Abstract
This report concerns an immunohistochemical investigation on RNA-related proteins in the basophilic inclusions (BIs) from patients with adult-onset atypical motor neuron disease. Formalin-fixed, paraffin-embedded sections of the motor cortex and the lumbar spinal cord were examined. The BIs appeared blue in color with H&E and Nissl stain, and pink with methylgreen–pyronin stain. Ribonuclease pretreatment abolished the methylgreen–pyronin staining, suggesting that the BIs contained RNA. Immunohistochemically, the BIs were distinctly labeled with the antibodies against poly(A)-binding protein 1, T cell intracellular antigen 1, and ribosomal protein S6. These proteins are essential constituents of stress granules. In contrast, the BIs were not immunoreactive for ribosomal protein L28 and decapping enzyme 1, which are core components of transport ribonucleoprotein particles and processing bodies, respectively. Moreover, the BIs were not immunopositive for TDP-43. Our results imply that translation attenuation could be involved in the processes of BI formation in this disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The classic type of sporadic amyotrophic lateral sclerosis (ALS) usually affects middle-aged or older people, and shows clinical manifestations confined to the motor neuron system with characteristic pathological features such as motor neuron loss and presence of Bunina bodies, hyaline inclusions, and skein-like inclusions.
On the other hand, besides the classic ALS, the El Escorial criteria proposed for the diagnosis of ALS include those for ALS-plus syndromes, which are defined by an association of ALS with extrapyramidal features, dementia, ocular movement abnormalities, or autonomic dysfunction [6]. Neuropathologically, certain characteristic inclusions such as ubiquitin-positive intraneuronal inclusions have been reported in occasional cases with ALS-plus syndromes in addition to the motor neuron degeneration [29].
Another example of such inclusions is round intracytoplasmic basophilic inclusions (BIs), designated as type R-III by Munoz [23], which are characterized by their staining property of being basophilic with hematoxylin and eosin (H&E) stain, poorly argyrophilic, reactive with Nissl stain, and immunohistochemically negative for tau and giving weak staining, if any, for ubiquitin [9, 18, 19, 31]. These histological features clearly differentiate this type of inclusion from Pick bodies and ubiquitin-positive intraneuronal inclusions [29]. These distinctive BIs have been identified in patients with juvenile-onset motor neuron disease [26], with adult-onset atypical motor neuron disease with ophthalmoplegia or gaze palsy and dysuria [18, 19, 31], with a frontal lobe syndrome [22], and with a combination of motor and cognitive dysfunction [10, 12]. However, these BIs have not been observed in several systematic studies on cases of classic ALS [11, 24]. Ultrastructurally, this type of inclusion has been reported to consist of thick filamentous structures of 12–25 nm in diameter studded with electron-dense ribosome-like granules [10, 19, 22, 31].
The detailed constituents of the BIs and pathomechanism responsible for their formation remain to be elucidated. Considering their basophilic nature it is conceivable that the BIs would contain RNA and its related proteins. Therefore, in the present study we investigated, immunohistochemically, the possible presence of RNA-related proteins in the BIs from two autopsied patients previously reported as having had adult-onset motor neuron disease with BIs (MND–BIs) [18, 19].
Materials and methods
We investigated autopsied material from two patients with MND–BIs, three cases with classical ALS, and two cases with frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusions with motor neuron disease (FTLD-U with MND) [9] for comparison.
Both patients with MND–BIs were male, one 45 years old (Case 1) [18] and the other 58 years old (Case 2) [19]. Case 1 manifested right upper weakness at age 37, followed by slowly progressive generalized muscular weakness. He died of respiratory failure 8.5 years after onset. Throughout the clinical course, there were no signs other than upper and lower motor neuron involvement. Case 2 presented right foot weakness at age 53, followed by slowly progressive generalized weakness. At age 58, downward gaze paresis and double incontinence developed. Artificial ventilation was performed for 1 month due to respiratory failure, and then he died of bronchopneumonia. In both cases no family history of neurological disorders was noted. Neuropathological examinations of these cases revealed loss of upper and lower motor neurons, and the presence of neuronal intracytoplasmic BIs with widespread distribution throughout the central nervous system.
