Abstract
The immunoreactivity of the serotoninergic receptor subtype 1A (5HT1AR) was quantitatively analyzed in the human infant brainstem medulla (caudal and rostral levels). We hypothesized that immunoreactivity of 5HT1AR would be reduced in infants diagnosed with sudden infant death syndrome (SIDS). In particular that those infants with known clinical risk factors (including cigarette smoke exposure, bed sharing and sleep position) would have greater changes than those without clinical risks. Comparing SIDS (n = 67) to infants who died suddenly with another diagnosis (non-SIDS, n = 25), we found decreased 5HT1AR immunoreactivity in the majority of the nuclei studied at the rostral medulla level including dorsal motor nucleus of the vagus (DMNV), nucleus of the solitary tract, vestibular, and inferior olivary nucleus (ION). There was a significant relationship with all risk factors for 5HT1AR, especially for DMNV, suggesting that 5HT1ARs are highly vulnerable to various insults within the SIDS DMNV. This study not only provides further evidence of abnormalities within the brainstem serotoninergic system of SIDS infants, but also shows that these changes may be associated with exposure to clinical risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sudden infant death syndrome (SIDS) is defined as “the sudden unexplained death of an infant less than 1-year of age after a thorough case investigation, including a complete autopsy, examination of a death scene, and review of the clinical history” [33]. In most developed countries, SIDS is the most common single cause of death in the post-neonatal period (1–12 months). However, the specific cause of death remains unknown. One fundamental hypothesis is that SIDS infants have abnormal brainstem control of cardiorespiratory function during hypoxia [9]. Thus, one avenue of SIDS research is to identify abnormal brainstem pathology.
The serotoninergic (5-HT) system is a productive area of current research in SIDS. There are seven sub-families of 5-HT receptors, 5-HT1–7, consisting of a total of 14 structurally and pharmacologically distinct mammalian 5-HT receptor subtypes [5]. The small amount of information available regarding the regional distribution of 5-HT receptors in the infant human brainstem shows that these receptors are highly expressed during early development and decreases with age [12]. The receptors are expressed in most nuclei of the brainstem, predominantly related to serotonin-containing cells in the raphe nuclei [12, 20].
Comparing SIDS to non-SIDS brainstems, abnormal 5-HT receptor expression and binding have been reported; 5-HT1A-D and 5HT2 receptor binding densities are reduced in the arcuate nucleus, nucleus of raphé obscurus, inferior olivary nucleus, nucleus of the paragigantocellularis lateralis, nucleus of the gigantocellularis and intermediate reticular zone [13, 21, 23]. 5-HT1A and 5-HT2A receptor immunoreactivity are also decreased in the dorsal motor nucleus of the vagus, nucleus of the solitary tract, and ventrolateral medulla in SIDS infants [20]. Finally, a positive association between serotonin transporter (5HTT) promoter polymorphism and SIDS has been reported [19, 32].
Several risk factors have now been associated with SIDS. There is a male predominance, and epidemiological associations have been found with sleep position (prone sleeping) and cigarette smoke exposure (reviewed in [6]). Despite strong epidemiological evidence for these risk factors, pathological associations with these risk factors are rare. Receptor binding studies of the serotoninergic system found positive associations with gender [23] and cigarette smoke exposure [13].
Studies at the protein level using immunohistochemistry have the advantage over receptor binding studies in that they can identify and localize the cellular distribution of proteins as well as allowing visual identification of specific cell types. By undertaking immunohistochemistry, this study provides an investigation of the protein immunoreactivity for the serotoninergic receptor subtype 1A (5HT1AR) in the human infant brainstem. We focused on the 5HT1AR subtype because this subtype appears to be one of the main ones involved in brain development [2], has a high distribution and expression in medullary regions of cardiorespiratory regulation [13, 20, 23], and known roles in cardiorespiratory control [14, 15], sleep regulation [8], neural development, and apoptosis (reviewed in [1]). Of particular interest is its inhibitory actions in the central respiratory network; it suppresses hypoxic activation of respiratory neurons [25], and decreases ventilatory responses to hypercapnia [29], two physiological consequences thought to occur in the eitiology of SIDS. Also, because of previous findings that this subtype is abnormally expressed in SIDS; immunoreactivity [20] and binding density [23] were significantly reduced in multiple medullary nuclei of SIDS cases.
