Abstract
Defects of major histocompatibility complex (MHC) class I antigen-processing machinery (APM) components have been shown to contribute to immune escape of malignant cells. We investigated the expression of APM components in astrocytomas without detectable defects in HLA class I antigen expression and correlated it with grade of malignancy. Quantitative immunohistochemical analysis of astrocytomas revealed reduced expression of the cytosolic proteasome subunit low molecular weight protein 2 (LMP2), the endoplasmatic reticulum (ER) transporter associated with antigen processing-1 (TAP1), and the ER chaperone β2-microglobulin (β2m) in astrocytoma cells when compared to astrocytes from nonpathological brain. Among human WHO grade II–IV astrocytomas, downregulation of LMP2, TAP1 and β2m correlated with grade of malignancy. Furthermore, astrocytoma cell lines (n = 12) expressed all APM components analyzed at levels comparable to dendritic cells (DC), which were used for comparative purposes. However, upregulation of β2m after stimulation with inflammatory cytokines was significantly lower in astrocytoma cell lines than in control cells. Our results support the hypothesis that coordinated downregulation or impaired upregulation of certain HLA class I APM components may serve as a mechanism for astrocytoma cells to evade the host’s immune response, even if HLA class I antigen surface expression is not altered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumor cells express antigens that enable their immunological recognition as “altered-self” cells. These so-called tumor antigens are not expressed or they are expressed much less in nonmalignant cells, or their expression is considered to be limited to certain developmental stages [41]. One hypothesis for how tumors are able to escape from immune surveillance postulates the inadequate activation of cytotoxic T lymphocytes (CTL) that recognize tumor antigens [7]. Efficient recognition and elimination of cells expressing tumor antigens by CTL is dependent on proper processing and presentation of the respective antigens by an intact major histocompatibility complex (MHC) class I APM [37].
During the last decade, various components of the MHC class I antigen-processing machinery (APM) have been identified, and their functions in the processing of endogenous proteins have been characterized [20, 30]. After ubiquitination, proteins are catalytically degraded by the cytosolic multiproteasome complex, which comprises the constitutive subunits delta, MB1, and Z, as well as the interferon-γ-inducible subunits LMP2, LMP7, and LMP10 [13]. The newly generated peptides are subsequently transported to the endoplasmatic reticulum (ER) by TAP, which consists of TAP1 and TAP2 [14]. Within the ER, the chaperones calnexin, calreticulin, tapasin, and β2m ensure proper loading of the peptides onto MHC class I molecules [2]. Subsequently, β2-microglobulin (β2m) stabilizes the newly synthesized MHC class I–peptide complex during translocation to the cell surface via the Golgi compartment [26]. MHC–peptide complexes can then be recognized by the T cell receptor in the context of CD8 (trimolecular complex), eventually leading to T-cell receptor activation and cytolytic damage of the presenting target cell. A prerequisite for the “immune” elimination of transformed cells, therefore, is an intact MHC class I APM, which ensures appropriate processing of antigens expressed by tumor cells.
MHC class I APM alterations have been documented in many malignancies and proposed as a potential mechanism for tumor escape [9]. Interestingly, dysregulations of MHC class I APM components can also occur in tumor cells expressing normal levels of MHC class I molecules [36]. Intact MHC class I surface expression in combination with defective antigen processing might protect tumor cells from natural killer cell (NK cell)-mediated lysis without enabling target cell recognition by T cells [4, 10, 23].
Among nonmetastatic brain tumors, high-grade astrocytomas carry the poorest prognosis. Despite resection, radiotherapy, and chemotherapy, the median survival of glioblastomas (WHO grade IV) is approximately 12 months [42]. The malignancy of these tumors can to some extent be ascribed to their ability to effectively evade the immune system, although infiltration by lymphocytes and macrophages is frequently found within the lesions [44]. The lack of efficient immune responses can partly be attributed to tumor-derived immunosuppressive molecules such as TGF-β, HLA-G or HLA-E [44, 47, 49–51]. It has recently been reported that the grade of malignancy in human brain tumors correlates with decreased MHC class I antigen expression [8]. However, MHC class I molecules were lost and downregulated in 46.2 and 36.2%, respectively, of the glioblastomas analyzed. MHC class I expression was unaltered in 17.6% of the lesions. Furthermore, no correlation between MHC class I expression and disease-free interval and survival was found. It can therefore be hypothesized that failure of the immune system to recognize and eliminate MHC class I positive astrocytoma cells may contribute to inadequate presentation of tumor antigens, as has been reported for other MHC class I positive tumors [36]. In this work we assessed the expression and regulation of various molecules of the MHC class I APM in astrocytomas positive for MHC class I in vivo and in vitro.