Paraffin-embedded 7-μm-thick sections of formalin-fixed motor cortex and the lumbar spinal cord of both cases were stained with H&E, Nissl stain, and methylgreen–pyronin stain with or without bovine ribonuclease (Wako Pure Chemical Industries, Ltd, Osaka, Japan) pretreatment for 1 h at 37°C. For immunohistochemistry, we employed primary antibodies against the representative RNA-related proteins listed in Table 1. For all the primary antibodies, after pretreatment with hydrating autoclaving (10 min at 121°C in 10 mM sodium citrate buffer), the sections were incubated with a given primary antibody overnight at 4°C. Bound primary antibodies were detected with the appropriate Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA), with 3,3′-diaminobenzidine tetrahydrochloride used as the chromogen. All sections were counterstained with hematoxylin after immunohistochemistry. The tissues from three age-matched neurologically normal subjects served as controls.
The classical ALS cases (63-year-old male and 78- and 81-year-old females) were characterized histologically by loss of the spinal anterior horn cells and the appearance of Bunina bodies, round hyaline inclusions, and skein-like inclusions in the cytoplasm of the remaining ones. In the FTLD-U with MND patients (62-year-old male and 63-year-old female) the presence of intracytoplasmic inclusions in the granular cells of the hippocampal dentate gyrus was a feature additional to those features of classical ALS. For investigation of these inclusions immunohistochemically, formalin-fixed paraffin-embedded sections of the lumbar spinal cords from the ALS cases and those of the hippocampus from the FTLD-U with MND cases were first stained with H&E, photographed, decolorized with 70% ethanol, and then immunostained with the same antibodies used in the present study.
The staining specificity was assessed by replacing the primary antibodies with the appropriate amount of phosphate-buffered saline solution containing 3% bovine serum albumin or by pre-incubating the primary antibodies with an excess of their respective peptide immunogen. No reaction–product deposits were seen in the sections thus treated.
Results
In both cases of MND–BIs, histological examinations demonstrated BIs in the cytoplasm of neurons in the motor cortex and lumbar anterior horn. There were no essential differences between these BIs in these two locations in terms of morphology and histochemical staining properties.
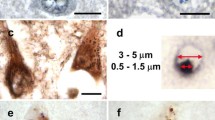
The BIs appeared blue in color with H&E (Fig. 1a) and with Nissl stain. We occasionally encountered some BIs with small vacuoles within their structure. When methylgreen–pyronin staining was done, the BIs had a pink color (Fig. 1b). Pretreatment of the sections with ribonuclease abolished the staining of these BIs (Fig. 1c), suggesting that the BIs contained RNA.
Representative photomicrographs of basophilic inclusions (BIs) in neurons in the motor cortex (Case 1). a An intracytoplasmic BI is stained blue with H&E (arrow). b A BI shows pink staining with the methylgreen–pyronin technique (arrow). c Pretreatment with ribonuclease abolishes the pink color of the methylgreen–pyronin-stained BI (arrow). Scale bar 10 μm
Poly(A)-binding protein 1 (PABP1) immunohistochemistry of sections of the cerebral cortex and spinal cord of the control subjects showed the neuronal cytoplasm being more intensely labeled than the nucleus (Fig. 2a). In both MND–BIs cases, virtually all the BIs observed in the cerebral cortex and the spinal cords were obviously labeled by the anti-PABP1 antibody (Fig. 2b–d). The BIs showed a homogeneous staining pattern at their entire structure or somewhat stronger staining at their periphery. The vacuoles observed in some BIs were devoid of PABP1-immunolabeling. On the other hand, in the cytoplasm of the neurons containing BIs, the PABP1 immunoreactivity appeared to be reduced (Fig. 2b–d).