Another major abnormal finding in the SIDS brainstem is increased apoptosis [17, 18, 31]. Several regulatory pathways may be involved in this process, including serotoninergic and/or glutamatergic receptors. For example, activation of 5HT1AR results in cell survival against certain insults including ischemia and NMDA mediated activities [1]. Thus, the 5HT1ARs act as neuroprotectants. Based on these regulatory roles, our hypothesis is that the immunoreactivity of 5HT1AR is reduced (consistent with loss of protective activity) in SIDS infants compared to non-SIDS infants. To test this hypothesis, we compared the immunoreactivity results from SIDS infants to those from infants who died of known causes (non-SIDS). Additional analyses examined associations between these brainstem changes and characteristics of the infants including age, gender, and history of cigarette smoke exposure, prone sleeping, or bed sharing, where we postulated that there would be an association between abnormalities of the immunoreactivity of 5HT1AR and these clinical risks.
Materials and methods
Data and tissue collection
Each subject included in this study had a death scene report, clinical history, and autopsy. These data and brain tissue were obtained from the Department of Forensic Medicine, Glebe, NSW, Australia. All cases were number coded and any identifying information was removed.
The dataset was sourced from all cases of sudden infant deaths (1–12 months of age) between January 1997 and December 2002. From 196 cases collected over the period of study only 92 cases were eligible for inclusion. Cases were excluded if they were known to have cause for neurological damage such as head injury or evidence of shaken impact syndrome (n = 7), if a diagnosis of SIDS was doubted (positional asphyxia, or a bed-sharing (sleeping) accident where the clinical history or death scene investigation suggested possible overlaying, or ‘undetermined/other’; n = 28), or if brain tissue was not available (n = 17). Moreover, only cases for which the brain was fixed in the same fixative, either 10 or 20% neutral buffered formalin (NBF), were included in this study. This resulted in the exclusion of another 52 cases for which the brain was fixed in a solution of NBF and glacial acetic acid.
Cases were included in this study if the death scene report, clinical history, and autopsy were complete and paraffin blocks of tissue from the medulla were available after the brain had been examined by a neuropathologist. Thus, tissue used in this study was residual in diagnostic tissue blocks after the post-mortem examination was complete. Coronal sections of the brainstem were cut at 7 μm thickness and mounted onto silanized glass slides. Approval was obtained from the Sydney Human Ethics Committee and Central Sydney Area Health Service.
Immunohistochemistry
Immunostaining was performed using a guinea pig polyclonal antibody against 5HT1AR (BD Pharmingen, catalogue no. 550469). This antibody has been well characterized and its specificity established in brain tissue by the manufacturer and by Collin et al. [7]. The immunohistochemistry method was performed as reported in detail previously [26]. All steps were undertaken at room temperature unless otherwise noted. In brief, all the tissue sections went through deparaffinisation, rehydration of ethanols to water, and antigen retrieval by microwaving in a Tris/EDTA buffer (1 mM EDTA, 1 mM sodium citrate, 2 mM Tris; pH 9.0) on high power (Black & Decker, Maryland, 700 W, USA) for 14 min. Sections were quenched for endogenous peroxidase activity, blocked in 10% normal horse serum (NHS) for 30 min and then incubated in the primary antibody (1:500 in 1% NHS) overnight at 4°C. Sections were then incubated in the biotinylated anti-guinea pig secondary antibody (Vector Laboratories Inc., BA-7000) for 40 min, followed with the avidin/biotin-horseradish peroxidase enzyme reagent (Vector Laboratories, VEPK-4000) for 30 min. Brown color reaction was created with diaminobenzidine (DAB) (Dako Laboratories) for 4 min, counterstained with Harris’ Hematoxylin, and then mounted in DPX. Negative controls consisted of sections immunostained as mentioned above except that the primary antibody was replaced with 1% NHS.
