Abstract
Medulloblastoma (WHO grade IV) is an embryonal tumour of the cerebellum and the most common malignant central nervous system tumour in children. Despite significant advances in treatment, 5-year survival rates are still less than 70%, suggesting the presence of subgroups with different response to radio/chemotherapy. In the present study, we re-evaluated a series of 347 medulloblastomas from the SIOP II clinical trial of the International Society of Paediatric Oncology to identify features predictive of clinical outcome. Relapse free survival for medulloblastomas with severe anaplasia [5-year rate: S(60)=49.5%], was significantly shorter than for tumours with moderate or mild anaplasia S(60)=65.4%; P=0.001). The difference between both groups was even larger when the presence or absence of extensive apoptosis was included (46.5 vs. 66.7%; P=0.0216). Other histological features including nodularity, necrosis, vascular proliferation and the presence of β-catenin mutations (7% of cases) were not predictive for relapse free survival. These findings indicate that degree of anaplasia is the most significant histologic feature predictive of the survival of medulloblastoma patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
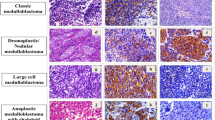
The World Health Organization (WHO) classification of the tumours of the nervous system defines four subtypes of medulloblastomas, i.e. classic medulloblastoma, desmoplastic medulloblastoma, medulloblastoma with extensive nodularity, and large cell medulloblastoma [25]. All these variants are graded as grade IV. However, approximately 60% of patients survive for more than 5 years [36], suggesting that medulloblastomas represent a heterogeneous group of neoplasms. The explanation of such variable biological behaviour can be related to the extent of resection, adverse molecular features, or histological aggressiveness. Although the risk stratification for response to therapy and relapse is mainly done on the basis of clinical parameters (location, extent of resection, metastatic spread along CSF pathways), there is growing evidence that histopathological as well as molecular genetic features may constitute a more useful tool to predict the behaviour of medulloblastoma [9, 31, 40]. For example, desmoplastic medulloblastoma has been considered to behave less aggressive than classic medulloblastomas, while large cell medulloblastoma has been associated with poor prognosis [4, 9, 31].
Recently, the concept of large cell medulloblastomas has been enlarged to include tumours showing highly anaplastic pleomorphic cells [4]. However, medulloblastomas are histologically not homogeneous but may have gradations of anaplasia within the same lesion. Histopathological features of anaplasia such as increased nuclear size and marked cytological pleomorphism can be found focally in classic medulloblastoma, and less frequently in desmoplastic medulloblastoma. Grading of medulloblastomas by identifying areas of high grade anaplasia appears to be prognostically most significant [31].
In the present study, we focused on histological criteria for medulloblastoma grading, by analyzing different parameters in a large group of medulloblastomas. Specifically, we correlated the degree of anaplasia and clinical outcome. We also evaluated necrosis and vascular proliferation, since these features are generally correlated with aggressiveness in brain tumours, including medulloblastomas [40].
The Wnt pathway plays a crucial role during embryonal development and organogenesis through the control of cell proliferation and apoptosis [22, 29, 39]. Wnt signalling stabilizes and accumulates β-catenin which, after transfer from the cytoplasms to the nucleus, interacts with members of the LEF-TCF family of transcription factors, leading to activation of various target genes, including c-myc and cyclin D1 [3, 33]. The products of APC gene, originally identified as the target of germline mutations that cause familial adenomatous polyposis (FAP) [19] and of the AXIN gene are negative regulators of the Wnt pathway. Several studies have indicated the involvement of the Wnt pathway through either β-catenin, APC, or AXIN1 mutations in a small, but significant fraction (10–15%) of medulloblastomas [1, 6, 11, 20, 41]. The most extensively studied gene is the β-catenin, which has been reported to be mutated in 5–10% of medulloblastomas [1, 11, 20, 41]. However, it remains unclear whether the disruption of the Wnt pathway is associated with clinical outcome of medulloblastoma patients. In the present study, we therefore screened for β-catenin mutations in a large number of medulloblastomas, to assess their predictive value in medulloblastoma patients.
