Abstract
Under autoimmune inflammatory conditions within the brain, evidence suggests that neurons downregulate microglial activation through CD200/CD200R interaction, which reduces disease severity. To gain insight into the regulation of intracerebral immune reactions by resident brain cells in chronic cerebral infections, the expression of the CD200 antigen and the CD200R as well as the functional role of CD200/CD200R interactions were characterized in murine Toxoplasma encephalitis. In the normal brain of C57BL/6 wild type mice, CD200 was ubiquitously expressed on neurons, their axons, cerebral endothelial cells, and plexus macrophages. CD200R was expressed at very low levels on cerebral macrophages and microglia without differences between CD200−/− and wild type mice. Infection of C57BL/6 mice with Toxoplasma gondii induced an upregulation of CD200R on microglia and of CD200 on blood vessel endothelial cells. In Toxoplasma encephalitis of CD200−/− mice, microglial cell numbers strongly increased due to an enhanced proliferation indicated by increased Ki-67 immunoreactivity. In addition, microglial activation was increased in CD200−/− mice as evidenced by a further upregulation of already high MHC class II levels as well as an increased expression of the anti-parasitic effector molecules, TNF and iNOS. The increased microglial cell activation resulted in a reduced intracerebral parasite burden and an increased survival rate. Thus, in Toxoplasma encephalitis, microglial activity was regulated via CD200/CD200R-mediated interaction further pointing to an intrinsic regulation of brain resident cells under inflammatory CNS conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The existence of a tissue-specific regulation of immune responses has been claimed for the CNS, which is characterized by a physiologically downregulated immunological phenotype. Even under inflammatory circumstances, intracerebral (i.c.) immune reactions must be tightly regulated in order to prevent immunopathology.
The obligate intracellular parasite Toxoplasma (T.) gondii infects up to 80% of the human population and persists asymptomatically in the brain of its host. Both in humans and mice, the course of toxoplasmosis is regulated by genes of the major histocompatibility complex [4, 5, 11, 13, 15, 28]. Studies in T. gondii-resistant mouse strains, e.g. BALB/c mice, which develop a non-lethal chronic encephalitis, and T. gondii-susceptible strains, e.g. C57BL/6 mice, which succumb to a chronic progressive encephalitis, have identified interferon (IFN)-γ-producing CD4 and CD8 T cells, which induce anti-parasitic effector mechanisms in infected resident brain cells, as the major mechanism of i.c. control of T. gondii [9, 14, 29].
In Toxoplasma encephalitis (TE), T cells and microglia reciprocally regulate each other with respect to proliferation and apoptosis, expression of immunologically relevant cell surface molecules, production of pro- and anti-inflammatory mediators and anti-parasitic effector molecules finally resulting in a finely tuned balance preventing immunopathology while simultaneously ensuring parasite control [23, 24].
In addition to microglia, other resident cell populations of the CNS including neurons and blood vessel endothelial cells are also involved in the regulation of immune reactions. Neurons may contribute to the i.c. immune reaction to T. gondii by secretion of soluble mediators such as interleukin (IL)-1, IL-6, IL-10, MIP-1α, and MIP-1β [22]. These data are of considerable relevance for TE, since neurons are the target cell of T. gondii in the CNS [8].
A group of cell surface molecules is shared by cells of the nervous system and of the immune system including the neural cell adhesion molecule NCAM, Thy-1, L1, the heat stable antigen HSA, and GL7, which are involved in antigen presentation, cell adhesion, signal transduction, and activation [7]. Recently, the CD200 (Ox2) protein and its receptor CD200R, both of which belong to the immunoglobulin superfamily, have been identified as further members of this group of molecules [30]. While the CD200 protein is widely expressed on a variety of cell populations including neurons of the central and peripheral nervous system, expression of the CD200R is more restricted and predominantly observed on cells of the myeloid lineage [1, 2, 17, 30]. This specific distribution pattern has led to the hypothesis that CD200R engagement may lead to intracellular signals affecting macrophage function [17] and, functionally important, that neurons may regulate microglial cells via CD200/CD200R interactions [12].
In the absence of the CD200 protein, mice developed an increased susceptibility to autoimmune disorders. In CD200-deficient (CD200−/−) mice, MOG-induced EAE showed a more rapid onset and microglial activation was enhanced [12]. In addition, in the model of facial nerve transection microglial responses were accelerated in the absence of CD200 [12]. While such an accelerated microglial response may be harmful in autoimmune disorders, the effect of a disrupted CD200/CD200R axis on the course of a chronic infectious encephalitis, such as TE, of which a strong microglial activation is a hallmark, and in which neurons provide the parasitic target cell, has not yet been defined.
To gain further insight into the regulation of i.c. immune responses by resident cell populations of the CNS, we characterized the expression of CD200 and CD200R, and analyzed the functional importance of CD200/CD200R interactions in T. gondii-infected CD200−/− mice. Upon infection, the CD200R was upregulated on microglia. The absence of CD200 resulted in an increased proliferation of microglia with the number of microglial cells being increased in infected CD200−/− mice. Although lack of CD200 still exerted a further moderately stimulatory effect on microglia as evidenced by an increased expression of MHC class II antigens as well as an increased expression of the anti-parasitic effector molecules, iNOS and TNF. This enhanced microglial activation was associated with a reduced parasite burden and a reduced mortality up to day 70 p.i. of CD200−/− C57BL/6 as compared to wild type (WT) mice, but finally all CD200−/− mice either died or were critically ill indistinguishable from WT animals.