Brainstem nuclei of interest
Two brainstem medullary levels (rostral and caudal) were studied. Tissue sections from the rostral level corresponded to Fig. 21 of the Atlas of the human brainstem [24], while those from the caudal level corresponded to Fig. 15. Not all infant cases had tissue sections available at both levels. The nuclei quantified at both levels included the hypoglossal (XII), dorsal motor nucleus of the vagus (DMNV), inferior olivary nucleus (ION), nucleus of the solitary tract (NTS), cuneate nucleus (CUN), raphé nucleus (RAPHE) and arcuate nucleus (AN). The vestibular nucleus (VEST) was studied in the rostral medulla. The RAPHE included the raphé obscurus and raphé pallidus, thus, the entire midline raphé.
Quantification
All quantification was performed with the use of computerized image analysis systems at the Australian Key Centre for Microscopy and MicroAnalysis, University of Sydney. Images were captured at 10× magnification using the Nikon Eclipse E800 light microscope and sensicam CCD camera. The number of images captured per nucleus ranged from 2 to 6, depending on the size of each nucleus. All slides were coded so that analysis was performed blinded to the diagnosis.
A manual cell count included only neurons to identify and report the percent of positive stained neurons. The presentation of 5HT1AR counts as a percentage, standardizes the results amongst the different cases, consistent with our previous published data [16, 17]. The correlation between our results and global neuronal density within the nuclei, which would require stereotactic counting, is not known.
Statistical analysis
Data were collated and exported to SPSS for Windows (V14.0, Chicago, USA) for statistical analysis. Clinical and autopsy characteristics were compared between groups (SIDS and non-SIDS) using Student’s t test, and results presented as mean ± SD.
For 5HT1AR staining quantification, results are presented as the mean percentage of positively stained neurons (% positive) ± SEM. Since all data were normally distributed, two-way analysis of variance (ANOVA) with post-hoc between group comparisons was used.
To assess whether any changes could be attributed to developmental processes, analyses were undertaken for all infants. Subgroup analyses were then undertaken to compare diagnostic groups (SIDS vs. non-SIDS). Where a variable was found to have positive correlation, analysis of covariance (ANCOVA) was applied using co-variates to compare the diagnostic groups. A P value of ≤ 0.05 was considered statistically significant.
Results
Dataset characteristics
The detailed characteristics of this infant dataset were reported recently [17, 18]. In brief, the SIDS infants included n = 67 (39 males and 28 females) and the non-SIDS included n = 25 (18 males and 7 females). The causes of death of infants in the non-SIDS group included pneumonia (n = 6), Waterhouse-Friderichsen syndrome ± septicaemia (n = 4), pneumococcal meningitis (n = 1), Chiari type II malformation (n = 1), lower respiratory tract infection (RTI) (n = 1), brompheniramine toxicity and RTI (n = 1), aspiration of gastric contents (n = 1), myocarditis (n = 3), dilated cardiomyopathy (n = 1), congenital heart disease (n = 2), gastroenteritis (n = 1), leukemia (n = 1), drowning (n = 1), and necrotising enterocolitis (n = 1).
The ranges in age and birth weight were comparable between the SIDS and non-SIDS groups (P > 0.05 for both), as were autopsy characteristics such as body and brain weight, head circumference and body length (summarized in Table 1). Tissue-related factors that had the potential to affect staining such as post-mortem interval (PMI) and fixation time, did not differ between the two diagnostic groups; non-SIDS versus SIDS PMI = 21.3 ± 13.4 versus 25.5 ± 21.6 h (P = 0.4), fixation time = 7.1 ± 2.9 versus 6.3 ± 3.7 weeks (P = 0.3).