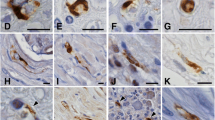
Immunostaining of motor cortical neurons and lumbar spinal neurons from control subjects (a, e, g, i, l) and patients with MND–BIs (b, c, d, f, h, j, k, m, n). a A neuron in the motor cortex from a control subject shows the cytoplasm diffusely labeled with the anti-PABP1 antibody, and the nucleus stained less intensely. b–d Neurons in the motor cortex from Case 1 (b), and neurons in the spinal anterior horn from Case 1 (c) and Case 2 (d), immunostained for PABP1. The BIs are evidently immunopositive for PABP1 (arrows). Note the reduced immunoreactivity in the cytoplasm of the neurons containing BIs. e, g In neocortical neurons from control subjects, immunoreactivity indicating ribosomal protein S6 (e) and ribosomal protein L28 (g) is seen in the cytoplasm, but not in the nucleus. f, h A BI in the motor cortex from an MND–BIs case (Case 1) is immunolabeled by the anti-rpS6 antibody, with the staining being somewhat weaker at the center (f, arrow). In contrast, the anti-rpL28 antibody does not bind to the BI (h, the motor cortex from Case 1, arrow). i A neuron in the motor cortex from a normal individual shows diffuse staining in the cytoplasm with the antibody against Dcp1. j A BI (arrow) at the motor cortex from the MND–BIs Case 1, showing no reactivity for Dcp1. k A BI in a cortical neuron from Case 1, showing no reactivity for TDP-43 (arrow). l A neuron in the motor cortex from a control subject, stained with the anti-goat polyclonal antibody against TIA-1 and showing the predominant labeling of the nucleus (arrowhead). m, n BIs within a cortical neuron from Case 1 (m) and within a lumbar anterior horn neuron from Case 2 (n) are evidently reactive with the same anti-TIA-1 antibody employed in “l” (arrows). The nuclei of the neurons containing BIs show reduced immunoreactivity for TIA-1 (arrowheads). Scale bar 10 μm
In the sections from control cases incubated with the anti-ribosomal protein S6 (rpS6; Fig. 2e) or anti-ribosomal protein L28 (rpL28; Fig. 2g) antibody, the immunoreaction product deposits were recognizable as clusters of very fine granules in the cytoplasm, but were not observed within the nucleus, of the neocortical and spinal anterior horn neurons. In both MND–BIs cases, the BIs were labeled by the anti-rpS6 antibody (Fig. 2f) similarly as with the anti-PABP1 antibody; and the rpS6-immunoreactivity in the BIs appeared to have a homogeneous distribution pattern throughout their entire structure or was somewhat less intense at the center. On the other hand, the staining intensity with the anti-rpS6 antibody in the cytoplasm of the neurons containing BIs appeared to be decreased. In contrast, the anti-rpL28 antibody did not bind to the BIs at all (Fig. 2h).
The immunoreactivity for decapping enzyme 1 (Dcp1) was diffusely recognizable in the cytoplasm, but not in the nucleus, of the neurons examined in the control subjects (Fig. 2i). The BIs were not distinctly labeled by this anti-Dcp1 antibody (Fig. 2j).
The antibody against TDP-43, another member of RNA-binding protein family [4, 7, 8, 27], did not react with the BIs of our cases (Fig. 2k), being consistent with findings presented recently [9].
The three distinct anti-T cell intracellular antigen-1 (TIA-1) antibodies employed in the present study gave similar staining results for both the control subjects and the MND–BI cases. The TIA-1 immunoreactivities were identified principally within the nucleus of the cortical and spinal neurons from the normal individuals (Fig. 2l). In the MND–BI patients, virtually all the BIs were definitely labeled throughout their entire structure by all three anti-TIA-1 antibodies recognizing a distinct epitope within the TIA-1 protein (Fig. 2m, n). In addition, the nucleus of the BI-containing neurons appeared to show reduced TIA-1-immunoreactivity (Fig. 2m, n; arrowheads).
