corresponding to 4.1 ± 2.0 vertical cell layers.
Bilayered dentate gyrus
A bilaminar dentate gyrus was characterized by two parallel layers of granule cells, the inner band considered as remnant of the original dentate gyrus and an additional ectopic outer band towards the molecular layer. This pattern was recognized in 14 patients. Both layers are separated by a cell free gap (mean width 97.3 ± 46.5 μm; Fig. 1h). Compared to the inner layer, the outer band was broader (mean width 102.3 ± 43.8 μm, corresponding to 6.3 ± 1.4 cell rows). The mean width of the inner band reached 74.2 ± 35.7 μm, corresponding to 4.0 ± 0.9 rows of granule cells.
Relationship between specific dentate gyrus pathologies
The most frequent pattern of dentate gyrus pathology was a thinning of the dentate granule cell layer in 78 of all investigated patients (81.3%). Another frequent finding was granule cell dispersion, which occurs in half of our patient cohort (n = 49,51.0%). A bilaminar dentate gyrus and ectopic clusters presented rare findings, which occurred only in 14 (14.6%) or 19 (19.8%) patients, respectively. Noteworthy, individual specimens demonstrated several lesion patterns in parallel. There was a frequent association between thinning of the granule cell layer and occurrence of cell free gaps (p = 0.051). Both patterns were regarded to represent granule cell loss (GCP Type 1). Granule cell dispersion, ectopic granule cells, ectopic clusters and a bilaminar dentate gyrus were significantly associated (GCP Type 2) and may thus represent variants of a compromised architectural organization of the dentate gyrus. While patterns of granule cell loss occurred isolated, patters of architectural abnormalities came always along with cell loss. We built, therefore, three different categories. A dentate gyrus without pathological changes was identified in 11 (11.5%) cases (no-GCP). Granule cell loss either presenting with thinning and/or cell free gaps was detected in 36 (37.5%) patients (GCP Type 1). A combined dentate gyrus pathology with cell loss and one or more architectural disturbances was visible in a total of 49 cases (51.0%; GCP Type 2, respectively). As a rule, we classified a given specimen into GCP Type 2, if one or more of the features “granule cell dispersion, ectopic granule cells, ectopic clusters or bilaminar dentate gyrus” was developed (irrespective of its extent).
There was no statistical difference between the occurrence of dentate gyrus pathologies along the three anatomical subfields, i.e., internal, medial and external limbs (Table 2). However, thinning of the dentate gyrus occurred more often at the internal or external limbs, whereas dispersion affected more often medial sites.
Dentate gyrus pathology in MTS
All hippocampal specimens were analyzed according to the new MTS classification system [4]. In MTS type 1a and 1b, combined dentate gyrus pathology (Type 2) with significant loss of granule cells and granule cell dispersion was more common (Table 3). In atypical MTS, especially in MTS type 3, the majority of specimens presented with dentate gyrus pathology Type I. All 11 patients without dentate gyrus pathology showed an otherwise microscopically normal hippocampus (no MTS; Table 3).
Clinical correlations
The mean age at surgical resection was 38.2 ± 13.5 years in our patient cohort. Patients without dentate gyrus pathology were, however, significantly younger (28.9 ± 13.8 years) than patients with isolated dentate gyrus cell loss (Type 1). Dentate gyrus pathology with dispersion was observed in older patients (41.7 ± 10.7 years; p = 0.008). The mean duration of seizures was 24.3 ± 13.7 years in all patients. It was shortest in patients without dentate gyrus pathology (15.6 ± 12.6 years) and longest in patients with GCP Type 2 (28.6 ± 13.2 years), whereas patients with granule cell loss had an intermediate epilepsy duration (21.1 ± 13.2 years; p = 0.004). However, mean age at epilepsy onset (13.6 ± 11.5 years) did not differ between these groups.
Twenty-one out of 86 patients with sufficient clinical data experienced febrile seizures in the early childhood (<4 years). All of them displayed severe dentate gyrus pathology (p = 0.034), eight with granule cell loss (Type 1) and 13 with a combination of granule cell dispersion (Type 2).
Six months after operation, seizure relief was achieved in 74 patients (78.7%; Engel class 1). There was no statistically significant correlation with pathology patterns of the dentate gyrus. However, only one-third (33.3%) of patients without dentate gyrus pathology became seizure-free (Engel 1), whereas 70.4% with isolated granule cell loss (Type 1) and 73.0% with combined granule cell dispersion (Type 2) had no seizures 1 year after resection (Table 4). The association between favorable postsurgical outcome and granule cell pathology (Type 1 or Type 2) was statistically significant (p = 0.028).