The clinical risk factors analyzed in this study included a history of prone sleeping, bed sharing, and cigarette smoke exposure. Not all data were available for all cases. Sleep position data included the usual sleep position and the position found prior to death, categorized into prone and non-prone. Data regarding cigarette smoke exposure was obtained via parental self report and did not discriminate between active or passive exposure. Thus, infants with any exposure to cigarette smoke (from any person in the household) were analyzed as having a positive history of smoke exposure. Amongst SIDS infants, 7 out of 57 (12%) usually slept prone, but 33 out of 59 SIDS cases (56%) were found prone. Similar percentages were also seen for the non-SIDS group [usually slept prone = 1/12 (8%), found prone = 4/12 (33%)]. Bed sharing was reported in 32% of SIDS cases (n = 20/62) [compared to 8% (1/13) of non-SIDS cases]. Most of the deaths occurred at night whether they were of the SIDS (39/64; 61%) or non-SIDS group [night time of death = 10/16 (63%)]. The majority of our SIDS cases (43/53; 81%) had a history of cigarette smoke exposure, and although this was higher than the number of non-SIDS cases (7/12; 58%), the difference was not statistically significant (P = 0.1).
Staining pattern
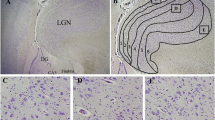
Immunoreactivity for 5HT1AR was seen in all the nuclei studied at both medullary levels. Staining was specifically localized to the cytoplasmic membrane and/or cytoplasm of neurons and was visualized by a dark brown color (Fig. 1). Thus, all neurons with brown colour staining darker than the surrounding tissue in the cytoplasmic membrane (with or without staining in the cytoplasm), were counted as positive. Light stained neurons, similar to the background, and with no obvious membrane staining were considered negative. Regionally, 5HT1AR immunoreactivity was highest in the XII and lowest in the RAPHE (Table 2).
5HT1AR staining in the DMNV and ION of a SIDS infant compared to a non-SIDS infant at the rostral medulla level. Positive neurons for 5HT1AR are characterized by a dark brown stain (black arrows) and negative neurons are characterized by a light brown stain (white arrows), similar to the background. Note the decrease in the number of positive neurons in the SIDS tissue compared to non-SIDS tissue. Scale bar 30 μm for panels a and b, and 50 μm for panels c and d
Quantitative analysis for developmental characteristics, SIDS versus non-SIDS, and risk factors
Within the entire dataset, there was no relationship between postconceptional age and 5HT1AR immunoreactivity. However, a positive correlation was found with postconceptional age in the SIDS group, evident for the DMNV (caudal DMNV P = 0.01, r 2 = 0.4; rostral DMNV P = 0.01, r 2 = 0.37) (Fig. 2). Gender differences were seen in the non-SIDS group where males had higher 5HT1AR immunoreactivity than females in the caudal AN (P = 0.01), but a lower immunoreactivity than the females in the rostral NTS (P = 0.008) (Table 2).
A scatter plot of 5HT1AR immunoreactivity in the dorsal motor nucleus of the vagus at the a caudal medulla level and b rostral medulla levels in the SIDS (solid squares) and non-SIDS (solid diamonds) according to postconceptional age, with added regression lines for comparison (SIDS, dotted line; non-SIDS, solid line)
Comparing the SIDS to non-SIDS infants, 5HT1AR immunoreactivity was significantly reduced in the SIDS infants in the caudal ION, and in the rostral DMNV, NTS, CUN, VEST, and ION compared to non-SIDS group (Table 2). Subgroup analysis by gender (SIDS compared to non-SIDS) showed that compared to non-SIDS males, SIDS males had decreased immunoreactivity in the ION (both levels) and in the rostral DMNV, and VEST. Compared to non-SIDS females, SIDS females had decreased immunoreactivity in the rostral DMNV, NTS, and CUN, but increased immunoreactivity in the AN (Table 2).