Materials and methods
Patients
Between January 1, 1984 and December 31, 1989, a total of 364 medulloblastoma patients had been recruited in the SIOP II clinical trial, conducted by the International Society of Paediatric Oncology [2]. The histological slides collected at the pathology reference center in Zurich (P.K.) were histopathologically re-evaluated (F.G.). Specimens with features suggestive of atypical teratoid/rhabdoid tumour, ependymoma, choroid plexus carcinoma, or gliomas were excluded. In the total sample of 364 cases, 231 (63.5%) were male and 133 (36.5%) were female patients with a male/female ratio of 1.74. The mean age of patients at study entry was 7.1±3.7 years (range 0–16 years). The mean follow-up time was 63.7±40.1 months.
Histopathological parameters
The histological analyses were performed in two steps. The first histological survey of all cases was carried out to define criteria for degree of anaplasia. In this first evaluation, we realized that criteria for anaplasia would be somewhat subjective and difficult to define since medulloblastomas fall along a continuous histologic spectrum of anaplasia and cytologic differentiation [8]. However, we considered medulloblastoma a tumour always showing a certain degree of anaplasia. On the basis of this first evaluation, we chose a three-tiered grading i.e.: mild, moderate, and severe anaplasia. It was noted that variations in anaplasia could be present in the same lesion and that severe anaplasia was also observed in both classic and desmoplastic lesions. The second histological review was performed, applying the previously defined criteria for three grades, and then assigned a grade to each lesion. The extent of anaplasia was also semiquantified.
Anaplasia
The general criteria were similar to those reported by Eberhart et al. [9], i.e. increased nuclear size, numerous mitoses, numerous apoptoses, and either sheets or nodules of large cells with round nuclei and prominent nucleoli or angular, crowded, pleomorphic nuclei in large cells wrapping around one another. The degree of anaplasia i.e. mild, moderate, or severe, was based on increasing degrees of these features (Fig. 1). Mildly anaplastic lesions contained cells with modestly enlarged nuclei, often with angular molding of nuclei against one another, and scattered mitotic and/or apoptotic figures. Moderately anaplastic tumours were those with enlarged nuclei generally twice the size of a red blood cell. Wrapping of tumour cells also usually was identified, and mitotic and apoptotic figures were common. Severely anaplastic medulloblastomas contained cells with nuclei often twice the size of those with mild anaplasia and two to three times the size of a red blood cell. In almost all tumours, wrapping of cells was identified. Mitoses were frequent, and large clusters of apoptotic cells were seen often. Regions with marked apoptosis often were present. Large cell/anaplastic medulloblastomas were all graded as severely anaplastic. In all tumours, the highest possible grade was used. Loss of cohesiveness of neoplastic cells was observed only in neoplasms with moderate and severe anaplasia. Extent of anaplasia was assessed for each tumour in which mild, moderate, or severe anaplasia was identified. Anaplasia was considered diffuse when it was present in all fields examined; otherwise, it was designated as focal.
Nodularity
Nodules were defined as well-circumscribed, pale regions of tumour with fibrillary cytoplasm. Tumours were divided based on the amount of tumour nodules, including the following categories: extensive nodularity, diffuse nodularity, focal nodularity and absence of nodules. The total percentage of tumour area comprised of nodules was estimated for the entire available specimen by review of representative hematoxylin and eosin-stained sections. Pale areas of tumour with a loose architecture but without well-defined border were not considered nodules.
Extent of apoptosis was determined semi-quantitatively
Focal (+) when single scattered occasional apoptotic bodies were present; diffuse (++) when small clusters of apoptotic bodies were distributed within the neoplasm, and extensive (+++) when large areas of apoptosis were present. The histological distinction between extensive apoptosis and coagulative necrosis, although not always straightforward, was based on the appearance of the apoptotic cells as round oval masses of intensely eosinophilic cytoplasms with dense nuclear chromatin fragments in contrast to acidophilic, coagulated anucleate cells present within the foci of coagulative necrosis.