Quantitative data from conscious, declarative memory testing (i.e., semantic memory for facts and concepts, episodic memory and spatial memory) were available from 26 patients which underwent intracarotid amobarbital anesthesia (WADA). There was a significant better memory performance in patients without dentate granule cell loss or dispersion (p < 0.05). However, no significant differences were observed between Type 1 and Type 2 pathology patterns, although patients with GCP Type 2 were likely to suffer from worse memory scores (Fig. 2).
Correlation between granule cell pathology and memory performance. WADA memory testing (IAT) was obtained in 26 epilepsy patients. Declarative memory performance was examined from isolated hemispheres and transferred into z-scores (0 = normal; −2 = severe deficits). “Ipsilateral” refers to the side of epileptogenic area. All patients with granule cell pathology (either Type 1 or 2) revealed severe loss of memory (p < 0.05)
Discussion
Two major lesion patterns can be identified from our systematic neuropathological survey of the dentate gyrus obtained from patients with temporal lobe epilepsies, either characterized by granule cell loss or granule cell dispersion. Although these pathologies were mutually associated with mesial temporal sclerosis (MTS), similar disturbances also occurred without microscopic evidence for hippocampal sclerosis [22].
Mesial temporal sclerosis (MTS) is the most common pathological finding in temporal lobe epilepsies and accompanied by architectural disturbances of the dentate gyrus (DG) in approximately half of this patient cohort [16, 25, 34]. A hallmark of granule cell pathology (GCP) was previously described as granule cell dispersion [25], but remained histopathologically unspecified so far [49]. Such a neuropathological definition will be, however, an important prerequisite to further address its pathogenic origin and functional impact on temporal lobe epilepsy and/or compromised memory acquisition [37]. Our neuropathological definition of granule cell dispersion includes: (1) blurring of the outer boundary towards the molecular layer (2) increased distances between individual granule cells (3) a dramatically increased vertical width of 400 μm (corresponding to 16 rows of granule cells) compared to 120 μm (8 rows of granule cells, respectively) in unaffected specimens (4) granule cell loss (5) association with other lesion patterns including single or clusters of ectopic neurons, or bilamination. The latter is compatible with a broad spectrum of architectural abnormalities associated with migration and/or proliferation deficits in granule cells.
One hypothesis describes granule cell dispersion as a developmental defect associated with early precipitating injuries before the age of 4 years, i.e., febrile seizures [25, 31]. The latter can partially be confirmed in our patient cohort. Moreover, granule cell dispersion has been discussed as a result of early seizure onset or status epilepticus at an initial stage of the disease [26]. We and others did not find a correlation between dentate granule cell dispersion and early onset of chronic seizures [6, 34], but rather observed an association between dentate gyrus pathology with higher age of individual patients at epilepsy surgery and longer epilepsy duration, respectively. The association of (1) early precipitating events and (2) long seizure duration with granule cell dispersion would indeed support a two-hit hypothesis. Whereas the early injury may induce long-lasting network reorganization and increased seizure susceptibility, granule cell loss and/or migration abnormalities may result rather from epilepsy-associated secondary changes, i.e., degeneration or compromised regeneration capacity. Recent experimental findings support this notion. Granule cells are generated throughout life in the subgranular zone of dentate gyrus and functionally integrated into the hippocampal circuitry [18, 47]. Animal studies suggest that epileptic activity increases neurogenesis in the dentate gyrus [36, 39]. Also, granule cell dispersion may thus develop in adult epilepsy patients due to increased neurogenesis. Newly build granule cells may than aberrantly migrate beyond the granule cell layer. Whether seizures similarly induce neurogenesis in humans is a matter of ongoing debate [42]. Granule cell proliferation cannot be studied by bromodeoxyuridine application and available markers of proliferation and immature neurons revealed inconsistent results. Such newborn granule cells were not detectable beyond the age of 2 years [5, 35], whereas other studies report an increased number of proliferating cells and neuronal precursors in granule cell dispersion [11, 45]. More recent findings further challenge this observation. Neurogenesis in the subgranular zone of the dentate gyrus seems to depend on the patient’s age and is down-regulated during adulthood [19]. The latter findings lead to the hypothesis, that rather abnormal migration of mature granule cells result in granule cell dispersion. Local reelin deficiency in the hippocampus of TLE patients, which significantly correlates with the degree of granule cell dispersion, is likely to represent a major molecular pathomechanism [21]. Animal studies in the reeler mouse revealed altered granule cell migration directing to aberrant positioning of granule cells during development compatible to human GCD [12, 20, 38]. Kainate-induced epilepsy in adult mice was also associated with loss of reelin expression and concomitant granule cell dispersion [23]. Recent evidence points also to an epilepsy-associated increase of promoter methylation in human TLE specimens, which represent another important mechanism to down-regulate reelin gene expression [29].