For the entire group, cigarette smoke exposure was associated with decreased 5HT1AR immunoreactivity in the rostral DMNV (P = 0.05) and ION (P = 0.03), and in the caudal CUN (P = 0.03). Within the SIDS cohort, cigarette smoke exposure was associated with decreased 5HT1AR in the rostral AN (P = 0.04) (Table 3). Infants in the SIDS group who were found prone had lower 5HT1AR immunoreactivity compared to SIDS infants found non-prone, in the caudal DMNV (P = 0.02) and CUN (P = 0.02) (Table 4). Finally, SIDS infants with a history of bed sharing had significantly higher 5HT1AR immunoreactivity compared to the SIDS infants with no history of bed sharing in the caudal XII (yes vs. no 80.3 ± 2.6 vs. 71.9 ± 2.3 respectively; P = 0.045).
Discussion
This study describes and quantifies the immunoreactivity of the serotoninergic receptor subtype 1A in several nuclei of the caudal and rostral medulla of human infants. The expression of this receptor was compared between non-SIDS and SIDS infants. Additional analyses examined for associations with age, gender, and the clinical risk factors of cigarette smoke exposure, sleep position, and bed sharing. The most important findings were that (a) there was decreased neuronal 5HT1AR immunoreactivity in SIDS infants compared to non-SIDS infants, affecting the majority of nuclei studied, with many more nuclei affected at the rostral than caudal medulla level and (b) changes in 5HT1AR immunoreactivity, predominantly in the DMNV, showed a significant relationship with all of the clinical risk factors.
The cellular localization of 5HT1AR immunostaining has been reported as predominantly somatic and dendritic, with occasional observations in the axonal hillock [1]. We found that 5HT1AR immunostaining within neurons was strongest along the cytoplasmic membrane and within the cytoplasm (i.e. somatic). Some fibrous, presumably dendritic, staining was also occasionally seen in some medullary regions. This distribution is thus consistent with the recognized role of the 5HT1AR as a somato-dendritic autoreceptor [4]. The predominant somatic versus dendritic expression that we observed may be representative of the immaturity of many of the neurons expressing 5HT1ARs, based on the findings that expression of 5HT1AR in the hippocampus is initially localized to the cell body and gradually shifts to the dendrites during postnatal development as the neurons mature [22]. It seems likely that they are newly synthesized and are not fully functional as they seem to still be internalized, but this assumption needs to be verified using a different antibody to 5HT1AR. Differences in cellular staining distributions have been reported using different antibodies and are thought to be due to region specific masking of the epitope, membrane anchoring, or chain modification [3].
Regionally, 5HT1AR immunostaining was seen in all nuclei of the medulla, and is consistent with previous IHC [20] and binding [13, 21, 23] reports. However, in contrast to binding studies, we found that the RAPHE had the lowest quantitative 5HT1AR immunostaining expression compared to the other nuclei. This discrepancy in expression between binding and immunostaining may be because our staining is detecting autoreceptors (with somatic distribution), whereas the binding studies are detecting both auto- and hetero 5HT1A receptors.
Our laboratory previously reported an increase in apoptosis in the SIDS medulla [17, 18, 31]. In addition, we previously reported changes in the NMDA system [16]. Our present findings support a role of the serotoninergic system in generating or promoting the increased apoptosis. Our findings also support our hypothesis that SIDS infants have decreased 5-HT1AR (indicative of a loss of protective activity) because of the known role of 5HT1ARs in protecting against cell death (reviewed in [3]). Decreased 5HT1AR immunoreactivity was consistent in the rostral and the caudal ION, but for the other nuclei, the decrease was statistically evident only at the rostral medulla level, and only in the DMNV, NTS, vestibular and cuneate nuclei. Similar decreases in 5HT1AR immunoreactivity have been reported in a Japanese cohort of SIDS infants in the DMNV and NTS [20]. In an American SIDS cohort, decreased 5HT1AR binding was also found in the NTS, but in contrast to our findings, not in the ION [23]. Another contrast is that we did not find decreased 5HT1AR immunoreactivity in the AN and RAPHE of SIDS infants despite the prior report of reduced 5HT1AR binding found in these nuclei [23]. Although the difference in results could be due to the difference in methodology, binding versus immunohistochemistry, it may also be indicative that changes in 5HT1AR are more evident at the functional, rather than expressional level; i.e. changes are only evident in the receptor once it is functional and not at the early stage of its protein expression. However, regional dependency cannot be excluded since expressional changes for 5HT1ARs correlate with functional changes in the NTS of SIDS infants.