Material and methods
Patients and tissue specimens
Brain tumor specimens from 16 patients were examined. Diagnosis and histological grading was done by two independent neuropathologists according to the World Health Organization (WHO) grade I–IV classification [18]. Our study included four patients with WHO grade I astrocytoma (pilocytic astrocytoma): two females and two males, mean age of 26 years, range 9–38 years; four patients with WHO grade II astrocytoma (diffuse astrocytoma): one female and three males, mean age of 65 years, range 43–84 years; four patients with WHO grade III astrocytoma (anaplastic astrocytoma): two females and two males, mean age of 44 years, range 35–67 years; four patients with WHO grade IV astrocytoma (glioblastoma): four males, mean age of 56 years, range 41–73 years). Two nonpathological brain tissue specimens obtained from the brain bank of the Brain Research Institute of the University of Tübingen served as controls.
Monoclonal and polyclonal antibodies and reagents
The mAb HC-10 which recognizes a determinant expressed on β2m-free HLA-A10, -A28, -A29, -A30, -A31, -A32 and -A33 heavy chains and on all β2m-free HLA-B and C heavy chains [31], the mAb HCA-2 which recognizes a determinant expressed on β2m-free HLA-A (except -A24), -B7301 and -G heavy chains [39], the β2m-specific mAb L368 [22], the delta-specific mAb SY-5, the MB-1-specific mAb SJJ-3, the Z-specific mAb NB1, the LMP2-specific mAb SY-1, the LMP7-specific mAb HB2, the LMP10-specific mAb TO-6, the TAP1-specific mAb N0B1, the TAP2-specific mAb, N0B2, the calnexin-specific mAb TO-5, the calreticulin-specific mAb TO-11, and the tapasin-specific mAb TO-3 [1, 29, 45] were developed and characterized as described. The HLA-DR-specific PE mAb L243, the CD80-specific mAb BB-1, and the CD86-specific mAb B-T7 were purchased from BD PharMingen, Heidelberg, Germany. Biotinylated rabbit anti-mouse F(ab’)2 fragments was purchased from Dako, Glostrup, Denmark. Goat anti-mouse-phycoerythrin (PE) IgG (H + L) F(ab’)2 fragments were purchased from Dianova, Hamburg, Germany. Mouse IgG2a G155-178 was purchased from BD PharMingen, and mouse IgG1κ-FITC and mouse IgG1κ-PE MOPC-21 were purchased from Sigma, Deisenhofen, Germany.
Cell lines
The human glioma cell lines A172, D247MG, LN-18, LNT-229, LN-308, LN-319, LN-428, T98G, U87MG, U138MG, U251MG, and U373MG (kindly provided by Dr. N. de Tribolet, Neurosurgical Service, Centre Hospitalier Universitaire, Vaudois, Lausanne, Switzerland) were cultured in 75 cm3 Falcon plastic flasks (BD Biosciences, Heidelberg, Germany) using DMEM supplemented with 1% glutamine (Life Technologies, Paisley, UK), 10% FCS (Biochrom, Berlin, Germany), and penicillin (100 IU/ml)/streptomycin (100 μg/ml) (Life Technologies) [49].
Culture of dendritic cells (DC)
Dendritic cells (DC) were cultured from peripheral blood mononuclear cells (PBMC) as described previously [34]. In brief, PBMC were isolated from peripheral blood of healthy volunteers by density gradient centrifugation using PAA Separating Solution (PAA Laboratories, Cölbe, Germany). Monocytes were depleted by adhesion to plastic flasks for 1 h. Monocytes, >90% pure, as assessed by flow cytometry, were cultured in RPMI 1640 medium supplemented with 10% FCS, GM-CSF (100 ng/ml) (Leukomax, Sandoz, Germany) and IL-4 (40 ng/ml) (PeproTech EC LTD, London, UK). After a six-day incubation at 37 °C, cells exhibited an immature DC phenotype (MHC class II, CD86low, CD80low). Maturation was induced by incubation of immature DC with LPS (5 μg/ml, S. thyphi, Sigma L-7261), TNF-α (10 ng/ml), and IFN-γ (1000 U/l) (both from PeproTech EC Ltd., London, UK) [48]. High levels of surface MHC class II and costimulatory molecules (CD86, CD80) identified mature DC.