An association between the severity of hippocampal cell loss, i.e., mesial temporal sclerosis, and favorable postsurgical seizure relief was already demonstrated in prior studies [4, 13, 33]. The precise nature of this association remains poorly understood considering the huge number of related factors, including different approaches and extent of surgical resections, presence/absence of extra-hippocampal pathologies as well as cellular and molecular reorganization patterns, which all likely to play a role [3, 4, 7, 8, 27]. Only few studies have so far systematically studied dentate gyrus pathology and compared these data with postsurgical outcome [33, 34]. Similar to our present data, the correlation between severe dentate gyrus pathology and a favorable 1-year postsurgical seizure relief was observed and most likely associates with that of related hippocampal sclerosis. Similarly, one-third of patients without a lesion pattern within the dentate gyrus became seizure-free and had also no related pathology. It remains an intriguing observation that dentate granule cell loss but not pyramidal cell loss associates with deficient memory acquisition in TLE patients [37, 44]. This further highlighted the impact of granule cells within hippocampal circuitries and that the anatomical integrity of the human dentate gyrus significantly correlates with the capacity to acquire new memories. Interestingly, professional training of spatial memory may increase hippocampal volumes [32]. Also, functional imaging has indicated the involvement of the hippocampal subregions including dentate gyrus into the formation of new memories [50], but structural resolution remains too coarse to precisely differentiate between anatomical subregions in the range of 2–5 mm. Animal studies most likely identified the dentate gyrus to harbor molecular and physiological mechanisms of memory formation pointing to the necessity for the recruitment of new granule cells for learning. Our data confirm this previous conclusion. Those patients with large numbers of granule cells perform a high memory score while those suffering from severe cell loss, i.e., depletion of granule cells and/or the regenerative stem cell pool, as well as architectural disturbances do not achieve sufficient memory scores (Fig. 2). Different time courses in the generation of external and internal limbs neurons should substantially add to this model suggesting higher vulnerability of the ontogenetically newer dentate cells of the internal limb. Whether disturbances in the late fetal period or early childhood also account to reduced cell numbers of the dentate gyrus, thus aggravating memory deficits in further life remains to be shown. Thus, a focus on pathogenic mechanisms of granule cell loss or neurogenesis will be fundamental to gain therapeutic concepts against memory dysfunction under physiological or pathophysiological conditions.
In conclusion, our clinico-pathological investigation characterized two patterns of dentate gyrus pathology, i.e., granule cell loss (GCP Type 1) and granule cell dispersion (GCP Type 2), which suggests a prevalent association with MTS, although DG pathology also occurred in an otherwise “normal” hippocampus. The latter observation points to different pathomechanisms associated with architectural abnormalities of the dentate gyrus in focal temporal lobe epilepsies. In addition, compromised memory performance was significantly associated with an altered granule cell layer. These data were compatible with recent animal studies, proposing that memory formation critically depends on the integrity of the dentate gyrus and its capability to maintain and recruit new neurons. A similar mechanism may thus operate also in humans.