Material and methods
Animals
CD200−/− mice, generated at DNAX (Palo Alto, CA, USA), and C57BL/6 WT mice, obtained from Harlan-Winkelmann (Borchen, Germany) of either sex were used for the experiments. Mice were kept under standard conditions in an isolated facility before and throughout the experiments.
T. gondii infection
Parasites were harvested from the brains of mice chronically infected with the DX strain of T. gondii. Brain tissue of these animals was dispersed in 0.1 M PBS pH 7.4. The final concentration of the infectious agents was adjusted to a dose of 5 cysts/0.5 ml, which was administered intraperitoneally (i.p.) to the experimental animals.
Experimental procedure and tissue processing
Uninfected and T. gondii-infected (21, 23, 42, 54, and 71 d after infection, p.i.) CD200−/− and WT mice were studied. At the respective days, animals were perfused intracardially with 0.9% saline in deep Metofane (Janssen, Neuss, Germany) narcosis.
For immunohistochemistry on frozen sections and reverse transcription (RT)-PCR, brains of four mice per experimental group were dissected and blocks were mounted on thick filter paper with O.T.C. compound (Miles Scientific, Naperville, IL, USA), snap-frozen in isopentane (Fluka, Neu-Ulm, Germany) precooled on dry ice, and stored at −80°C.
For flow cytometry analysis of brain-derived leukocytes, the brain of three to six animals per experimental group were pooled and prepared as described [23, 24]. Brain tissue was passed through a sieve, and leukocytes were separated by Percoll gradient centrifugation (Amersham-Pharmacia, Freiburg, Germany).
Monoclonal and polyclonal antibodies
The following rat anti-mouse monoclonal antibodies were used: CD45 (LCA, clone M1/9.3.4.HL.2), I-A (b, d, q haplotypes, clone M5/114.15.2), H-2 (all haplotypes, clone M1/42.3.9.8.HLK), F4/80 (clone F4/80), LFA-1 (clone FD441.8, all from the American Type Culture Collection, ATCC, Manassas, VA, USA), CD45-PE/Cy5, I-A-PE, H-2b-PE, CD80 (B7.1)-PE, CD86 (B7.2)-PE, LFA-1 (CD11a)-PE CD4, CD4-PE, CD8, CD8-FITC, B220, B200-FITC, TNF (all from BD, Heidelberg, Germany), monoclonal rat anti-mouse Ki-67 (DakoCytomation, Hamburg, Germany), monclonal rabbit anti-Ki-67 (DCS, Hamburg, Germany), monoclonal mouse anti-neuronal nuclei (NeuN, Chemicon, Hofheim, Germany), F4/80-FITC, CD200 (Serotec, Oxford, UK).
The following polyclonal antibodies were used: rabbit anti-iNOS (Alexis Biochemicals, Grünberg, Germany), monoclonal hamster anti-mouse CD11c (Endogen, Woburn, MA, USA), polyclonal anti-T. gondii antiserum (Biogenex, Duiven, The Netherlands), polyclonal rabbit anti-factor VIII (Sigma, Deisenhofen, Germany) and polyclonal rabbit anti-GFAP (Dako, Hamburg, Germany). In addition, anti-rabbit/anti-rat avidin-biotinylated horseradish peroxidase complex (ABC-Kit, Vector, Burlingame, CA, USA), BSP-conjugated F(ab)2 fragment mouse anti-rat IgG (Dianova, Hamburg, Germany), BSP-conjugated F(ab)2 fragment goat anti-rabbit IgG (Dianova), Texas Red-conjugated goat anti-rabbit IgG F(ab)2 fragments (Dianova), BSP-conjugated goat anti-hamster IgG (H+L) (Dianova), fluorescein-conjugated F(ab)2 fragment goat anti-rabbit IgG (Dianova), extravidin-Cy3 (Sigma), FITC-conjugated avidin (Extravidin-FITC, Sigma), goat anti-rat-PE (Serotec), avidin-PE/Cy5 (BD), and the M.O.M immunodetection kit (Vector) were employed.
Rat anti-mouse CD200 and CD200R (Ox90 and Ox110, IgG2a and IgG1) were kindly provided by Dr. Neil Barclay and Dr. Michael Puklavec (Sir William Dunn School of Pathology, University of Oxford, Oxford, UK).
Immunohistochemistry
Immunohistochemistry was performed on 10 μm frozen sections as described [19]. For detection of CD45, CD4, CD8, and B220, an indirect immunoperoxidase protocol using sheep anti-rat and goat anti-rabbit IgG F(ab′)2, respectively, was applied, while for demonstration of MHC class I and MHC class II antigens, LFA-1, F4/80, TNF, iNOS, CD200, Ki-67, and T. gondii antigen the avidin–biotin complex technique was used. Peroxidase reaction products were visualized using 3,3′-diaminobenzidine (Sigma) and H2O2 as co-substrate. Sections were lightly counterstained with hemalum (Merck, Darmstadt, Germany).