Immunohistochemistry
All tissues were fixed in buffered 4% formalin (pH 7.4) and paraffin-embedded by routine methods. Washing steps, dilution of antibodies and addition of peroxidase-conjugated avidin–biotin complex were performed in Tris-buffered saline (pH 7.5) with 0.2% Tween. Samples were cut into 4-μm slices, deparaffinized with chloroform, and rehydrated with alcohol in descending order (100–70%). Antigens were retrieved by microwave treatment for 8 min at 700 W in citrate buffer (pH 6.0) followed by blockade of endogenous peroxidase with 3% H2O2 in methanol for 15 min at room temperature. After washing, tissue sections were preincubated with tenfold-diluted normal swine serum (Seromed, Berlin, Germany) prior to incubation with MHC class I- and APM component-specific monoclonal antibodies or control antibodies at 20 μg/ml in a humified chamber overnight at 4°C. Tissue sections were then washed and incubated for 30 min at room temperature with biotinylated rabbit anti-mouse F(ab’)2 fragments. The peroxidase-conjugated avidin–biotin complex (ABC) technique (Dako) with 3,3-diaminobenzidine (DAB) (Sigma) used as chromogen was utilized to visualize the specific antigen binding. Tissue sections were counterstained with hemalaun. Two neuropathologists analyzed tissue sections independently. Quantification of immunoreactivity was performed by determining the proportion of positively stained astrocytes among 100 cells in six microscopic fields (magnification: 40×) in the respective areas of the brain specimen. Microglia cells and infiltrating lymphocytes have been reported to account for 0–2% of the cells in WHO grade IV astrocytomas and were excluded via histopathology [3]. All histological examinations were performed and photographical documentation obtained with an Olympus AX70 microscope with a digital camera device (Hamburg, Germany); digital photographs were adjusted for brightness and contrast with Jasc Paint Shop Pro (Corel, Minneapolis, MN, USA).
Flow cytometry
The staining procedure for DC and glioma cell lines with the APM component-specific mAbs was performed as described previously [28]. To obtain intracellular staining of APM components, cells were washed in PBS containing 1% bovine serum albumin (BSA) and subsequently fixed with 2% paraformaldehyde for 20 min at room temperature. After washing in PBS–BSA, cells were resuspended in 10 ml of PBS–BSA buffer and subjected to microwave treatment for 60 s at low power (700 W). Cells were then chilled on ice for 10 min, washed, permeabilized using 0.1% (w/v) saponin in the PBS–BSA buffer, and incubated with human IgG (Alphaglobin, Langen, Germany) at 1.25 mg/ml for 10 min at 4 °C to minimize binding to Fc receptors. After washing, unlabeled or labeled primary antibody (f.c. 10–25 μg/ml) diluted in saponin-containing buffer was placed on ice for 45 min. Monoclonal isotype control antibodies were used at the same concentration as the primary antibodies. Incubation was performed on ice for 45 min, followed by two washes. After incubation with secondary antibodies, cells were washed in saponin buffer, fixed with 1% (w/v) paraformaldehyde, and measured in a FACSCalibur cytometer (Becton Dickinson BD, Heidelberg, Germany) using CellQuestR software for analysis. Histograms of stained cells were analyzed by calculating the specific fluorescence index (SFI: geometric mean of the specific antibody divided by geometric mean of the isotype control antibody). Flow cytometry of cell surface markers was performed essentially as described in [34, 49].
Statistical analysis
Data nested in the different groups were analyzed by Wilk–Shapiro W-test and did not show any signs of non-normality. Therefore, statistical significance was assessed by paired Student’s two-sided t test followed by Bonferroni correction in order to adjust the alpha level to minimize the type I error rate. Since 15 specific antibodies plus isotype control were measured, only a corrected P-value of P < 0.05/16 was considered to be significant.