Extent of necrosis in medulloblastomas was divided into three groups, i.e. absence of necrosis, focal necrosis, extensive necrosis. With respect to vascular proliferation, cases were divided in two groups, i.e. absence or presence of vascular proliferation, with the latter including the presence of vascular glomeruloid structures.
SSCP and direct DNA sequencing for β-catenin mutations
Pre-screening for mutations in exon 3 of human β-catenin gene, which contains the four potential GSK-3β phosphorylation sites [7], was carried out by PCR–SSCP analysis as previously reported [20]. Briefly, PCR was performed in a total volume of 10 μl, consisting of 2 μl of DNA solution, 0.5 U of Taq DNA polymerase (Sigma, St. Louis, MO, USA), 0.5 μCi of [α-33P]-dCTP (ICN Biomedicals, Inc., Costa Mesa, CA, USA; specific activity, 3,000 Ci/mmol), 1.5 mM MgCl2, 0.2 mM of each dNTP, 0.4 μM of both sense and antisense primers, 10 mM Tris–HCl, pH 8.3, and 50 mM KCl in the RoboCycler Gradient 96 (Stratagene, La Jolla, CA, USA), with an initial denaturing step at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 1 min, annealing at 53°C for 1 min, and polymerization at 72°C for 1 min, and a final extension of 5 min at 72°C. After PCR, 5 μl of PCR products were mixed with 12.5 μl loading buffer (95% formamide, 20 mM EDTA, 0.05% xylene cyanol and bromophenol blue), denatured at 95°C for 10 min, and quenched on ice. Four microliter of the above mixture were run on a 6% polyacrylamide non-denaturing gel containing 8% glycerol at 4 W for 14 h at room temperature and/or on a 6% polyacrylamide non-denaturing gel containing 6% glycerol at 40 W for 3.5 h with cooling by fan. Gels were dried at 80°C and autoradiographed for 12–48 h. Primer sequences used were 5′-ATG GAA CCA GAC AGA AAA G-3′ (nt 254–272) and 5′-TAC AGG ACT TGG GAG GTA TC-3′ (nt 386–405).
Samples which showed mobility shifts in the SSCP analysis were further analysed by direct DNA sequencing. PCR was carried out as described above in the absence of [α-33P]-dCTP. Five microliter of PCR products were digested with 1 U of shrimp alkaline phosphatase and 5 U of exonuclease I at 37°C for 15 min. After inactivation of these enzymes at 80°C for 15 min, primers (the same primers for PCR, 15 pmol) and 2 μl of 5× sequenase buffer (200 mM Tris–HCl, pH 7.5, 100 mM MgCl2, 250 mM NaCl) were added. Template-primer mixture was heated at 100°C for 5 min and then placed in ice-cold water. 0.1 M dithiothreitol, 3 U Sequenase version 2.0 (USB, Cleveland, USA), and 0.5 μCi [α-33P]-dATP or [α-33P]-dCTP were added to samples, which were then divided into four wells each containing termination mixture. Samples were incubated at 42°C for 6 min and mixed with 5 μl stop solution (USB). After heating at 80°C for 3 min, samples were loaded onto a 6% polyacrylamide/7 M urea gel and run at 70 W for 1.5–3 h. Gels were dried at 80°C and autoradiographed for 12–48 h. Samples were considered mutated only if the mutations were confirmed on two independent PCRs.
Statistical analysis
In order to assess the histopathological criteria for possible association with the risk of relapsing, for each subgroup defined by a single or a combination of several histopathological parameters, the survival function was estimated by means of the product-limit method of Kaplan and Meier [24]. Differences between survival curves were tested for significance by means of the log-rank statistic [23]. Tests for associations between different histopathological parameters were carried out by means of the usual chi-square statistic for 2-way contingency tables [14].