The co-expression of CD200 and GFAP, NeuN, and factor VIII, respectively, was studied using double immunofluorescence with detection of CD200 by extravidin-FITC after incubation with biotinylated mouse anti-rat IgG F (ab)2 fragments, and detection of GFAP and factor VIII, respectively, by incubation with Texas red-conjugated goat anti-rabbit IgG. For detection of NeuN, the M.O.M. immunodetection kit was employed according to the manufacturer’s instructions. Furthermore, the co-expression of CD200 and CD4, CD8, F4/80, and B220, respectively, was analyzed with detection of CD200 by goat–anti-rat FITC after incubation with biotinylated mouse anti-rat IgG F (ab)2 fragments and detection of CD4, CD8, F4/80, and B220, respectively, by incubation with extravidin-Cy3.
Double immunofluorescence studies for the Ki-67 and the F4/80 and I-A antigen, respectively, were performed with labeling of Ki-67 with extravidin-Cy3 after incubation with biotinylated goat anti-rabbit IgG, followed by exposure to the F4/80 or the I-A antibody, respectively, and goat anti-rat FITC.
Appropriate positive and negative controls were included in all immunohistochemical reactions.
Flow cytometry analysis
Brain-derived leukocytes were analyzed by double-immunofluorescence staining followed by flow cytometry as described [22, 23]. Microglia and macrophages were identified by staining with anti-CD45 biotin and anti-F4/80-FITC followed by avidin-PE/Cy5. Microglia is F4/80+ CD45low, and macrophages are F4/80+ CD45high [23, 26]. Co-staining was performed with I-A-PE, H-2b-PE, LFA-1PE, CD80-PE, CD86-PE, respectively. In addition, microglia and macrophages were co-stained with rat anti-mouse CD200R followed by goat anti-rat-PE. T cells were identified by co-staining with anti-CD4-PE and anti-CD8-FITC. CD200R expression on T cells was analyzed by staining with rat anti-mouse CD200R and goat anti-rat-PE followed by blocking with rat IgG and CD4-FITC or CD8-FITC, respectively. B cells were stained with anti-B220-FITC and anti-CD45 biotin followed by avidin PE/Cy5. Granulocytes were stained with anti-Ly6G-PE, anti-CD11b-FITC, and anti-CD45 biotin followed by avidin PE/Cy5 and were defined as Ly6Ghigh CD11b+ CD45high. TNF expression of brain-derived leukocytes was analyzed by incubating isolated leukocytes with Golgi-Plug (1 μl/ml, BD) containing brefeldinA in MEM-α at 37°C for 3 h. Thereafter, cells were stained with rat anti-mouse TNF (BD). Controls included staining of unstimulated cells and staining with isotype-matched control antibodies. Data were analyzed with Cell Quest or WinMDI software.
Statistical evalution
For statistical evaluation of the i.c. parasitic load at the various time points of TE, the number of parasites was determined on anti-T. gondii stained immunostained sections in CD200−/− and WT mice. In these experiments, T. gondii positive cells containing either bradyzoites in cysts or tachyzoites in parasitophorous vacuoles were counted without differentiation between these two parasitic stages. In addition, the number of Ki-67 F4/80 double positive cells was evaluated on sections labeled simultaneously for the Ki-67 and the F4/80 antigen. At least 100 high power fields (HPF) randomly selected from all areas of the brain were analyzed per section in three animals per group. The statistical significance of the differences was evaluated by the Wilcoxon Ranksum test. To test for statistical differences in the survival rate and TNF expression of CD200−/− and WT mice, the χ2 test was used. P values <0.05 were accepted as significant. Experiments were performed in duplicate.
Results
Expression of CD200 and CD200R in the normal and T. gondii-infected murine brain of CD200−/− and WT mice
In accordance with data reported previously [12, 31], in the brain of uninfected WT mice, CD200 was ubiquitously expressed on neurons and their axons, cerebral endothelial cells, and plexus macrophages, but not on microglia, astrocytes, and oligodendrocytes. As expected, CD200 was consistently absent from the brain of CD200−/− mice (data not shown).
Upon T. gondii infection, CD200 expression was upregulated on cerebral endothelial cells and remained unchanged on neurons and their axons, while double immunofluorescence studies did neither detect CD200 co-expression on CD4 and CD8 T cells nor on B220+ B cells.
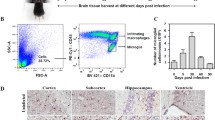
In the normal brain of CD200−/− and WT mice, the CD200R was expressed at very low levels on cerebral macrophages and microglia, and was absent from the few CD4 and CD8 T cells present i.c. under physiological conditions without differences between the two strains (Fig. 1). In TE, there was a strong upregulation of the CD200R on myeloid cells, which was most pronounced on microglial cells in both CD200−/− and WT mice, an intermediate upregulation on macrophages and a slight increase on CD4 and CD8 T cells in both CD200−/− and WT mice (Fig. 1).