Results
Association of WHO grade and MHC class I APM molecule downregulation in human astrocytomas
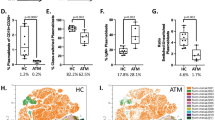
Expression of the MHC class I APM molecules delta, MB1, Z, LMP2, LMP7, LMP10, TAP1, TAP2, calnexin, calreticulin, tapasin, and β2m was found in astrocytes of nonpathological brain specimens (Fig. 1). MHC class I APM molecule expression in astrocytomas with different grades of malignancy was compared to values assessed in astrocytes in nonpathological brain controls. In specimens of MHC class I positive astrocytic brain tumors of WHO grade I–IV, expression of immunoreactive cells for various APM components varied considerably. In grade I astrocytomas, expression of the examined MHC class I APM molecules was comparable to that in astrocytes from nonpathological brain. In contrast to this, in grade IV glioblastomas only 17% ± 5 (S.E.M), 27% ± 3 (S.E.M.), and 33% ± 6 (S.E.M.) immunoreactive cells were found for LMP2 (P = 0.0004), β2m (P = 0.00005), and TAP1 (P = 0.03), whereas the expression of delta, MB1, Z, LMP7, LMP10, TAP1, TAP2, calnexin, calreticulin, and tapasin was comparable to that in grade I astrocytomas and astrocytes of nonpathological brain tissues. Interestingly, the frequencies of β2m- and LMP2-positive cells (P = 0.0001 and 0.003) were also reduced in high-grade astrocytomas compared with low-grade astrocytic tumors, therefore suggesting that alterations of these APM components correlate with the grade of malignancy (Fig. 1). Representative examples of staining patterns of LMP2, β2m, and HLA class I heavy chains in a WHO grade IV glioblastoma and nonpathological brain tissue are illustrated in Fig. 2.
Characterization of MHC class I APM molecule expression in vivo. Results from immunohistochemistry performed on tissue specimens of nonpathological brain tissue (n = 2) and astrocytomas (WHO grade I–IV, four samples each) are presented. Columns indicate mean percentages of positive cells, and error bars represent ±S.E.M.
Expression and regulation properties of MHC class I APM molecules in human astrocytoma cell lines in vitro
We then investigated the APM in astrocytoma cell lines in vitro. Using flow cytometry, we assessed the expression and regulation of MHC class I APM molecules in 12 astrocytoma cell lines and compared these to DC as a paradigm for professional APC. MHC class I APM molecule expression was measured in astrocytoma cell lines cultured in the presence or absence of inflammatory cytokines, which increase the expression of MHC class I APM molecules [48]. To ensure the efficacy of the intracellular staining procedure, MHC class I APM molecule expression was determined in immature DC (iDC) and mature DC (mDC), and expression patterns with a 1.5- to 6-fold increase of expression during DC maturation were observed (data not shown). Next, the basal expression of MHC class I APM molecules was determined in astrocytoma cell lines, which were previously reported to express varying levels of MHC class I molecules [49]. All APM components were found in astrocytic cells, and the expression levels did not reveal significant differences when compared to iDC (Figs. 3 and 4). However, astrocytoma cell lines showed impaired upregulation of β2m upon exposure to MHC class I APM-inducing cytokine stimulation (P = 0.003) (Figs. 4, 5).
Flow cytometric analysis of the MHC class I APM components LMP2, TAP2, calnexin, and β2 microglobulin in cells cultured in the presence and absence of LPS, TNF-α, and IFN-γ. Changes in APM component expression are illustrated as histograms comparing isotype control (solid gray), MHC class I APM component expression after culture with media only (shaded fill), and after culture with LPS, TNF-α, and IFN-γ (open bold line). y-axis: number of events, x-axis: fluorescence. Histograms show one representative experiment (n = 3)
Inducibility of MHC class I APM molecule expression when cultured in the presence of LPS, TNF-α, and IFN-γ was assessed in dendritic cells (DC) and in astrocytoma cell lines using flow cytometry. Columns represent the inducibility (obtained by calculating ratios of specific fluoresecence indices) of MCH class I APM molecule expression in maturing DC (DC; n = 3) and glioma cell lines (glioma; n = 12) after treatment with LPS, TNF-α, and IFN-γ for 24 h. Error bars represent ±S.E.M.