One hypothesis for the mechanism of SIDS is that the infants have abnormal brainstem control of cardiorespiration [9]. Our current findings in the DMNV and NTS support this, as do those of Ozawa and Okado [20] and Paterson et al. [23]. Amongst other functions, these nuclei are known to regulate heart rate, blood pressure, and respiratory drive. For example, in rats, activation of 5HT1AR in DMNV increased central respiratory drive, evidenced by increased activity in the parasympathetic cardiac [28] and phrenic nerves [30]. Thus, a decrease in 5HT1AR in the DMNV and NTS could contribute to the abnormal heart rate [10, 27] and respiratory responses [11] that have also been reported in (future) SIDS infants.
Changes that predominated in one or the other gender included decreased 5HT1AR in the DMNV, NTS, and increased 5HT1AR in the AN of females. Amongst males, the ION showed decreased 5HT1AR. Such gender-related differences are not unusual and have been reported for example in the RAPHE where 5HT1AR binding was lower in male SIDS compared with female SIDS infants [23]. The 5HT1AR findings from this current study closely mimic the 5HT1AR findings we recently reported from our piglet model of intermittent hypercapnic hypoxia (IHH) [26]. In that study, we reported that after IHH there was decreased 5HT1AR immunoreactivity in the DMNV of female piglets, while decreased immunoreactivity occurred in the ION in male piglets. These findings thus suggest that females are more vulnerable to hypoxia-induced changes in 5HT1AR expression within the DMNV, while for the males, it is the ION that appears to have greater vulnerability. The different direction of change in the female SIDS AN (increased) was unexpected and seems to be due to the fact that baseline 5HT1AR immunoreactivity is lower in females than in males (when comparing non-SIDS females to males). This result needs to be interpreted with caution since this baseline difference between the genders was only evident at the caudal AN, with no difference at rostral AN, and may be due to the lower number of female cases (n = 4) at the caudal level.
Only two previous studies have examined the relationships between epidemiological risk factors for SIDS and changes in serotoninergic expression [13, 23]. The one positive association found was of decreased 3H-lysergic acid diethylamide (LSD) binding (which binds to all 5HT1A-D and 5HT2 receptor subtypes) in the AN of infants exposed to cigarette smoke [13]. No associations were found for PH8 (a marker of serotonin containing neurons) immunoreactivity or for specific 5HT1AR binding using 3H8-hydroxy-2-[di-N-propylamino]-tetralin (3H-8-OH-DPAT) [23]. In contrast, we found positive associations with exposure to cigarette smoke, prone sleep position and bed sharing. One likely explanation for this is our larger dataset which allowed us to reach statistical power, and our specific targeting of the 1A receptor protein. These are important findings because they provide biological correlates with epidemiological findings, and offer a potential etiological mechanism between SIDS and these risk factors.
Exposure to cigarette smoke was associated with decreased 5HT1AR immunoreactivity in most of the nuclei we studied, with statistically significant changes in the DMNV (rostral level only), ION and cuneate nucleus of cigarette smoke exposed infants, regardless of their diagnosis. A decreased pattern in rostral DMNV and ION was still evident when studying the SIDS group alone, although smoke-exposed SIDS infants also demonstrated decreased immunoreactivity in the rostral AN. Cigarette smoke exposure appears to affect 5HT1ARs in several medullary nuclei, with the caveat that smoke exposure was self-reported, and does not differentiate between active or passive exposure. Regardless, the finding that the 5HT1A changes were associated with smoke exposure is significant and again highlights the vulnerability of 5HT1ARs, especially in the DMNV of SIDS infants. Interestingly, in our animal studies, piglets exposed to postnatal nicotine also had significant reduction in 5HT1AR immunoreactivity in the DMNV [26]. This raises the possibility that nicotine contributes to the 5HT1AR changes seen in SIDS infants, and that postnatal cigarette smoke exposure, as opposed to a purely prenatal exposure, may also precipitate the changes.