Expression of the CD200R on macrophages, microglia, and CD4 and CD8 T cells in the normal and T. gondii-infected brain of CD200−/− and WT mice. Cerebral leukocytes were isolated from uninfected and T. gondii-infected (day 23 p.i.) CD200−/− and WT mice. Microglia, macrophages, as well as CD4 and CD8 T cells were stained for CD200R expression and analyzed by flow cytometry. a Microglia (dotted circle) and macrophages (solid circle) were identified by their F4/40 und CD45 expression as illustrated for both WT and CD200−/− mice at day 23 p.i. b Histograms of CD200R expression are shown, and the mean fluorescence intensity of CD200R expression is given for each cell type and time point. Microglia and macrophages were stained for CD200R expression as described in Materials and methods including negative control stainings with isotype-matched control antibodies. Negative control stainings are shown for microglial cells (dotted lines) as an example including the mean (in italics). Pooled brains of six mice per time point and group were studied
Characteristics of microglia in the normal brain of CD200−/− mice
Morphologically, microglia of uninfected mice expressed low levels of F4/80, CD45, LFA-1 (CD11a) and CD11b, and was negative for MHC class I and II antigens; TNF, iNOS, and Ki-67 without differences between CD200−/− and WT mice. CD11c was expressed on exceptional microglial cells in the periventricular brain parenchyma in WT mice (Fig. 2a). In CD200−/−, but not in WT mice, some CD11c+ microglial cells formed small clusters in the basal ganglia (Fig. 2b).
Expression of CD11c on microglia of uninfected CD200−/− and WT mice. The CD11c antigen is expressed on occasional microglial cells and a small cluster of microglial cells in the basal ganglia of uninfected WT (a) and CD200−/− mice (b). Anti-CD11c immunostaining, slight counterstaining with hemalum, original magnification ×200
Macrophages of both strains of mice expressed identical levels of LFA-1, CD11b, MHC class I and II antigens (data not shown). Furthermore, identical numbers of leukocytes and microglial cells were isolated from the uninfected brain of CD200−/− and WT mice (Fig. 3a, b).
Numbers of macrophages and microglial cells isolated from the brain of normal and T. gondii-infected brain of CD200−/− and WT mice. Leukocytes were isolated from uninfected (day 0) and T. gondii-infected (day 23 and 42 p.i.) CD200−/− and WT mice. Six brains of each group were pooled per time point, and the total number of i.c. leukocytes was determined by cell counting (a). Flow cytometry of stained leukocytes revealed that the number of microglia was elevated in T. gondii-infected CD200−/− mice (b). The number of CD4 and CD8 T cells, macrophages, and B cells did not differ between CD200−/− and WT mice (not shown). In a and b data represent the mean of two independent experiments with six mice each. Standard deviation was below 15%
CD200 negatively regulates microglial cell numbers in TE
To determine whether CD200 regulates recruitment of leukocytes to the T. gondii-infected CNS, the numbers of i.c. leukocytes were determined. In uninfected and T. gondii-infected mice at days 23 and 42 p.i., the number of i.c. leukocytes did not differ between CD200−/− and WT animals (Fig. 3a). However, a detailed subanalysis of i.c. leukocytes showed that microglial cells were markedly increased in number in the brains of T. gondii-infected CD200−/− mice (Fig. 3b), whereas the numbers of CD4 and CD8 T cells, B cells, granulocytes, and macrophages did not differ between the two experimental groups (data not shown). In addition, T cells of both strains were CD44+ and CD62L− compatible with an effector/memory phenotype (data not shown). These findings argue that CD200 primarily acts on myeloid cells and does not affect the phenotype of i.c. CD4 and CD8 T cells in TE.
Therefore, the expression of the proliferation-associated Ki-67 antigen by F4/80+ cells, which include microglia and macrophages, was analyzed. In WT mice, Ki-67+ F4/80+ cells increased during TE with peak levels at day 42 p.i. and declining thereafter (Table 1). In addition to being located in meningeal infiltrates, Ki-67+ leukocytes were present in perivascular cuffs and also scattered throughout the brain parenchyma (Fig. 4a, b). While Ki-67+F4/80+ cells were equally distributed within the brain of CD200−/− and WT mice, they were increased in number in mice lacking CD200 throughout the infection (Table 1). In both strains of mice, most Ki-67+ F4/80+ cells harbored short, thin, ramified processes, and a small elongated nucleus, i.e. the morphological characteristics of activated microglia. Collectively, these findings indicate that CD200 strongly downregulates proliferation of microglia.
Proliferative activity in T. gondii-infected CD200−/− and WT mice. While there are only very few Ki-67+ cells in a T. gondii-associated infiltrate in the brain of a WT mouse (a), many cells with rod shaped nuclei are Ki-67+ in the vicinity of an inflammatory infiltrate in a CD200−/− mouse (b) at day 54 p.i. Anti-Ki-67 immunostaining, slight counterstaining with hemalum, original magnification ×100. At day 54 p.i., cells in a characteristic small inflammatory infiltrate are iNOS+ in a WT mouse (c), whereas a large number of cells in an infiltrate of typical size express the iNOS protein in a CD200−/− mouse (d), original magnification ×200
Impact of CD200 on microglial cell and macrophage immune reactions
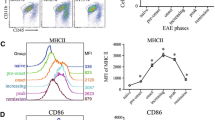
Microglial cells were strongly activated in both strains of mice as evidenced by an ubiquitous upregulation of F4/80, LCA, LFA-1, as well as a strong induction of MHC class I and II antigens. There were no regional differences in the activation pattern of microglia between the experimental groups. A detailed quantitative flow cytometric analysis demonstrated an increased expression of MHC class II antigen on microglia, but not on macrophages of CD200−/− mice as compared to WT animals at days 23 and 42 p.i. In addition, MHC class I expression was slightly increased on microglia and macrophages of CD200−/− mice. While induction of CD80 and CD86 expression on microglia was equally strong in T. gondii-infected CD200−/− and WT mice, expression of LFA-1 was slightly reduced on microglia of CD200−/− mice (Fig. 5). In general, CD200−/− macrophages showed the same alterations as CD200−/− microglia (Fig. 5) indicating that the effect of CD200 on the expression of these cell surface molecules is shared between these two closely related myeloid cell populations.