Results
Anaplasia
For 257 cases, anaplasia data was available. Of these, mild, moderate, and severe anaplasia was observed in 18 cases (7%), 152 cases (59%), and 87 cases (34%), respectively. The patients with severely anaplastic tumours had the poorest outcome, while patients with mild or moderate anaplasia had a better outcome, in terms of relapse free survival (Fig. 2a). The 5-year relapse-free survival rate of patients with medulloblastomas with severe anaplasia was 49.5% and thus, significantly lower than those with tumours showing moderate anaplasia at most (65.4%; P=0.0010). Similarly, overall 5-year survival rate of patients with medulloblastomas with severe anaplasia was 50.6%, significantly lower than those with tumours showing mild or moderate anaplasia (67.4%; P=0.0026).
The patients with severely anaplastic tumours had the poorest outcome, while patients with moderate or mild anaplasia had a better outcome, in terms of relapse-free survival (P=0.001) (a). Combination of extent of anaplasia and apoptosis was significant predictive factor for relapse-free survival time of medulloblastoma patients (P=0.003) (b)
Apoptosis
Data on apoptosis was available for 248 cases. Focal area of apoptosis was observed in 182 cases (74%), diffuse area of apoptosis was in 56 cases (23%), and extensive apoptosis was in 10 cases (4%). Survivals of patients with medulloblastoma showing focal versus diffuse or extensive area of apoptosis were significantly different in the log-rank test (P=0.0097). Similarly, 5-year overall survival rate of patients with medulloblastomas with diffuse or extensive area of apoptosis was 47.7%, significantly lower than those with tumours showing focal apoptosis (67.1%; P=0.0033).
Anaplasia and apoptosis
The combination of extent of anaplasia and apoptosis was a significant predictive factor for relapse free survival time of medulloblastoma patients. The survival curves for the four subgroups obtained by distinguishing between mild/moderate and severe anaplasia on the one hand, and focal versus diffuse or extensive area of apoptosis are shown in Fig. 2b. The log-rank test gave a P value of 0.0216 so that the null hypothesis of homogeneity of all 4 survival curves could be rejected. In the most favourable group (mild or moderate anaplasia combined with focal area of apoptosis), the 5-year survival rate was markedly higher than for the group with the worst prognosis, i.e. severe anaplasia combined with diffuse or extensive apoptosis [S(60)= 46.5 vs. 66.7%; P=0.0216]. Similarly, overall survival rate of patients with medulloblastomas showing severe anaplasia and diffuse or extensive area of apoptosis was 45.3%, significantly lower than those with tumours showing focal apoptosis (68.7%; P=0.0033).
Nodularity
For 251 tumours, data of nodularity was available. Of these, 30 (12%) tumours contained regions of nodularity, with focal (16 cases; 6%), diffuse (10 cases; 4%), and extensive (4 cases; 2%) nodularity. All these 30 cases containing “bona fide” nodules would be diagnosed as nodular/desmoplastic medulloblastoma according to the WHO classification [16]. There was no evidence of association between nodularity and outcome of medulloblastoma patients (log-rank test; P=0.1098). There was also no association between anaplasia and nodularity and the frequency of severe anaplasia was similar in nodular (8/30; 27%) and in non-nodular tumours (76/220; 34.5%; P=0.3914).
Necrosis
For 251 cases, data on necrosis was available. Necrosis was not observed in the large majority of the cases (221 cases; 88.1%), while 16 cases (6.4%) and 14 cases (5.6%) showed extensive or focal necrosis, respectively. There was no statistically significant relationship between the presence or absence of necrosis and relapse free survival time (P=0.1848).
Vascular proliferation
For 250 cases, the vascular proliferation was assessed: 22 cases (9%) showed vascular proliferation, whereas 228 (91%) cases showed no vascular proliferation. Between the corresponding relapse-free survival curves, there was no statistical difference (P=0.7677).
β-catenin mutations
In 146 cases, PCR amplification of exon 3 of the β-catenin gene from DNA extracted from archival sections was successful. Of these, 10 cases (7%) contained a β-catenin mutation (Table 1). They were located at codon 32 (3 cases), codon 33 (6 cases), and codon 34 (one case). All except for one medulloblastoma patient with β-catenin mutations were females. Although patients with medulloblastoma with β-catenin mutations survived longer (5-year overall survival 83.3±10.8%; relapse-free survival 83.3±10.8%) than those without β-catenin mutations (overall survival 61.8 ± 4.3%; relapse-free survival 62.7±4.4%), the differences were not statistically significant (overall survival, P=0.1501; relapse-free survival, P=0.1605).