In both MND–BI cases the BIs were not labeled with the anti-alpha-internexin antibody (data not shown).
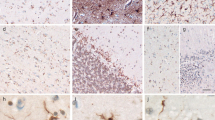
Neither the round hyaline inclusions and the skein-like inclusions of the anterior horn cells of the classical ALS cases nor the intracytoplasmic inclusions in the dentate granular cells of the FTLD-U with MND patients showed immunoreactivities for any of the antibodies employed in the present study (Fig. 3; arrows). In contrast, these inclusions were invariably labeled by the anti-TDP-43 antibody (data not shown).
Representative photomicrographs of lumbar anterior horn cells from patients with classical ALS. a, c Round hyaline inclusions identified by H&E are depicted (arrows). b, d The same sections as “a” and “c” were decolorized and re-stained with the anti-PABP1 and anti-TIA-1 antibodies, respectively. Note that the inclusions are negative for reactivity with either antibody (arrows). Scale bar 10 μm
Discussion
In the present study, we for the first time demonstrated the presence of immunoreactivities indicating the presence of certain mRNA-related proteins in the BIs from the two cases with MND–BIs examined; in both cases the BIs in the motor cortex and the lumbar anterior horn were invariably immunopositive for PABP1, rpS6, and TIA-1, but devoid of labeling with antibodies against rpL28 or Dcp1.
We previously reported that the BIs in our patients showed only partially positive immunoreactivity for ubiquitin and that they were negative for tau [18, 19]. In the present study, we demonstrated that the antibody against TDP-43 did not label the BIs. Recent reports [9, 33] indicate that neurodegenerative disorders characterized by the presence of ubiquitin-, tau-, and TDP-43-negative intracytoplasmic inclusions include neuronal intermediate filament inclusion disease (NIFID) and basophilic inclusion body disease (BIBD). These reports recommend that apha-internexin immunohistochemistry is useful for differentiating these disorders. In the present study, we performed alpha-internexin immunohistochemistry and demonstrated that the inclusions of our patients did not react with the antibody. These results indicate that our patients are different from NIFID, but would be closely related to the entity of BIBD.
It was earlier shown, using histochemical procedures, that the BIs from a sporadic juvenile ALS case include RNA [26]. This notion is supported by the present methylgreen–pyronin-staining results with and without ribonuclease in our MND–BI cases. Additionally, Oda et al. [28] ultrastructurally examined the BIs of the sporadic juvenile ALS case, and reported that the BIs contained ‘ribosomes’. In the present study, we demonstrated that the BIs of our cases were immunohistochemically positive for rpS6, one of the ribosomal proteins, thus implying that at least a part of the RNA included in the BIs would have been derived from ribosomes.
To infer the possible processes involved in BI formation, here we further looked for the presence of three representative mRNA-binding proteins, each having a distinct function, and two ribosomal proteins in the BIs. As depicted in Fig. 4, PABP1 binds to the poly(A) tail of mRNA and plays a significant role in mRNA stabilization and translation [20]. Dcp1 is a decapping enzyme that initiates the actual mRNA decay by removing the cap structure from the 5′ end of the mRNA. TIA-1 is supposed to bind a glutamine-rich prion-related domain, and promotes general translational arrest in response to environmental stress [15], as also shown in Fig. 4. On the other hand, a ribosome consists of a small 40S subunit and a large 60S subunit; ribosomal protein S6 is a major component of the 40S subunit of the eukaryotic ribosome, and rpL28 is that of the 60S subunit. Using specific antibodies for these proteins, we found that PABP1, TIA-1, and the small 40S subunit of ribosomes would be involved in the BI formation, whereas Dcp1 and the large 60S subunit of ribosomes would not. These results strongly suggest that, besides ribosomal RNA, translationally silenced mRNA would be included in the BIs of our cases. The relationship between these RNA-binding proteins identified in the present study and transferrin, recently demonstrated within the BIs [21], remains to be elucidated.
