Abstract
Guam parkinsonism-dementia complex (PDC) is a neurodegenerative tauopathy in ethnic Chamorro residents of the Mariana Islands that manifests clinically with parkinsonism as well as dementia and is characterized neuropathologically by prominent cortical neuron loss in association with extensive telencephalic neurofibrillary tau pathology. To further characterize cortical gray and white matter tau, alpha-synuclein and lipid peroxidation pathologies in Guam PDC, we examined the brains of 17 Chamorro PDC and control subjects using biochemical and immunohistological techniques. We observed insoluble tau pathology in both gray and white matter of PDC and Guam control cases, with frontal and temporal lobes being most severely affected. Using phosphorylation dependent anti-tau antibodies, abundant tau inclusions were detected by immunohistochemistry in both neuronal and glial cells of the neocortex, while less alpha-synuclein pathology was observed in more limited brain regions. Further, in sharp contrast to Alzheimer’s disease (AD), levels of the lipid peroxidation product 8, 12–iso-iPF2α-VI isoprostane were not elevated in Guam PDC brains relative to controls. Thus, although the tau pathologies of Guam PDC share similarities with AD, the composite Guam PDC neuropathology profile of tau, alpha-synuclein and 8, 12-iso-iPF2α-VI isoprostane reported here more closely resembles that seen in other tauopathies including frontotemporal dementias (FTDs), which may imply that Guam PDC and FTD tauopathies share underlying mechanisms of neurodegeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Guam parkinsonism-dementia complex (PDC) is a neurodegenerative disorder of the indigenous Chamorro population of the Mariana Islands in the Western Pacific [34, 36]. The incidence and prevalence of this progressive tauopathy among the native inhabitants of Guam, the largest island in the Mariana group, has been well documented for over 60 years. However, while from 1956–1965, the prevalence of PDC among Chamorros in the Mariana Islands was estimated to be extremely high, i.e., ~250/100,000 (reviewed in [9, 16, 31, 53, 54]), for unknown reasons, the incidence of Guam PDC has declined dramatically over the last two decades [9, 41, 54]. To date no genetic abnormalities (e.g., mutations in the tau gene) have been shown to be pathogenic for PDC [25, 26, 33]. However, tau gene polymorphisms associated with two sporadic frontotemporal dementia (FTD) tauopathies in populations outside Guam, i.e., progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD), are in linkage disequilibrium in affected Charmorros thereby implicating tau gene polymorphisms in the susceptibility to Guam PDC [43]. Furthermore, it has been proposed that environmental factors (e.g., cycad toxicity, high levels of aluminum) contribute to the onset and/or progression of PDC, however this evidence remains controversial [5, 10, 23, 51, 56].
Clinically, patients afflicted with Guam PDC present with rigidity, tremor, bradykinesia and dementia. Dementia in PDC patients consists of prominent memory deficits, disorientation and deterioration of intellectual function as well as variable personality and behavior alterations [17, 18]. Neuropathologically, PDC is characterized by the presence of cortical atrophy, neuronal loss, depigmentation of the substantia nigra and locus ceruleus, in addition to extensive neurofibrillary tangles (NFTs) throughout the neocortex, hippocampus and brain stem [18, 20, 35, 37, 38]. Similar to Alzheimer’s disease (AD), the NFTs in PDC are intraneuronal aggregates of paired helical filaments (PHFs) comprised of hyperphosphorylated forms of the microtubule associated protein tau [4, 28]. In the adult human central nervous system (CNS), six tau isoforms are generated by alternative splicing, and similar to AD, all six isoforms are found in the NFTs of Guam PDC patients [3, 30]. Thus, like other neurodegenerative diseases characterized by prominent tau pathologies, including PSP, CBD and AD as well as several additional sporadic and familial forms of FTD, Guam PDC is classified as a tauopathy [28, 57].