Discussion
Characterization of MHC class I APM molecule expression revealed reduced levels of three APM components in human astrocytomas (LMP2, TAP1, β2m) compared with normal astrocytes in vivo. Importantly, APM expression levels were negatively correlated with the grade of malignancy, grade IV tumors showing the lowest levels of expression. Experiments in 12 astrocytoma cell lines in vitro partly confirmed our results in vivo: while APM components are abundantly expressed in astrocytoma cell lines, upregulation of β2m is significantly impaired.
It has recently been reported that reduced expression of MHC class I molecules correlates with increasing grade of malignancy in human gliomas [8]. Nevertheless, Facoetti et al. found that more than 50% of human glioblastomas express MHC class I molecules. Since MHC class I expression on the cell surface does not necessarily correlate with effective antigen presentation in tumors [25], we focused here on the question of APM alteration in MHC class I molecule-positive astrocytoma specimens. These specimens were defined in terms of their MHC class I expression status by mAb HC10, which exclusively detects free HLA class I heavy chains (HC) that are not loaded with immunogenic peptides [40]. In our study, expression of MHC class I APM molecules was studied in WHO grade I–IV astrocytomas and astrocytes from nonpathological brain, which were used as controls as astrocytic progenitor cells are considered to be the key cellular source of malignant transformation in various gliomas [18].
The three molecules (LMP2, TAP1, β2m) found to be downregulated in high-grade astrocytomas in our study have highly important functions in the complex cascade where peptides are processed and loaded on MHC class I molecules [19]. Consequently, reduced expression or altered regulation of LMP2, TAP1, and β2m in astrocytoma cells expressing MHC class I affects immune recognition of tumor cells by antigen-specific T cells and at the same time promotes MHC-I-mediated inhibition of NK cell immunity. Such “non-antigen-related” peptide-free expression of MHC class I molecules could be employed by astrocytoma cells in two ways: first, it avoids activation and antigen-specific recognition by CD8+ cytotoxic T lymphocytes (CTLs), since T cell receptors only interact with MHC class I molecules that carry a suitable peptide in their binding cleft [11]. Second, activation of NK cells, which might potentially counteract tumor proliferation, is potently hindered by the expression of MHC class I molecules [17, 24]. Accordingly, astrocytomas expressing “peptide-free” MHC class I molecules due to impairment of their APM may enforce immune paralysis.
Furthermore, in addition to the lowered expression of β2m, LMP2, and TAP1 but not that of other molecules of the MHC class I APM in human astrocytomas in vivo, we found that the alterations of APM components correlate with the grade of malignancy (Fig. 1): β2m and LMP2 were expressed in significantly lower frequencies of cells in astrocytomas of WHO grade II–IV than in pilocytic astrocytomas of WHO grade I. This finding is remarkable, as pilocytic astrocytomas have a much better prognosis and are histogenetically not comparable to diffuse astrocytomas and their higher grade variants. In addition to lower levels of β2m- and LMP2-positive cells, high-grade astrocytomas were also characterized by reduced levels of TAP1-positive cells when compared with nonpathological brain astrocytes. To date, only one study has correlated the alterations of MHC class I APM molecules with tumor differentiation in squamous cell carcinomas [27]. Here, we provide the first study to support the hypothesis that expression of MHC class I APM components correlates with the grade of tumor malignancy. Clearly, higher patient numbers are needed to evaluate the correlation of APM alterations with tumor grade and clinical outcomes in glioma patients as assessed in follow-up studies.
Furthermore, simultaneous downregulation of β2m and LMP2 in tumors has not been reported so far. This is of special interest, as the expressions of these substantial players of the MHC class I APM, which are located in different cellular compartments, are regulated independent of each other. This observation illustrates the complex changes that occur in gene expression profiles during the malignant transformation of astrocytomas. Downregulation of the inducible immunoproteasome subunit LMP2 inhibits the processing of antigens at an early or initial point in the cytoplasm, whereas downregulation of β2m and TAP1 impedes proper loading of MHC molecules in the ER. With respect to the immune inhibitory capabilities of astrocytoma cells, the simultaneous downregulation of two APM molecules involved in the loading of MHC molecules in the ER is noteworthy. Since malignant cells are capable of presenting immunogenic antigens such as the Lass5 protein, even if the function of TAP is disturbed [43], multiple alterations or the “coordinated” downregulation of the APM in astrocytomas underline their potential to interfere with alternative forms of antigen presentation.