Schematic representation of messenger RNA (mRNA) translation processes in the neuronal cytoplasm, under situations with or without stress. Messenger RNAs (mRNAs) having their poly(A) tail bound by poly (A)-binding proteins (PABP) are capped and interact with eukaryotic initiation factors (eIFs) 4E/4G. Then, the small ribosomal subunit associated with other eIFs binds to the 5′ end of an mRNA molecule. In the physiological state without any stress, a ternary complex consisting of the eIFs 2B/5, GTP, and initiator transfer RNA (tRNA) coupled to methionine, is also assembled at the 5′ end of the transcript to form the canonical 48S preinitiation complex, and scanning starts. Recognition of the initiation codon triggers displacement of eIFs by the 60S ribosomal subunit. The mRNA molecules being under translation are usually found in the form of polyribosomes (also known as polysomes). After completion of translation, Cap is dissociated from the transcript by decapping enzymes such as Dcp1, and the mRNA is transferred into the processing body for degradation (not shown). On the other hand, under certain stress conditions, stress-activated kinases such as PERK and PKR inhibit the formation of eIF 2B/5-GTP-tRNA complexes. Consequently, TIA-1 is included in a 48S* complex that is deficient in the ternary complex. These 48S* preinitiation complexes are translationally silent, and thus the 60S ribosomal subunits do not interact with them. The self-aggregation tendency of TIA-1 then promotes the accumulation of the complexes at discrete cytoplasmic foci known as stress granules
In the neuronal cytoplasm, some mRNAs are programmed to be immediately translated, but others are programmed for delayed translation and are transported into dendrites and stored until cues call for their translation [5, 17]. After productive translation, polysomes are disassembled and mRNAs are deadenylated, decapped, and then degraded [2]. When cells are exposed to stress, the translation of mRNAs encoding enzymes involved in damage repair is enhanced, whereas the translation of those encoding housekeeping proteins is aborted [2]. These translationally silenced mRNAs are known to be packaged into neuronal RNA granules. There are at least three types of neuronal RNA granules identified to date: transport ribonucleoprotein particles (transport RNPs), processing bodies, and stress granules [16]. Different types of neuronal RNA granules share some protein components, but also contain certain unique markers specific to each granule [2, 16].
Transport RNPs contain, besides mRNA, small 40S and large 60S ribosomal subunits, translation initiation factors, and certain RNA-binding proteins such as Staufen and survival of motor neurons protein, which regulate mRNA function [2]. However, this type of granule definitely lacks PABP1 [17]; and thus the mRNAs within the transport RNPs are not translated despite the presence of intact ribosomes. The processing bodies are structures associated with mRNA decay, and thus certainly contain proteins involved in mRNA degradation such as Dcp1, GW182, and Lsm proteins, as their core components [2, 16, 30]. On the other hand, translation initiation factors and ribosomes are generally lacking from the processing bodies [3]. These findings imply that, considering our immunohistochemical results, the BIs of our cases would be distinct from transport RNPs because of the presence of PABP1 immunoreactivity and absence of that for 60S ribosomal proteins. Similarly, the BIs would be different from the processing bodies because the BIs were positive for 40S ribosomal proteins and negative for Dcp1. Moreover, a very recent report demonstrated that TDP-43 is localized in the dendritic processing bodies [32]. The lack of TDP-43 immunoreactivity in the BIs of our cases further supports the notion that the BIs would be structures distinct from the processing bodies.