Activation of microglia and macrophages in the normal and T. gondii-infected brain of CD200−/− and WT mice. Cerebral leukocytes were isolated from uninfected (d0) and T. gondii-infected (days 23 and 42 p.i.) CD200−/− and WT mice. Microglia and macrophages were stained for cell surface expression of MHC class II antigens, MHC class I antigens, LFA-1, B7-1 (CD80) and B7-2 (CD86), as well as with a rat IgG control antibody. The mean fluorescence intensity of the respective antigens is shown for microglia (left column) and macrophages (right column). Pooled brains of six mice per time point and group were studied. In a second experiment, similar findings and differences were observed
Histology revealed that CD11c was markedly and widely upregulated on perivascular CD11b+F4/80+ macrophages and microglia, which also formed clusters of CD11c+ cells, however, without prominent differences between the two strains of mice.
In accordance with earlier observations [20], a panel of cytokines was induced in the brain of T. gondii-infected CD200−/− and WT mice with levels paralleling disease activity. Transcription levels of IFN-γ, IL-12p40, IL-10, IL-15 as well of IDO, TNF, and iNOS did not differ in brain tissue homogenates between the two strains of mice (data not shown). TNF and iNOS, two important pro-inflammatory and effector molecules known to be produced by microglia and other i.c. macrophages, were further investigated at the protein level. Immunohistochemistry demonstrated expression of TNF predominantly on perivascular and T. gondii-associated leukocytes in inflammatory infiltrates and on microglia in their vicinity without differences in their topography. Further FACS analysis confirmed the expression of TNF by both macrophages and microglial cells. While macrophages of uninfected mice (day 0) did not express TNF, a small number of microglial cells (5%) was TNF+ (Fig. 6). In acute TE (day 23 p.i.), both strains strongly upregulated TNF expression of microglia (P<0.01 for both strains) and macrophages (P<0.005 for both strains, Fig. 6). In the chronic stage of TE (day 42 p.i.), more microglial cells, but less macrophages of CD200−/− mice expressed TNF as compared to WT mice (P<0.01 for microglial cells, P<0.05 for macrophages; Fig. 6). INOS was expressed in perivascular cuffs and in parasite-associated infiltrates and also by microglial cells in their vicinity, increasing in both strains of mice up to day 54 p.i. In the absence of CD200, iNOS expression by both microglia and leukocytes was more pronounced in the vicinity of T. gondii-associated infiltrates up to day 54 p.i. (Fig. 4c, d), but this regionally increased iNOS expression did not result in a more pronounced iNOS mRNA expression in brain tissue homogenates of CD200−/− mice.
TNF expression by i.c. macrophages and microglia in the normal and T. gondii-infected brain of CD200−/− and WT mice. Cerebral leukocytes were isolated from uninfected (d0) and T. gondii-infected (days 23 and 42 p.i.) CD200−/− and WT mice. Microglia and macrophages were stained for intracellular TNF expression and analyzed by flow cytometry. F4/80+ cells were gated and dot plots show TNF and CD45 expression of F4/80+ cells. Microglia is CD45low and macrophages are CD45high. The percentage of TNF positive and negative microglia (upper and lower left quadrant) and macrophages (upper and lower right quadrant) is given for each time point. Pooled brains of six mice per time point and group were studied
Taken together, these findings indicate that CD200 selectively regulates a restricted number of microglial cell reactions including expression of MHC antigens, cell adhesion molecules, and anti-parasitic effector molecules.
Reduced intracerebral parasitic load in T. gondii-infected CD200−/− WT mice
Following infection with T. gondii, both strains developed TE with the same kinetics. In the acute stage of the infection, numbers of i.c. parasites increased, declining with development of chronic TE. At all time points of the disease, WT animals harbored increased numbers of parasites in their brains as compared to CD200−/− mice (Fig. 7a), with an accelerated rate of parasite elimination from the brains of CD200−/− mice, finally achieving a significantly lower i.c. parasitic load (P<0.05 at days 21 and 71 p.i., Fig. 7a).