The finding that all components of the MHC class I APM investigated (delta, Z, LMP2, LMP7, LMP10, TAP1, TAP2, calnexin, calreticulin, tapasin, β2m, MB1, and HLA class I heavy chains) were expressed in the majority of nonpathological brain astrocytes in vivo is noteworthy in view of the potential of glial cells to process and present antigens. The brain has long been considered an immunologically privileged site. However, numerous studies in infectious, autoimmune, and tumor models have challenged this view by showing that potent immune reactions can and do occur in the CNS. Recently, Höftberger et al. provided evidence that various CNS cells can express MHC class I molecules under basal or inflammatory conditions in vivo, indicating that all cells of the CNS are potential targets for class I MHC restricted cytotoxic T cells [15]. Our data underline this notion, although we detected a considerably higher proportion of astrocytes staining positive for MHC class I molecules, and are thus in line with the findings of Facoetti et al. [8]. This discrepancy may have methodological explanations. We and Facoetti et al. used different monoclonal antibodies than Höftberger et al., and the latter only considered cells with simultaneous immunoreactivity for β2m and α-chain as being positive for MHC class I. Our data thus extend these findings by providing evidence of the principal possibility that astrocytes are able to process and present endogenous antigens, a potential function of astrocytes that has probably been underestimated [46]. This may underline the notion of the brain as being immunoprivileged in the way that immunological activity is not absent but rather well controlled [32].
Our in vivo observations with respect to MHC class I APM molecule downregulation in human astrocytomas are substantiated by our in vitro data on the expression and regulation of APM MHC class I components in 12 astrocytoma cell lines. Comparison with the behavior of MHC class I APM in DC provided further evidence for substantial dysregulation of APM components in astrocytoma cells. Upon adequate stimulation with the proinflammatory cytokines TNF-α, IFN-γ, and LPS, upregulation of β2m, LMP2, and delta was reduced in astrocytoma cell lines as compared with DC (Figs. 4, 5). Our results are in accordance with earlier findings showing altered MHC class I APM expression in various malignant cell lines and tumors (breast cancer, renal cancer, bladder cancer, lung cancer, or melanoma) [5, 6, 9, 12, 16, 21, 33, 35, 38]. However, a coincident study correlating the status of MHC class I APM in vivo and in vitro has not yet been performed.
Taken together, our results extend existing data attributing immunological alterations to the immune-deviating nature of malignant astrocytomas [10]. Further studies are required to investigate the extent to which alterations in MHC class I APM may correlate with patient outcome and response to therapy. In view of the concepts considered for immunoselective therapeutic approaches in future glioma therapy (e.g., tumor vaccination), the problem of altered MHC class I APM molecule expression as a potential mechanism for immune escape must be taken into account. The assessment of MHC class I and APM expression may provide valuable information for the selection of patients that are likely to benefit from these strategies.
References
Bandoh N, Ogino T, Cho HS, Hur SY, Shen J, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S (2005) Development and characterization of human constitutive proteasome and immunoproteasome subunit-specific monoclonal antibodies. Tissue Antigens 66:185–194
Bouvier M (2003) Accessory proteins and the assembly of human class I MHC molecules: a molecular and structural perspective. Mol Immunol 39:697–706
Brat DJ, Castellano-Sanchez AA, Hunter SB, Pecot M, Cohen C, Hammond EH, Devi SN, Kaur B, Van Meir EG (2004) Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res 64:920–927
Colonna M, Navarro F, Bellon T, Llano M, Garcia P, Samaridis J, Angman L, Cella M, Lopez-Botet M (1997) A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med 186:1809–1818
Cromme FV, van Bommel PF, Walboomers JM, Gallee MP, Stern PL, Kenemans P, Helmerhorst TJ, Stukart MJ, Meijer CJ (1994) Differences in MHC and TAP-1 expression in cervical cancer lymph node metastases as compared with the primary tumours. Br J Cancer 69:1176–1181