References
Becker AJ, Gillardon F, Blümcke I, Langendorfer D, Beck H, Wiestler OD (1999) Differential regulation of apoptosis-related genes in resistant and vulnerable subfields of the rat epileptic hippocampus. Mol Brain Res 67:172–176
Bien CG, Schulze-Bonhage A, Deckert M, Urbach H, Helmstaedter C, Grunwald T, Schaller C, Elger CE (2000) Limbic encephalitis not associated with neoplasm as a cause of temporal lobe epilepsy. Neurology 55:1823–1828
Blumcke I, Beck H, Lie AA, Wiestler OD (1999) Molecular neuropathology of human mesial temporal lobe epilepsy. Epilepsy Res 36:205–223
Blumcke I, Pauli E, Clusmann H, Schramm J, Becker A, Elger C, Merschhemke M, Meencke HJ, Lehmann T, von Deimling A, Scheiwe C, Zentner J, Volk B, Romstock J, Stefan H, Hildebrandt M (2007) A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol 113:235–244
Blumcke I, Schewe JC, Normann S, Brustle O, Schramm J, Elger CE, Wiestler OD (2001) Increase of nestin-immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus 11:311–321
Blumcke I, Thom M, Wiestler OD (2002) Ammon’s horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol 12:199–211
Blumcke I, Zuschratter W, Schewe JC, Suter B, Lie AA, Riederer BM, Meyer B, Schramm J, Elger CE, Wiestler OD (1999) Cellular pathology of hilar neurons in Ammon’s horn sclerosis. J Comp Neurol 414:437–453
Clusmann H, Kral T, Gleissner U, Sassen R, Urbach H, Blumcke I, Bogucki J, Schramm J (2004) Analysis of different types of resection for pediatric patients with temporal lobe epilepsy. Neurosurgery 54:847–859 discussion 859-860
Clusmann H, Kral T, Schramm J (2006) Present practice and perspective of evaluation and surgery for temporal lobe epilepsy. Zentralbl Neurochir 67:165–182
Clusmann H, Schramm J, Kral T, Helmstaedter C, Ostertun B, Fimmers R, Haun D, Elger CE (2002) Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurg 97:1131–1141
Crespel A, Rigau V, Coubes P, Rousset MC, de Bock F, Okano H, Baldy-Moulinier M, Bockaert J, Lerner-Natoli M (2005) Increased number of neural progenitors in human temporal lobe epilepsy. Neurobiol Dis 19:436–450
D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T (1995) A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374:719–723
de Lanerolle NC, Kim JH, Williamson A, Spencer SS, Zaveri HP, Eid T, Spencer DD (2003) A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: evidence for distinctive patient subcategories. Epilepsia 44:677–687
Del Rio JA, Heimrich B, Borrell V, Förster E, Drakew A, Alcantara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Derer P, Frotscher M, Soriano E (1997) A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature 385:70–74
Duvernoy HM (2005) The human hippocampus: functional anatomy, vascularization and serial sections with MRI. Springer, Berlin
El Bahh B, Lespinet V, Lurton D, Coussemacq M, Le Gal La Salle G, Rougier A (1999) Correlations between granule cell dispersion, mossy fiber sprouting, and hippocampal cell loss in temporal lobe epilepsy. Epilepsia 40:1393–1401
Engel J Jr, Van Ness PC, Rasmussen TB, Ojemann LM (1993) Outcome with respect to epileptic seizures. In: Engel J Jr (ed) Surgical treatment of the epilepsies. Raven Press, New York, pp 609–621
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317
Fahrner A, Kann G, Flubacher A, Heinrich C, Freiman TM, Zentner J, Frotscher M, Haas CA (2007) Granule cell dispersion is not accompanied by enhanced neurogenesis in temporal lobe epilepsy patients. Exp Neurol 203:320–332
Frotscher M, Haas CA, Forster E (2003) Reelin controls granule cell migration in the dentate gyrus by acting on the radial glial scaffold. Cereb Cortex 13:634–640
Haas CA, Dudeck O, Kirsch M, Huszka C, Kann G, Pollak S, Zentner J, Frotscher M (2002) Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy. J Neurosci 22:5797–5802
Harding B, Thom M (2001) Bilateral hippocampal granule cell dispersion: autopsy study of 3 infants. Neuropathol Appl Neurobiol 27:245–251
Heinrich C, Nitta N, Flubacher A, Muller M, Fahrner A, Kirsch M, Freiman T, Suzuki F, Depaulis A, Frotscher M, Haas CA (2006) Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus. J Neurosci 26:4701–4713
Houser CE, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta VA (1990) Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci 10:267–282
Houser CR (1990) Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res 535:195–204
Houser CR (1992) Morphological changes in the dentate gyrus in human temporal lobe epilepsy. Epilepsy Res Suppl 7:223–234
Janszky J, Janszky I, Schulz R, Hoppe M, Behne F, Pannek HW, Ebner A (2005) Temporal lobe epilepsy with hippocampal sclerosis: predictors for long-term surgical outcome. Brain 128:395–404
Kesner RP, Lee I, Gilbert P (2004) A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci 15:333–351