Discussion
Cytological pleomorphism, increased nuclear size, brisk mitotic activity, and cell wrapping are considered as the key features of anaplasia in medulloblastomas. Anaplastic features are observed in any of the medulloblastoma subtypes. Anaplasia has been reported to be associated with poor prognosis of medulloblastoma patients [9, 28, 31]. This correlation is confirmed in the present large cohort of children enrolled in a clinical trial. We clearly show that an increasing degree of anaplasia is significantly associated with shorter relapse-free survival time. Slight anaplasia appears not to influence prognosis, but patients with moderate and severe anaplasia had a significantly worse outcome.
The evaluation of anaplasia in medulloblastomas has added to the concept of large cell medulloblastomas, characterized by extremely large, round nuclei with prominent nucleoli, abundant mitoses, numerous apoptotic cells and cellular wrapping [4, 17, 25]. The survival of children bearing this variant is significantly reduced [34, 35]. The large cell variant represented only 10% of cases when diagnosed on the basis of the original description [17]. However, since large cell medulloblastomas mainly contain cells with anaplastic features, it has been suggested to merge large cell and anaplastic medulloblastomas into a single group, constituting approximately 20% of all medulloblastomas [28], although one study showed that large cell medulloblastomas appear to have a slightly worse prognosis than anaplastic medulloblastomas [4], and several studies showed that patients with anaplastic medulloblastoma have more variable prognosis than large cell medulloblastoma [8, 10, 28].
To grade anaplasia in a biologically highly malignant neoplasm as medulloblastoma may be predictably subjective and difficult. In the present study, we used a three tiered grading system in which according to our criteria, the majority of cases fell into the moderate (59%) and severe (34%) groups, whereas only a minor percentage (7%) showed mild anaplasia. These results are significantly different from those reported by Eberhart et al. [9]. These authors found no or slight anaplasia in 76% of cases, moderate in 14% and severe in only 10%. These discrepancies are mainly related to the differences in the histological evaluation of anaplasia. We consider medulloblastoma a tumour which always shows some degree of anaplasia. This attitude inevitably enlarged the group of neoplasms with moderate anaplasia. However, in both studies, medulloblastomas were stratified into two large groups, not numerically distant, with statistically different prognosis: Eberhat et al. [9] have a percentage of non-anaplastic and anaplastic respectively of 76 and 24%; whereas, in the present study, grouping together cases with mild/moderate anaplasia versus severely anaplastic, the percentages were 66 and 34%, respectively. This suggests that, despite the subjective evaluation of anaplasia, histology alone is able to segregate into two groups with statistically significantly different prognosis. Both studies, moreover, have enlarged the concept of large cell/anaplastic medulloblastoma, indicating that such “anaplastic” changes are more frequent than previously observed. On the basis of these considerations, pathological grading of medulloblastomas seems to be of clinical utility to stratify patients in low and high-risk groups.
In the present study, we also correlated apoptosis with anaplasia in medulloblastomas. Relapse free survival was significantly shorter in patients with medulloblastomas with severe anaplasia and extensive apoptosis than in those with mild or moderate anaplasia and diffuse apoptosis. Similarly, Korshunov et al. [27] reported that in multivariate analysis higher apoptotic index was a significant prognostic factor of poor outcome [27]. In contrast, the study by Schubert et al. [37] found no significant difference in numbers or in the pattern of apoptotic tumour cells among medulloblastomas with different degrees of differentiations.