On the other hand, stress granules are discrete cytoplasmic structures that appear under conditions of cellular stress [1]. This type of granule is composed largely of stalled preinitiation complexes, and contains mRNA, PABP1, TIA-1, and small 40S ribosomal subunits but not those of large 60S, as their principal components (Fig. 4). Among them, TIA-1 is essential to trigger stress granules formation. TIA-1 is concentrated within the nucleus under steady-state conditions, but is normally able to shuttle between the nucleus and the cytoplasm. Although its functions have not been precisely elucidated, this protein is thought to be associated with the signaling cascade regulating alternative splicing of some mRNAs in the nucleus [1, 13]; whereas in the cytoplasm it is considered to act as a translational suppressor [13]. In response to environmental stresses such as heat, hyperosmolarity, and oxidative conditions, TIA-1 is introduced into the cytoplasm and binds mRNAs to form a non-canonical, 48S* preinitiation complex that is translationally silent. TIA-1 self-aggregation then promotes the rapid accumulation of these complexes to form stress granules [15].
The novel constituents of the BIs identified in the present immunohistochemical study are consistent with those of the stress granules, and thus these inclusions might be equivalent to them. However, the stress granules induced in in vitro experiments are invariably multiple in number and small with a granular appearance [13]; whereas the BIs are found singly in a single neuron and comparable to the nucleus in size. These discrepancies might be attributable to the difference between cultured cells and human neuronal cells in vivo. On the other hand, stress granules are supposed to be induced within 15–20 min after cells are exposed to a general stress factor; and removal of the stress results in immediate dissolution of stress granules within 1.5–3.0 h, after which the cell returns to a translation-active state [1]. The persistence of stress leads to cell death [14]. However, in other experimental studies concerning real-time observations of stress granules, stress granule formation starts as minute foci that coalesce and enlarge with time [15]. Therefore, it is conceivable that the stress granules might aggregate to form the BIs under unique situations such as a pulsating or a weak stress continuing for a long time. These notions are advocated by our previous observations made on inclusion body myositis. We reported many miniature PABP1-positive foci being more often located in non-vacuolated fibers, and large PABP1-positive inclusions being more abundant in vacuolated fibers [25]. We speculate that the numerous miniature PABP1-positive deposits may correspond to the beginning of stress granule formation and that the dense aggregates of PABP1-positive deposits may the mature form of stress granules. This notion implies that stress granules can aggregate to form a larger structure. Further investigations are warranted to elucidate the involvement of stress granules in inclusion formation and neurodegeneration.
References
Anderson P, Kedersha N (2002) Stressful initiations. J Cell Sci 115:3227–3234
Anderson P, Kedersha N (2006) RNA granules. J Cell Biol 172:803–808
Andrei MA, Ingelfinger D, Heintzmann R, Achsel T, Rivera-Pomar R, Lührmann R (2005) A role for eIF4E and eIF4E-transporter in targeting mRNPs to mammalian processing bodies. RNA 11:717–727
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Bramham CR, Wells DG (2007) Dendric mRNA: transport, translation and function. Nat Rev Neurosci 8:776–789
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases (2000) El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Buratti E, Dörk T, Zuccato E, Pagani F, Romano M, Baralle FE (2001) Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J 20:1774–1784
Buratti E, Baralle FE (2001) Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem 276:36337–36343
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL 3rd, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM, Consortium for frontotemporal lobar degeneration (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol (Berl) 114:5–22