Kobow K, Jeske I, Hildebrandt M, Hauke J, Hahnen E, Buslei R, Buchfelder M, Weigel D, Stefan H, Kasper BS, Pauli E, Blumcke I (2009) Increased Reelin promoter methylation associates with granule cell dispersion in human temporal lobe epilepsy. J Neuropathol Exp Neurol (in press)
Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ (2004) Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci 24:7477–7481
Lurton D, El Bahh B, Sundstrom L, Rougier A (1998) Granule cell dispersion is correlated with early epileptic events in human temporal lobe epilepsy. J Neurol Sci 154:133–136
Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD (2000) Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA 97:4398–4403
Mathern GW, Babb TL, Vickrey BG, Melendez M, Pretorius JK (1995) The clinical-pathogenic mechanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilepsy. Brain 118:105–118
Mathern GW, Kuhlman PA, Mendoza D, Pretorius JK (1997) Human fascia dentata anatomy and hippocampal neuron densities differ depending on the epileptic syndrome and age at first seizure. J Neuropathol Exp Neurol 56:199–212
Mathern GW, Leiphart JL, De Vera A, Adelson PD, Seki T, Neder L, Leite JP (2002) Seizures decrease postnatal neurogenesis and granule cell development in the human fascia dentata. Epilepsia 43(Suppl 5):68–73
Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH (1997) Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 17:3727–3738
Pauli E, Hildebrandt M, Romstock J, Stefan H, Blumcke I (2006) Deficient memory acquisition in temporal lobe epilepsy is predicted by hippocampal granule cell loss. Neurology 67:1383–1389
Rakic P, Caviness VS Jr (1995) Cortical development: view from neurological mutants two decades later. Neuron 14:1101–1104
Scharfman HE, Goodman JH, Sollas AL (2000) Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci 20:6144–6158
Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21
Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410:372–376
Siebzehnrubl F, Blumcke I (2008) Neurogenesis in the human hippocampus and its relevance to temporal lobe epilepsies. Epilepsia 49:55–65
Squire LR, Stark CE, Clark RE (2004) The medial temporal lobe. Annu Rev Neurosci 27:279–306
Stefan H, Hildebrandt M, Kerling F, Kasper B, Hammen T, Doerfler A, Weigel D, Buchfelder M, Blumcke I, Pauli E (2009) Clinical prediction of postoperative seizure control: structural, functional findings and disease histories. J Neurol Neurosurg Psychiatry 80(2):196–200
Thom M, Martinian L, Williams G, Stoeber K, Sisodiya SM (2005) Cell proliferation and granule cell dispersion in human hippocampal sclerosis. J Neuropathol Exp Neurol 64:194–201
Thom M, Zhou J, Martinian L, Sisodiya S (2005) Quantitative post-mortem study of the hippocampus in chronic epilepsy: seizures do not inevitably cause neuronal loss. Brain 128:1344–1357
van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH (2002) Functional neurogenesis in the adult hippocampus. Nature 415:1030–1034
von Lehe M, Lutz M, Kral T, Schramm J, Elger CE, Clusmann H (2006) Correlation of health-related quality of life after surgery for mesial temporal lobe epilepsy with two seizure outcome scales. Epilepsy Behav 9:73–82
Wieser HG (2004) ILAE Commission Report: mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 45:695–714
Zeineh MM, Engel SA, Thompson PM, Bookheimer SY (2003) Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science 299:577–580
Zhao S, Chai X, Forster E, Frotscher M (2004) Reelin is a positional signal for the lamination of dentate granule cells. Development 131:5117–5125
Acknowledgments
We kindly acknowledge the technical assistance of Silke Sterner and Birte Rings. The work is supported by the German research council (DFG Bl 421/1-2; SFB TR3 A1 & C6), the European Community (LSH-CT-2006-037315 EPICURE), and Elan-Fond of the Faculty of Medicine (University of Erlangen-Nuremberg). The Neuropathological Reference Center for Epilepsy Surgery is a consortium of colleagues from the following German epilepsy centers: Berlin: H.J. Meencke, M. Merschhemke, N.T. Lehmann. Bielefeld: V. Hans, A. Ebner, H·W. Pannek, F. Woermann. Bonn: A. Becker, P. Niehusmann, C. Elger, C. G. Bien, C. Helmstaedter, J. Schramm, H. Clusmann, H. Urbach. Erlangen: I. Blümcke, M. Hildebrandt, R. Buslei, R. Coras, H. Stefan, B. Kasper, E. Pauli, M. Buchfelder, D. Weigel, A. Dörfler, T. Engelhorn. Freiburg/Kehl-Kork: B. Steinhoff, A. Schulze-Bonhage, S. Fauser, J. Zentner, C. Scheiwe. Greifswald: S. Vogelgesang. Marburg: F. Rosenow, H. Hamer, S. Knake. Munich: P.A. Winkler, S. Noachtar. Munster: W. Paulus. Radeberg: K. Grohme, T. Mayer. Stuttgart: P. Winkler. Ulm: H. Lerche, Y. Wagner. Vogtareuth: H. Holthausen, T. Pieper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blümcke, I., Kistner, I., Clusmann, H. et al. Towards a clinico-pathological classification of granule cell dispersion in human mesial temporal lobe epilepsies. Acta Neuropathol 117, 535–544 (2009). https://doi.org/10.1007/s00401-009-0512-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-009-0512-5