Desmoplastic medulloblastomas are characterized by nodular islands with reduced cellularity surrounded by densely packed, highly proliferative cells that produce a dense reticulin fibre network. Cells in the islands have round, regular nuclei and abundant fibrillar cytoplasm and show neuronal differentiation [25]. Desmoplastic medulloblastomas were associated with a somewhat better outcome in some studies [5, 30, 38], but not in others [9, 18, 21]. This discrepancy may be due to the fact that in some of these studies, tumours were classified as desmoplastic on the basis of an increased amount of collagen and reticulin fibres but without the typical nodular pattern of this variant. One study confirmed a favourable prognosis for the rare cases of extensive nodularity, while lesser degrees of nodularity or desmoplasticity were not associated with a statistically significant survival advantage [35]. In the present study, we found that nodularity in medulloblastomas had no significant predictive value, although this negative result may be explained by the lack of statistical power due to the extreme imbalance in the sub-sample sizes (only 12% of cases were nodular). It is noted that frequencies of severe anaplasia was not different in nodular tumours (27%) and in non-nodular tumours (35%; P=0.3914). Similarly, necrosis and vascular proliferations were not predictive for outcome of medulloblastoma patients in the present study. This result was consistent with a study by Miralbell et al. [32], in which microvessel density was not associated with survival of pediatric medulloblastoma patients.
The management of medulloblastoma will rely not only on improved histopathological grading schemes but on the incorporation of specific biologic markers as well [35]. These markers will provide not only prognostic information but also target for specific therapy [8, 15, 28]. Several molecular prognostic indicators have been proposed for medulloblastoma, including 17p loss [28], c-erbB2 overexpression [15], and c-myc amplification or overexpression [15, 28]. A correlation between 17p loss and high frequency c-myc amplification and between the large-cell/anaplastic variant and c-myc amplification was found [12]. In the present study, we evaluated the presence of β-catenin mutations as additional parameter that might be associated with anaplasia or worse prognosis in medulloblastoma. β-catenin, translocated into the nucleus, activates transcription of a number of gene targets, including, c-myc, that is overexpressed in anaplastic/large cell medulloblastoma. In the present study, we screened for mutations of β-catenin gene in 146 medulloblastomas. The frequency of β-catenin mutations in sporadic medulloblastoma (7%) was similar to that in other series with smaller numbers of cases [20, 26, 41]. We did not find a significant correlation between β-catenin mutations and anaplasia or other histological parameters. The presence of mutations was also not significantly associated with clinical outcome of patients, although the results showed that mean overall and relapse-free survivals were longer in patients with β-catenin mutations. This result is in contrast to a recent study by Ellison et al. [13] which showed that nucleopositive β-catenin immunophenotype (27/109; 25%) had significantly better overall and event free survival. However, in the study by Ellison et al. [13], mutational analyses of β-catenin gene were restricted to only 27 medulloblastomas with a nuclear β-catenin immunophenotype.
References
Baeza N, Masuoka J, Kleihues P, Ohgaki H (2003) AXIN1 mutations but not deletions in cerebellar medulloblastomas. Oncogene 22:632–636
Bailey CC, Gnekow A, Wellek S, Jones M, Round C, Brown J, Phillips A, Neidhardt MK (1995) Prospective randomised trial of chemotherapy given before radiotherapy in childhood medulloblastoma. International Society of Paediatric Oncology (SIOP) and the (German) Society of Paediatric Oncology (GPO): SIOPII. Med Pediatr Oncol 25:166–178