Dissemond J, Busch M, Kothen T, Mors J, Weimann TK, Lindeke A, Goos M, Wagner SN (2004) Differential downregulation of endoplasmic reticulum-residing chaperones calnexin and calreticulin in human metastatic melanoma. Cancer Lett 203:225–231
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3:991–998
Facoetti A, Nano R, Zelini P, Morbini P, Benericetti E, Ceroni M, Campoli M, Ferrone S (2005) Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res 11:8304–8311
Ferrone S, Marincola FM (1995) Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today 16:487–494
Friese MA, Platten M, Lutz SZ, Naumann U, Aulwurm S, Bischof F, Buhring HJ, Dichgans J, Rammensee HG, Steinle A et al (2003) MICA/NKG2D-mediated immunogene therapy of experimental gliomas. Cancer Res 63:8996–9006
Germain RN, Margulies DH (1993) The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol 11:403–450
Giorda E, Sibilio L, Martayan A, Moretti S, Venturo I, Mottolese M, Ferrara GB, Cappellacci S, Eibenschutz L, Catricala C, Grammatico P, Giacomini P (2003) The antigen processing machinery of class I human leukocyte antigens: linked patterns of gene expression in neoplastic cells. Cancer Res 63:4119–4127
Groettrup M, Soza A, Kuckelkorn U, Kloetzel PM (1996) Peptide antigen production by the proteasome: complexity provides efficiency. Immunol Today 17:429–435
Hill A, Ploegh H (1995) Getting the inside out: the transporter associated with antigen processing (TAP) and the presentation of viral antigen. Proc Natl Acad Sci USA 92:341–343
Höftberger R, Aboul-Enein F, Brueck W, Lucchinetti C, Rodriguez M, Schmidbauer M, Jellinger K, Lassmann H (2004) Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol 14:43–50
Kaklamanis L, Leek R, Koukourakis M, Gatter KC, Harris AL (1995) Loss of transporter in antigen processing 1 transport protein and major histocompatibility complex class I molecules in metastatic versus primary breast cancer. Cancer Res 55:5191–5194
Karre K, Ljunggren HG, Piontek G, Kiessling R (1986) Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319:675–678
Kleihues P, Cavenee W (2000) Pathology and genetics of tumours of the nervous system. IARC Press, Lyon
Kloetzel PM (2004) The proteasome and MHC class I antigen processing. Biochim Biophys Acta 1695:225–233
Kloetzel PM, Ossendorp F (2004) Proteasome and peptidase function in MHC-class-I-mediated antigen presentation. Curr Opin Immunol 16:76–81
Korkolopoulou P, Kaklamanis L, Pezzella F, Harris AL, Gatter KC (1996) Loss of antigen-presenting molecules (MHC class I and TAP-1) in lung cancer. Br J Cancer 73:148–153
Lampson LA, Fisher CA, Whelan JP (1983) Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol 130:2471–2478
Lanier LL (2005) NK cell recognition. Annu Rev Immunol 23:225–274
Lanier LL (2000) Turning on natural killer cells. J Exp Med 191:1259–1262
Lopez-Albaitero A, Nayak JV, Ogino T, Machandia A, Gooding W, DeLeo AB, Ferrone S, Ferris RL (2006) Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol 176:3402–3409
Momburg F, Tan P (2002) Tapasin—the keystone of the loading complex optimizing peptide binding by MHC class I molecules in the endoplasmic reticulum. Mol Immunol 39:217–233
Ogino T, Bandoh N, Hayashi T, Miyokawa N, Harabuchi Y, Ferrone S (2003) Association of tapasin and HLA class I antigen down-regulation in primary maxillary sinus squamous cell carcinoma lesions with reduced survival of patients. Clin Cancer Res 9:4043–4051
Ogino T, Wang X, Ferrone S (2003) Modified flow cytometry and cell-ELISA methodology to detect HLA class I antigen processing machinery components in cytoplasm and endoplasmic reticulum. J Immunol Methods 278:33–44
Ogino T, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S (2003) Endoplasmic reticulum chaperone-specific monoclonal antibodies for flow cytometry and immunohistochemical staining. Tissue Antigens 62:385–393
Paulsson KM, Wang P (2004) Quality control of MHC class I maturation. FASEB J 18:31–38
Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F (2003) Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol 171:1918–1926
Prins RM, Liau LM (2003) Immunology and immunotherapy in neurosurgical disease. Neurosurgery 53:144–152; discussion 152–143