Our model of IHH most closely represents rebreathing in prone sleeping infants, and we have found similar pathology in SIDS infants found prone and piglets exposed to IHH [26]. Here, decreased 5HT1AR immunoreactivity is present in the DMNV of both piglets and infants, so we postulate that a hypercapnic-hypoxic environment has a causal association with decreased 5HT1AR immunoreactivity. The implications of increased 5HT1AR in XII of SIDS infants who had a history of bed sharing remains unclear, and is complicated in our dataset by the fact that 94% of the bed sharing infants were also exposed to cigarette smoke.
In conclusion, using a large infant dataset, we present evidence of abnormal expression of the serotoninergic receptor 1A protein in the brainstem of SIDS compared to non-SIDS infants. Additional, novel data show that some of these findings are associated with exposure to the risk factors of cigarette smoke exposure and prone sleeping. These findings further support a role of the brainstem serotoninergic system in the etiology of SIDS.
References
Azmitia EC (2001) Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull 56:413–424
Azmitia EC, Whitaker-Azmitia PM (1997) Development and adult plasticity of serotoninergic neurons and their target cells. In: Baumgarten HG, Geothert M, Aghajanian GK (eds) Serotoninergic neurons and 5-HT receptors in the CNS. Springer, Berlin
Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM (1996) Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharm 14:35–46
Barnes NM, Sharp T (1999) A review of central 5-HT receptors and their function. Neuropharm 38:1083–1152
Baumgarten HG, Geothert M, Aghajanian GK (1997) Serotoninergic neurons and 5-HT receptors in the CNS. Springer, Berlin
Byard RW, Krous HF (2004) Research and sudden infant death syndrome: definitions, diagnostic difficulties and discrepancies. J Paediatr Child Health 40:419–421
Collin M, Backberg M, Onnestam K, Meister B (2002) 5-HT1A receptor immunoreactivity in hypothalamic neurons involved in body weight control. Neuroreport 13:945–951
Darnall RA, Harris MB, Gill WH, Hoffman JM, Brown JW, Niblock MM (2005) Inhibition of serotonergic neurons in the nucleus paragigantocellularis lateralis fragments sleep and decreases rapid eye movement sleep in the piglet: implications for sudden infant death syndrome. J Neurosci 25:8322–8332
Hunt CE (1992) The cardiorespiratory control hypothesis for sudden infant death syndrome. Clin Perinatol 19:757–771
Kahn A, Riazi J, Blum D (1983) Oculocardiac reflex in near miss for sudden infant death syndrome infants. Pediatrics 71:49–52
Kato I, Franco P, Groswasser J, Kelmanson I, Togari H, Kahn A (2000) Frequency of obstructive and mixed sleep apneas in 1, 023 infants. Sleep 23:487–492
Kinney HC (2005) Abnormalities of the brainstem serotonergic system in the sudden infant death syndrome: a review. Pediatr Dev Pathol 8:507–524
Kinney HC, Randall LL, Sleeper LA, Willinger M, Belliveau RA, Zec N, Rava LA, Dominici L, Iyasu S, Randall B, Habbe D, Wilson H, Mandell F, McClain M, Welty TK (2003) Serotonergic brainstem abnormalities in Northern Plains Indians with the sudden infant death syndrome. J Neuropath Exp Neuro 62:1178–1191
Lalley PM, Bischoff AM, Richter DW (1994) Serotonin 1A-receptor activation suppresses respiratory apneusis in the cat. Neurosci Lett 172:59–62
Lalley PM, Bischoff AM, Schwarzacher SW, Richter DW (1995) 5-HT2 receptor-controlled modulation of medullary respiratory neurones in the cat. J Physiol 487:653–661
Machaalani R, Waters KA (2003) NMDA receptor 1 expression in the brainstem of human infants and its relevance to the sudden infant death syndrome (SIDS). J Neuropathol Exp Neurol 62:1076–1085