Brantjes H, Barker N, van EJ, Clevers H (2002) TCF: lady justice casting the final verdict on the outcome of Wnt signalling. Biol Chem 383:255–261
Brown HG, Kepner JL, Perlman EJ, Friedman HS, Strother DR, Duffner PK, Kun LE, Goldthwaite PT, Burger PC (2000) “Large cell/anaplastic” medulloblastomas: a Pediatric Oncology Group Study. J Neuropathol Exp Neurol 59:857–865
Carrie C, Lasset C, Alapetite C, Haie Meder C, Hoffstetter S, Demaille MC, Kerr C, Wagner JP, Lagrange JL, Maire JP et al (1994) Multivariate analysis of prognostic factors in adult patients with medulloblastoma. Retrospective study of 156 patients. Cancer 74:2352–2360
Dahmen RP, Koch A, Denkhaus D, Tonn JC, Sörensen N, Berthold F, Behrens J, Birchmeier W, Wiestler OD, Pietsch T (2001) Deletions of AXIN1, a component of the WNT/wingless pathway, in sporadic medulloblastomas. Cancer Res 61:7039–7043
De La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, Perret C (1998) Somatic mutations of the b-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA 95:8847–8851
Eberhart CG, Cohen KJ, Tihan T, Goldthwaite PT, Burger PC (2003) Medulloblastomas with systemic metastases: evaluation of tumor histopathology and clinical behavior in 23 patients. J Pediatr Hematol Oncol 25:198–203
Eberhart CG, Kepner JL, Goldthwaite PT, Kun LE, Duffner PK, Friedman HS, Strother DR, Burger PC (2002) Histopathologic grading of medulloblastomas: a pediatric oncology group study. Cancer 94:552–560
Eberhart CG, Kratz J, Wang Y, Summers K, Stearns D, Cohen K, Dang CV, Burger PC (2004) Histopathological and molecular prognostic markers in medulloblastoma: c-myc, N-myc, TrkC, and anaplasia. J Neuropathol Exp Neurol 63:441–449
Eberhart CG, Tihan T, Burger PC (2000) Nuclear localization and mutation of b-catenin in medulloblastomas. J Neuropathol Exp Neurol 59:333–337
Ellison DW, Clifford SC, Gajjar A, Gilbertson RJ (2003) What’s new in neuro-oncology? Recent advances in medulloblastoma. Eur J Paediatr Neurol 7:53–66
Ellison DW, Onilude OE, Lindsey JC, Lusher ME, Weston CL, Taylor RE, Pearson AD, Clifford SC (2005) Beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children’s Cancer Study Group Brain Tumour Committee. J Clin Oncol 23:7951–7957
Fienberg SE (1980) The analysis of cross-classified data. MIT Press, Cambridge
Gajjar A, Hernan R, Kocak M, Fuller C, Lee Y, McKinnon PJ, Wallace D, Lau C, Chintagumpala M, Ashley DM, Kellie SJ, Kun L, Gilbertson RJ (2004) Clinical, histopathologic, and molecular markers of prognosis: toward a new disease risk stratification system for medulloblastoma. J Clin Oncol 22:984–993
Giangaspero F, Bigner SH, Kleihues P, Pietsch T, Trojanowski JQ (2000) Medulloblastoma. In: Kleihues P, Cavenee WK (eds) Pathology and genetics of tumours of the nervous system. IARC Press, Lyon, pp 129–137
Giangaspero F, Rigobello L, Badiali M, Loda M, Andreini L, Basso G, Zorzi F, Montaldi A (1992) Large-cell medulloblastomas. A distinct variant with highly aggressive behavior. Am J Surg Pathol 16:687–693
Giordana MT, Cavalla P, Chio A, Marino S, Soffietti R, Vigliani MC, Schiffer D (1995) Prognostic factors in adult medulloblastoma. A clinico-pathologic study. Tumori 81:338–346
Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, Burt R, Hughes JP, Warrington J, McPherson J, Wasmuth J, Le Paslier D, Abderrahim H, Cohen D, Leppert M, White R (1991) Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66:589–600
Huang H, Mahler-Araujo BM, Sankila A, Chimelli L, Yonekawa Y, Kleihues P, Ohgaki H (2000) APC mutations in sporadic medulloblastomas. Am J Pathol 156:433–437
Hubbard JL, Scheithauer BW, Kispert DB, Carpenter SM, Wick MR, Laws ER Jr (1989) Adult cerebellar medulloblastomas: the pathological, radiographic, and clinical disease spectrum. J Neurosurg 70:536–544