Romero JM, Jimenez P, Cabrera T, Cozar JM, Pedrinaci S, Tallada M, Garrido F, Ruiz-Cabello F (2005) Coordinated downregulation of the antigen presentation machinery and HLA class I/beta2-microglobulin complex is responsible for HLA-ABC loss in bladder cancer. Int J Cancer 113:605–610
Schreiner B, Mitsdoerffer M, Kieseier BC, Chen L, Hartung HP, Weller M, Wiendl H (2004) Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J Neuroimmunol 155:172–182
Seliger B, Atkins D, Bock M, Ritz U, Ferrone S, Huber C, Storkel S (2003) Characterization of human lymphocyte antigen class I antigen-processing machinery defects in renal cell carcinoma lesions with special emphasis on transporter-associated with antigen-processing down-regulation. Clin Cancer Res 9:1721–1727
Seliger B, Lichtenfels R, Kellner R (2003) Detection of renal cell carcinoma-associated markers via proteome- and other 'ome'-based analyses. Brief Funct Genomic Proteomic 2:194–212
Seliger B, Maeurer MJ, Ferrone S (2000) Antigen-processing machinery breakdown and tumor growth. Immunol Today 21:455–464
Seliger B, Wollscheid U, Momburg F, Blankenstein T, Huber C (2000) Coordinate downregulation of multiple MHC class I antigen processing genes in chemical-induced murine tumor cell lines of distinct origin. Tissue Antigens 56:327–336
Sernee MF, Ploegh HL, Schust DJ (1998) Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol Immunol 35:177–188
Stam NJ, Spits H, Ploegh HL (1986) Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol 137:2299–2306
Stevanovic S (2002) Identification of tumour-associated T-cell epitopes for vaccine development. Nat Rev Cancer 2:514–520
Surawicz TS, Davis F, Freels S, Laws ER Jr, Menck HR (1998) Brain tumor survival: results from the National Cancer Data Base. J Neurooncol 40:151–160
van Hall T, Wolpert EZ, van Veelen P, Laban S, van der Veer M, Roseboom M, Bres S, Grufman P, de Ru A, Meiring H, de Jong A, Franken K, Teixeira A, Valentijn R, Drijfhout JW, Koning F, Camps M, Ossendorp F, Karre K, Ljunggren HG, Melief CJ, Offringa R (2006) Selective cytotoxic T-lymphocyte targeting of tumor immune escape variants. Nat Med 12:417–424
Walker PR, Calzascia T, Dietrich PY (2002) All in the head: obstacles for immune rejection of brain tumours. Immunology 107:28–38
Wang X, Campoli M, Cho HS, Ogino T, Bandoh N, Shen J, Hur SY, Kageshita T, Ferrone S (2005) A method to generate antigen-specific mAb capable of staining formalin-fixed, paraffin-embedded tissue sections. J Immunol Methods 299:139–151
Weber F, Meinl E, Aloisi F, Nevinny-Stickel C, Albert E, Wekerle H, Hohlfeld R (1994) Human astrocytes are only partially competent antigen presenting cells. Possible implications for lesion development in multiple sclerosis. Brain 117(1):59–69
Weller M, Fontana A (1995) The failure of current immunotherapy for malignant glioma. Tumor-derived TGF-beta, T-cell apoptosis, and the immune privilege of the brain. Brain Res Brain Res Rev 21:128–151
Whiteside TL, Stanson J, Shurin MR, Ferrone S (2004) Antigen-processing machinery in human dendritic cells: up-regulation by maturation and down-regulation by tumor cells. J Immunol 173:1526–1534
Wiendl H, Mitsdoerffer M, Hofmeister V, Wischhusen J, Bornemann A, Meyermann R, Weiss EH, Melms A, Weller M (2002) A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol 168:4772–4780
Wischhusen J, Friese MA, Mittelbronn M, Meyermann R, Weller M (2005) HLA-E protects glioma cells from NKG2D-mediated immune responses in vitro: implications for immune escape in vivo. J Neuropathol Exp Neurol 64:523–528
Wischhusen J, Jung G, Radovanovic I, Beier C, Steinbach JP, Rimner A, Huang H, Schulz JB, Ohgaki H, Aguzzi A, et al (2002) Identification of CD70-mediated apoptosis of immune effector cells as a novel immune escape pathway of human glioblastoma. Cancer Res 62:2592–2599
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehling, M., Simon, P., Mittelbronn, M. et al. WHO grade associated downregulation of MHC class I antigen-processing machinery components in human astrocytomas: does it reflect a potential immune escape mechanism?. Acta Neuropathol 114, 111–119 (2007). https://doi.org/10.1007/s00401-007-0231-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-007-0231-8