Intracerebral parasitic load and survival rates of T. gondii-infected WT and CD200−/− mice. a Parasitic load in the brains of T. gondii-infected WT (filled bars) and CD200−/− (open bars) mice. The mean ± SEM of the number of T. gondii/HPF evaluated on immunostained sections is demonstrated (*P<0.05). b The survival of T. gondii-infected WT and CD200−/− mice was monitored up to day 71 p.i. At this stage of disease, surviving mice of both strains were severely ill and prone to death. For ethical reasons mice were killed at this time point. Ten mice were analyzed per group. At day 71 p.i., the difference in the survival rate was significantly different between WT and CD200−/− mice (P<0.025)
In addition, the survival rate of CD200−/− mice was significantly higher as compared to WT animals at day 71 p.i. (P<0.025, Fig. 7b), although the absence of CD200 did not prevent the progressive deterioration of mice with chronic TE, and at day 71 p.i. surviving mice of both strains were critically ill and killed for ethical reasons.
Discussion
The present study illustrates that brain resident cells participate in the control and regulation of i.c. immune responses during a chronic parasitic encephalitis via CD200/CD200R interactions. The CD200-mediated immunomodulatory effect was largely restricted to microglial cells which prominently expressed the CD200R, and to a lesser extent on macrophages, which also upregulated expression of the CD200R in TE. The only cell population expressing the CD200 antigen were neurons, blood vessel endothelial cells, and plexus macrophages indicating that CD200 deficiency of these cell populations caused the increase in microglial cell activation, proliferation and, finally, the improved parasite control.
While the CD200 antigen was widely expressed in the brain on neurons and their axons, cerebral endothelial cells, and plexus macrophages, it was absent on astrocytes, microglia, oligodendrocytes, and i.c. inflammatory leukocytes including T and B cells illustrating that specialized cell populations express this immunomodulatory molecule. In contrast, the CD200R was weakly expressed on microglia and macrophages of uninfected mice, but absent on CD4 and CD8 T cells, thereby confirming and extending recent reports [1, 2, 12, 17, 30, 31]. Interestingly, both the expression of the CD200 antigen as well as its receptor were upregulated in TE with a particularly prominent rise of CD200R on microglia, an intermediate increase on macrophages, and a small increase on CD4 and CD8 T cells already indicating that a CD200/CD200R interaction might regulate i.c. immune responses in TE and that regional changes in the expression level may provide a mechanism for the local regulation of myeloid cellular activity in the T. gondii-infected brain parenchyma. Since the increase of the CD200R expression was equally strong in T. gondii-infected CD200−/− and WT mice, expression and upregulation of CD200R is regulated independent of CD200.
Previous studies revealed an expansion of macrophage populations in the spleen and mesenteric lymph nodes in the absence of CD200 [12], the reason for which is still unknown. However, the number of i.c. macrophages and microglia in the normal, uninfected brain was not significantly altered by CD200-deficiency (Fig. 3). In addition, there were no differences in the basal levels of CD45, F4/80, LFA-1, MHC class I and II antigen, B7.1, B7.2, and TNF of microglia from uninfected CD200−/− mice, which, however, harbored elevated numbers of CD11c+ microglia in the basal ganglia. Resting microglia in the brain parenchyma of CD200−/− mice did not express iNOS, a finding which differs markedly from retinal microglia of CD200−/− mice, which responded to CD200-deficiency by a spontaneous expression of iNOS as well as a 30% increase in the proportion of resident retinal CD45+CD11b+ cells [3]. This latter observation supported the concept of CD200/CD200R-mediated signals suppressing the tonic activation of macrophages [3]. The divergent findings on the resting state of microglia in the brain and the retina may indicate that the immunological phenotype of the brain is more tightly downregulated to protect the highly vulnerable brain parenchyma.
Although microglial cell numbers increased upon T. gondii infection in both strains of mice, microglial cells raised to significantly elevated numbers in CD200−/− mice as compared to WT animals at days 23 and 42 p.i. At later stages of the infection, microglia cell numbers of WT mice had returned to the number of uninfected mice, whereas CD200−/− animals still harbored elevated numbers of microglial cells. Since macrophages recruited into the T. gondii-infected CNS do not transform into microglia [23], we evaluated whether the increased microglia cell numbers might be caused by a proliferation of this cell type. In fact, in CD200−/− mice an increased number of F4/80+ cells expressed the proliferation-associated Ki-67 antigen as compared to WT animals. In both strains of mice most Ki67+F4/80+ cells harbored short thin processes and an elongated nucleus indicating microglial cell proliferation in TE. In addition, it should be noted that inflammatory macrophages recruited to the CNS are a post-mitotic cell population, which does not transform into microglia in TE [23], and, therefore the combined flow cytometric and immunohistochemical analysis suggests that the increase of microglial cell numbers in CD200−/− mice is caused by an increased proliferation of this cell type.
In accordance with observations in EAE and in the model of facial nerve transection in CD200−/− mice [9], microglial cells exhibited features of an increased activation. In acute TE, microglia in both strains were already prominently activated at day 21 p.i. without significant differences in the levels of immunologically relevant cell surface molecules including CD45, F4/80, LFA-1, MHC class I and II antigens, CD11c, CD80, and CD86. However, in chronic TE, i.e. at day 42 p.i., expression of MHC class II antigen, the most sensitive parameter of microglial activation, was enhanced in microglia from CD200−/− mice as opposed to WT mice. In comparison to facial nerve transection and EAE, in which microglial activation is either strictly confined to the facial nucleus or shows regional differences with a pronounced activation in the spinal cord, regional differences of microglial cell activation are not a characteristic feature of TE irrespective of the mouse strain, also including CD200−/− mice. In general, CD200−/− macrophages showed the same up- and downregulation of cell surface molecules as microglia, but the extent of modulation was slightly different illustrating that CD200 has roughly the same either immunosuppressive, immunostimulatory or even no function with respect to the expression of immunoregulatory molecules on microglia and macrophages. Collectively, these observations obtained from various models of autoimmune, degenerative, and infectious diseases of the CNS highlight the strong impact of distinct features of the underlying pathogenic condition on microglial activation.