Machaalani R, Waters KA (2008) Neuronal cell death in the sudden infant death syndrome brainstem and associations with risk factors. Brain 131:218–228
Machaalani R, Rodriguez M, Waters KA (2007) Active caspase-3 in the sudden infant death syndrome (SIDS) brainstem. Acta Neuropathol 113(5):577–584
Narita N, Narita M, Takashima S, Nakayama M, Nagai T, Okado N (2001) Serotonin transporter gene variation is a risk factor for sudden infant death syndrome in the Japanese population. Pediatrics 107:690–692
Ozawa Y, Okado N (2002) Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics 33:142–149
Panigraphy A, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC (2000) Decreased serotonergic receptor binding lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol 59:377–384
Patel TD, Zhou FC (2005) Ontogeny of 5-HT1A receptor expression in the developing hippocampus. Brain Res Dev Brain Res 157:42–57
Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC (2006) Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA 296:2124–2132
Paxinos G, Huang X (1995) Atlas of the human brainstem. Academic Press, California
Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM (1999) Neurotransmitters and neuromodulators controlling the hypoxic respiratory responses in anaesthesized cats. J Physiol 514:567–578
Say M, Machaalani R, Waters KA (2007) Changes in serotoninergic receptors 1A and 2A in the piglet brainstem after intermittent hypercapnic hypoxia (IHH) and nicotine. Brain Res 1152:17–26
Schwartz PJ, Stramba Badiale M, Segantini A, Austoni P, Bosi G, Giorgetti R, Grancini F, Marni ED, Perticone F, Rosti D, Salice P (1998) Prolongation of the QT interval and the sudden infant death syndrome. N Engl J Med 338:1709–1714
Sporton SC, Shepheard SL, Jordan D, Ramage AG (1991) Microinjections of 5-HT1A agonists into the dorsal motor vagal nucleus produce a bradycardia in the atenolol-pretreated anaesthetized rat. Br J Pharmacol 104:466–470
Taylor NC, Li AH, Nattie EE (2005) Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol 566:543–557
Teng YD, Bingaman M, Taveira-DaSilva AM, Pace PP, Gillis RA, Wrathall JR (2003) Serotonin 1A receptor agonists reverse respiratory abnormalities in spinal cord-injured rats. J Neurosci 23:4182–4189
Waters KA, Meehan B, Huang JQ, Gravel RA, Michaud J, Cote A (1999) Neuronal apoptosis in sudden infant death syndrome. Pediatr Res 45:166–172
Weese-Mayer DE, Berry-Kravis EM, Maher BS, Silvestri JM, Curran ME, Marazita ML (2003) Sudden infant death syndrome: association with a promoter polymorphism of the serotonin transporter gene. Am J Med Genet 117:268–274
Willinger M, James LS, Catz C (1991) Defining the sudden infant death syndrome (SIDS): deliberations of an expert panel convened by the National Institute of Child Health and Human Development. Pediatr Pathol 11:677–684
Acknowledgments
This research was funded by the SIDS Foundation of South Australia, NH&MRC (#302006), and A/Prof. Waters was supported by an NH&MRC Practitioner Fellowship (#206507). The authors thank the staff of the Department of Forensic Medicine, Glebe, NSW, Australia, for their co-operation in this study, and acknowledge the facilities as well as scientific and technical assistance from the staff of the NANO Major National Research Facility at the Electron Microscope Unit, University of Sydney.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Machaalani, R., Say, M. & Waters, K.A. Serotoninergic receptor 1A in the sudden infant death syndrome brainstem medulla and associations with clinical risk factors. Acta Neuropathol 117, 257–265 (2009). https://doi.org/10.1007/s00401-008-0468-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0468-x