Itoh K, Krupnik VE, Sokol SY (1998) Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Curr Biol 8:591–594
Kalbfleisch JD, Prentice R (2002) The statistical analysis of failure time date. Wiley, New York
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Kleihues P, Cavenee WK (eds) (2000) WHO classification of tumours pathology and genetics of tumours of the nervous system. IARC Press, Lyon
Koch A, Waha A, Tonn JC, Sörensen N, Berthold F, Wolter M, Reifenberger J, Hartmann W, Friedl W, Reifenberger G, Wiestler OD, Pietsch T (2001) Somatic mutations of WNT/wingless signaling pathway components in primitive neuroectodermal tumors. Int J Cancer 93:445–449
Korshunov A, Savostikova M, Ozerov S (2002) Immunohistochemical markers for prognosis of average-risk pediatric medulloblastomas the effect of apoptotic index, TrkC, and c-myc expression. J Neurooncol 58:271–279
Lamont JM, McManamy CS, Pearson AD, Clifford SC, Ellison DW (2004) Combined histopathological and molecular cytogenetic stratification of medulloblastoma patients. Clin Cancer Res 10:5482–5493
Lee YJ, Swencki B, Shoichet S, Shivdasani RA (1999) A possible role for the high mobility group box transcription factor Tcf-4 in vertebrate gut epithelial cell differentiation. J Biol Chem 274:1566–1572
Maire JP, Guerin J, Rivel J, San Galli F, Bernard C, Dautheribes M, Caudry M (1992) Medulloblastoma in children. Prognostic incidence of vascular hyperplasia, coagulation necrosis and postoperative clinical state on survival. Neurochirurgie 38:80–88
McManamy CS, Lamont JM, Taylor RE, Cole M, Pearson AD, Clifford SC, Ellison DW (2003) Morphophenotypic variation predicts clinical behavior in childhood non-desmoplastic medulloblastomas. J Neuropathol Exp Neurol 62:627–632
Miralbell R, Tolnay M, Bieri S, Probst A, Sappino AP, Berchtold W, Pepper MS, Pizzolato G (1999) Pediatric medulloblastoma: prognostic value of p53, bcl-2, Mib-1, and microvessel density. J Neurooncol 45:103–110
Morin PJ (1999) Beta-catenin signaling and cancer. Bioessays 21:1021–1030
Ozer E, Sarialioglu F, Cetingoz R, Yuceer N, Cakmakci H, Ozkal S, Olgun N, Uysal K, Corapcioglu F, Canda S (2004) Prognostic significance of anaplasia and angiogenesis in childhood medulloblastoma: a pediatric oncology group study. Pathol Res Pract 200:501–509
Perry A (2002) Medulloblastomas with favorable versus unfavorable histology: how many small blue cell tumor types are there in the brain?. Adv Anat Pathol 9:345–350
Rood BR, MacDonald TJ, Packer RJ (2004) Current treatment of medulloblastoma: recent advances and future challenges. Semin Oncol 31:666–675
Schubert TE, Cervos-Navarro J (1998) The histopathological and clinical relevance of apoptotic cell death in medulloblastomas. J Neuropathol Exp Neurol 57:10–15
Sure U, Berghorn WJ, Bertalanffy H, Wakabayashi T, Yoshida J, Sugita K, Seeger W (1995) Staging, scoring and grading of medulloblastoma A postoperative prognosis predicting system based on the cases of a single institute. Acta Neurochir (Wien) 132:59–65
Tsuda M, Kamimura K, Nakato H, Archer M, Staatz W, Fox B, Humphrey M, Olson S, Futch T, Kaluza V, Siegfried E, Stam L, Selleck SB (1999) The cell-surface proteoglycan dally regulates wingless signalling in Drosophila. Nature 400:276–280
Urberuaga A, Navajas A, Burgos J, Pijoan JI (2005) A review of clinical and histological features of Spanish paediatric medulloblastomas during the last 21 years. Childs Nerv Syst 1–9
Zurawel RH, Chiappa SA, Allen C, Raffel C (1998) Sporadic medulloblastomas contain oncogenic b-catenin mutations. Cancer Res 58:896–899
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giangaspero, F., Wellek, S., Masuoka, J. et al. Stratification of medulloblastoma on the basis of histopathological grading. Acta Neuropathol 112, 5–12 (2006). https://doi.org/10.1007/s00401-006-0064-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0064-x