Hamada K, Fukazawa T, Yanagihara T, Yoshida K, Hamada T, Yoshimura N, Tashiro K (1995) Dementia with ALS features and diffuse Pick body-like inclusions (atypical Pick’s disease?). Clin Neuropathol 14:1–6
Hirano A, Iwata M (1979) Pathology of motor neurons with special reference to amyotrophic lateral sclerosis and related disease. In: Tsubaki T, Toyokura Y (eds) Amyotrophic lateral sclerosis. University of Tokyo Press, Tokyo, pp 107–133
Ishihara K, Araki S, Ihori N, Shiota J, Kawamura M, Nakano I (2006) An autopsy case of frontotemporal dementia with severe dysarthria and motor neuron disease showing numerous basophilic inclusions. Neuropathology 26:447–454
Ivanov PA, Nadezhdina ES (2006) Stress granules: RNP-containing cytoplasmic bodies arising in stress: structure and mechanism of organization. Mol Biol 40:844–850
Kedersha NL, Gupta M, Li W, Miller I, Anderson P (1999) RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol 147:1431–1442
Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, Golan DE, Anderson P (2000) Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol 151:1257–1268
Kiebler MA, Bassell GL (2006) Neuronal RNA granules: movers and makers. Neuron 51:685–690
Krichevsky AM, Kosik KS (2001) Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron 32:683–696
Kusaka H, Matsumoto S, Imai T (1990) An adult-onset case of sporadic motor neuron disease with basophilic inclusions. Acta Neuropathol (Berl) 80:660–665
Kusaka H, Matsumoto S, Imai T (1993) Adult-onset motor neuron disease with basophilic intraneuronal inclusion bodies. Clin Neuropathol 12:215–218
Mangus DA, Evans MC, Jacobson A (2003) Poly(A)-binding proteins: multi-functional scaffolds for the post-transcriptional control of gene expression. Genome Biol 4:223–236
Mizuno Y, Amari M, Takatama M, Aizawa H, Mihara B, Okamoto K (2006) Transferrin localizes in Bunina bodies in amyotrophic lateral sclerosis. Acta Neuropathol 112:597–603
Munoz-Garcia D, Ludwin SK (1988) Classic and generalized variants of Pick’s disease: a clinicopathological, ultrastructual, and immunocytochemical comparative study. Ann Neurol 16:467–480
Munoz DG (1998) The pathology of pick complex. In: Munoz DG (ed) Pick’s disease and pick complex. Wiley, New York, pp 211–241
Murayama S, Mori H, Ihara Y, Bouldin TW, Suzuki K, Tomonaga M (1990) Immunocytochemical and ultrastructural studies of lower motor neurons in amyotrophic lateral sclerosis. Ann Neurol 27:137–148
Nakano S, Shinde A, Ito H, Ito H, Kusaka H (2005) Messenger RNA degradation may be inhibited in sporadic inclusion body myositis. Neurology 65:420–425
Nelson JS, Prensky AL (1972) Sporadic juvenile amyotrophic lateral sclerosis. a clinic-pathological study of a case with neuronal cytoplasmic inclusions containing RNA. Arch Neurol 27:300–306
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Oda M, Akagawa N, Tabuchi Y, Tanabe H (1978) A sporadic juvenile case of the amyotrophic lateral sclerosis with neuronal intracytoplasmic inclusions. Acta Neuropathol (Berl) 44:211–216
Okamoto K, Hirai S, Yamazaki T, Sun XY, Nakazato Y (1991) New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett 129:233–236
Parker R, Sheth U (2007) P bodies and the control of mRNA translation and degradation. Mol Cell 25:635–646
Sasaki S, Toi S, Shirata A, Yamane K, Sakuma H, Iwata M (2001) Immunohistochemical and ultrastructual study of basophilic inclusions in adult-onset motor neuron disease. Acta Neuropathol (Berl) 102:200–206
Wang IF, Wu LS, Chang HY, Shen CK (2008) TDP-43, the signature protein of FTLD-U, is a neuronal activity-responsive factor. J Neurochem 105:797–806
Yokota O, Tsuchiya K, Terada S, Ishizu H, Uchikado H, Ikeda M, Oyanagi K, Nakano I, Murayama S, Kuroda S, Akiyama H (2008) Basophilic inclusion body disease and neuronal intermediate filament inclusion disease: a comparative clinicopathological study. Acta Neuropathol 115:561–575
Acknowledgment
We express our sincere appreciation to Miss Tomoko Takemi for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujita, K., Ito, H., Nakano, S. et al. Immunohistochemical identification of messenger RNA-related proteins in basophilic inclusions of adult-onset atypical motor neuron disease. Acta Neuropathol 116, 439–445 (2008). https://doi.org/10.1007/s00401-008-0415-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0415-x