This assumption is further supported by the T. gondii-associated increased iNOS and TNF expression of CD200−/− microglia in the chronic stage of infection. Both TNF and iNOS play an important role in the control of T. gondii [6, 10, 18, 21, 25] and, functionally important, the enhanced expression of the anti-parasitic effector molecule iNOS and of TNF by microglial cells resulted in a reduced i.c. parasitic load in chronically infected CD200−/− mice and a prolonged survival. The effect of iNOS may not only be limited to microglia and macrophages, which both can be infected with T. gondii and control the intracellular parasite by an iNOS-dependent pathway [14, 16], but may also extend to T. gondii-infected cells neighboring iNOS-expressing cells such as astrocytes and neurons. Such a mechanism has also been described in murine leishmaniasis [27]. Interestingly, the inhibitory effect of CD200 on TNF expression of myeloid cells was limited to microglia, since CD200−/− macrophages produced less TNF than WT macrophages, further illustrating that CD200 exerts different effects on microglia and macrophages.
The observation that the CD200/CD200R axis is of functional importance for parasite control is of key importance for both an understanding on how brain resident cells contribute to the control of an i.c. pathogen as well as for the potential use of CD200 or CD200R blocking for the treatment of autoimmune diseases. The latter bears the intrinsic risk for reactivation of latent toxoplasmosis by microglia/macrophage deactivation.
While T cells are important regulators of microglial activity in TE, microglia vice versa regulates T cells in the T. gondii-infected brain, irreversibly inhibiting T cell proliferation [23, 24]. Therefore, we also analyzed T cell responses in TE of CD200−/− mice. In CD200−/− and WT mice, equal numbers of i.c. CD4 and CD8 T cells were present and these cells had a similar topographical distribution indicating that the CD200 expression neither of cerebral blood vessel endothelial cells nor of neurons affects the recruitment, migration, and persistence of T cells in TE. Furthermore, the CD200R was equally expressed on i.c. CD4 and CD8 T cells of T. gondii-infected CD200−/− and WT mice, and both strains had an equally strong induction of IFN-γ mRNA in the T. gondii-infected brain. From these data it can be concluded that in TE CD200−/− mice generate equivalent i.c. T cell responses. These observations demonstrating unimpaired T cell responses in a model of CNS infection extend data on splenic T cells in experimental allergic uveitis (EAU) [3].
In conclusion, while the absence of the CD200 protein did not significantly alter the downregulated immunological phenotype of microglia in the normal brain, it increased the number of microglia under infectious conditions, induced a parasite-associated local increase in iNOS expression and an increase of microglial TNF production resulting in an improved parasite control and increased survival rate in late stages of TE. However, compared to autoimmune disorders of the brain and other organs, the CD200-mediated effects were more subtle. Whether this rather moderate effect is a characteristic feature of CNS infections as opposed to autoimmune disorders and whether CD200-mediated regulation of microglial cells plays a role in human CNS infections including TE remains to be elucidated.
References
Barclay AN (1981) Different reticular elements in rat lymphoid tissue identified by localization of Ia, Thy-1 and MRC Ox2 antigens. Immunology 44:727–736
Barclay AN, Ward H (1982) Purification and chemical characterisation of membrane glycoprotein from rat thymocytes and brain, recognised by monoclonal antibody MRC Ox2. Eur J Biochem 129:447–452
Broderick C, Hoek RM, Forrester JV, Liversidge J, Sedgwick JD, Dick AD (2002) Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am J Pathol 161:1669–1677
Brown CR, Hunter CA, Estes RG, Beckmann E, Forman J, David C, Remington JS, Mcleod R (1995) Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology 85:419–428
Deckert-Schlüter M, Schlüter D, Schmidt D, Schwendemann G, Wiestler OD, Hof H (1994) Toxoplasma encephalitis in congenic B10 and BALB mice: impact of genetic factors on the immune response. Infect Immun 62:221–228
Deckert-Schlüter M, Bluethmann H, Rang A, Hof H, Schlüter D (1998) Crucial role of TNF receptor type 1 (p55), but not of TNF receptor type 2 (p75), in murine toxoplasmosis. J Immunol 160:3427–3436
Deckert-Schlüter M, Buck C, Schlüter D (1999) Kinetics and differential expression of heat-stable antigen and GL7 in the normal and Toxoplasma gondii-infected murine brain. Acta Neuropathol (Berl) 98:97–106
Ferguson DJ, Hutchison WM (1987) The host–parasite relationship of Toxoplasma gondii in the brains of chronically infected mice. Virchows Arch A Pathol Anat Histopathol 411:39–43
Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A (1992) Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol 149:175–180
Gazzinelli RT, Eltoum I, Wynn TA, Sher A (1993) Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlated with the downregulated expression of inducible nitric oxide synthase and other markers of activation. J Immunol 151:3672–3681
Habegger de Sorentino A, Lopez R, Motta P, Marinic K, Sorrentino A, Iliovitch E, Rubio AE, Quarleri J, Salomon H (2005) HLA class II involvement in HIV-associated Toxoplasmic encephalitis development. Clin Immunol 115:133–137
Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom BH, Barclay AN, Sedgwick JD (2000) Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290:1768–1771
Johnson J, SuzukiY, Mack D, Mui E, Estes R, David C, Skamene E, Forman J, McLeod R (2002) Genetic analysis of influences on survival following Toxoplasma gondii infection. Int J Parasitol 32:179–185
Langermans JA, Van der Hulst ME, Nibbering PH, Hiemstra PS, Fransen L, Van Furth R (1992) IFN-gamma-induced l-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J Immunol 148:568–574
Mack DG, Johnson JJ, Roberts CW, Estes RG, David C, Grumet FC, McLeod R (1999) HLA-class II genes modify outcome of Toxoplasma gondii infection. Int J Parasitol 29:1351–1358
Nibbering PH, Langermans JA, van de Gevel JS, van der Hulst MB, van Furth R (1991) Nitrite production by activated murine macrophages correlates with their toxoplasmastatic activity, Ia antigen expression, and production of H2O2. Immunobiology 184:93–105
Preston S, Wright G, Starr K, Barclay N, Brown M (1997) The leukocyte/neuron cell surface antigen OX2 binds to a ligand on macrophages. Eur J Immunol 27(8):1911–1918
Scharton-Kersten TM, Yap G, Magram J, Sher A (1997) Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med 185:1261–1273
Schlüter D, Löhler J, Deckert M, Hof H, Schwendemann G (1991) Toxoplasma encephalitis of immunocompetent and nude mice: immunohistochemical characterisation of Toxoplasma antigen, infiltrates and major histocompatibility products. J Neuroimmunol 31:185–198
Schlüter D, Kaefer N, Wiestler OD, Hof H, Deckert-Schlüter M (1997) Expression pattern and cellular origin of cytokines in the normal and T. gondii-infected murine brain. Am J Pathol 150:1021–1035
Schlüter D, Deckert-Schlüter M, Lorenz E, Meyer T, Röllinghoff M, Bogdan C (1999) Inhibition of inducible nitric oxide synthase exacerbates chronic cerebral toxoplasmosis in Toxoplasma gondii-susceptible C57BL/6 mice but does not reactivate the latent disease in T. gondii-resistant BALB/c mice. J Immunol 162:3512–3518
Schlüter D, Deckert M, Hof H, Frei K (2001) Toxoplasma gondii infection of neurons induces neuronal cytokine and chemokine production, but gamma interferon- and tumor necrosis factor-stimulated neurons fail to inhibit the invasion and growth of T. gondii. Infect Immun 69:7889–7893
Schlüter D, Meyer T, Strack A, Reiter S, Kretschmar M, Wiestler OD, Hof H, Deckert M (2001) Regulation of microglia by CD4+ and CD8+ T cells: selective analysis of microglial cell surface antigen expression and cytokine production in CD45-congenic normal and Toxoplasma-infected bone marrow chimeras. Brain Pathol 11:44–55
Schlüter D, Meyer T, Kwok L, Montesinos-Rongen M, Lütjen S, Strack A, Schmitz L, Deckert M (2002) Phenotype and regulation of persistent T cells in murine Toxoplasma encephalitis. J Immunol 169:315–322
Schlüter D, Kwok LY, Lütjen S, Soltek S, Hoffmann S, Körner H, Deckert M (2003) Both lymphotoxin-alpha and TNF are crucial for control of Toxoplasma gondii in the central nervous system. J Immunol 170:6172–6182
Sedgwick JD, Schwender S, Imrich H, Dörries R, Butcher GW, ter Meulen V (1991) Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA 88:7438–7442
Stenger S, Thüring H, Röllinghoff M, Bogdan C (1994) Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J Exp Med 180:783–793
Suzuki Y, Wong SY, Grumet FC, Fessel J, Montoya JG, Zolopa AR, Portmore A, Schumacher-Perdreau F, Schrappe M, Koppen S, Brown BW, Remington JS (1996) Evidence for genetic regulation of susceptibility to toxoplasmic encephalitis in AIDS patients. J Infect Dis 173:265–268
Suzuki Y, Orellana MA, Schreiber RD, Remington JS (1998) Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516–518
Wright GJ, Puklavec MJ, Willis AC, Hoek RM, Sedgwick JD, Brown MH, Barclay AN (2000) Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity 13:233–242
Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, Song Y, Jenmalm M, Gorman D, McClanahan T, Liu M, Brown MH, Sedgwick JD, Phillips JH, Barclay AN (2003) Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol 171:3034–3046
Acknowledgement
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (grant no.: De 485/6-3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deckert, M., Sedgwick, J.D., Fischer, E. et al. Regulation of microglial cell responses in murine Toxoplasma encephalitis by CD200/CD200 receptor interaction. Acta Neuropathol 111, 548–558 (2006). https://doi.org/10.1007/s00401-006-0062-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0062-z