However, the relationship of mechanisms underlying Guam PDC to those underlying other tauopathies is not understood. For this reason, we examined the biochemical composition, distribution, and severity of tau pathologies in various brain regions of Guam PDC patients (n=9) and Chamorro controls (n=8) by Western blot and immunohistochemical methods. Further, since oxidative damage and cross seeding of other amyloidogenic brain proteins have been implicated in mechanisms underlying neurodegeneration in tauopathies [8, 29], similar studies were also performed on these brains to analyze alpha-synuclein pathologies as well as levels of 8, 12-iso-iPF2α-VI isoprotane, a marker of lipid peroxidation [15, 46, 47]. These data were compared with findings in AD and other tauopathies, and we found that although all six tau isoforms are accumulated as NFTs in Guam PDC similar to those observed in AD, the studies reported here suggest that the profile of tau, alpha-synuclein and 8, 12-iso-iPF2α-VI isoprostane neuropathology in Guam PDC brains more closely resembles that of FTD tauopathies.
Materials and methods
Subjects
Postmortem brain samples from 17 deceased Chamorro residents of Guam were obtained from the Guam Brain Bank at Mount Sinai School of Medicine (eight PDC cases and six Guam controls) or directly from the University of Guam (one PDC and two Guam controls). Chamorro Guam PDC patients and controls were evaluated by neurologists from the University of California at San Diego during regular study visits to Guam, and clinical diagnoses were established as described [9]. All patients had clinical features that included both dementia and parkinsonism, and had typical pathology of PDC at autopsy. Diagnosis of the cases studied here were confirmed neuropathologically according to previously reported methods [6, 40]. The age, gender, duration of the disease and confirmed diagnosis of all 17 cases used in this study are listed in Table 1. Guam PDC patients were divided into two subsets based on the duration of the disease, as determined by clinical diagnosis. The early PDC subset consisted of patients with duration of the disease from 1 to 7 years, whereas the late PDC subset was comprised of patients who had disease durations greater than 7 years. In addition, AD, CBD and normal control (without neurodegenerative disorders) brains also were studied here, and these samples were obtained and characterized by the University of Pennsylvania Center for Neurodegenerative Disease Research (CNDR) as described earlier [7].
Biochemical analysis of tau and alpha-synuclein
Frozen brain tissue from frontal, temporal, parietal and occipital lobes, as well as cerebellum, pons, thalamus, medulla and hippocampus, when available, were used for biochemical analysis. For all neocortical brain regions and cerebellum, gray and white matter were separated and processed individually. Phosphorylated and dephosphorylated sarkosyl-insoluble samples were prepared as previously described [22, 27]. Briefly, brain tissue was homogenized in high salt extraction buffer (0.75M NaCl, 0.02 M NaF, 100 mM MES or Tris buffer, pH 7.0–7.4, 1 mM EDTA, 0.5 mM MgSO4) at a ratio of 1 g: 0.8 ml of buffer and centrifuged at 40,000 rpm for 30 min. at 4°C. Pellets were then extracted with PHF extraction buffer (10 mM Tris, 0.85M NaCl, 1mM EDTA, 20 mM NaF, 10% sucrose) at a ratio of 1 g tissue to 5 ml buffer and centrifuged at 13,000 rpm for 25 min. at 4°C. This step was repeated twice and the supernatants were combined. The pooled supernatants were then incubated in a final concentration of 1% sarkosyl at room temperature for 1–3 h. After incubation, the sample was centrifuged at 40,000 rpm for 40 min. at 20°C and the resulting pellet was re-suspended in 1.5×sample buffer (1 g tissue : 100 μl buffer). Where indicated, tau was dephosphorylated by dialysis (50 mM Tris, 0.2 mM EDTA, pH 8.0) and treated with Escherichia coli alkaline phosphatase (Sigma, St. Louis MO).
To examine the solubility profile of tau and alpha-synuclein, sequential extractions of brain samples were performed with methods similar to those reported earlier [6]. Briefly, proteins were extracted by repeated homogenization and centrifugation steps in buffers of increasing extraction strength at a ratio of 1 g tissue to 2 ml buffer. Brain tissues were first homogenized in high salt buffer and centrifuged at 45,000 rpm for 30 min. at 4°C to generate high salt-soluble samples. To prevent carry over the resulting pellets were re-homogenized and re-centrifuged. Only supernatants from the first centrifugation were analyzed and all supernatants produced from the second wash step were discarded. High salt-insoluble pellets were then extracted in 1% Triton-X dissolved in high salt buffer and centrifuged at 45,000 rpm for 30 min. at 4°C, generating Triton-X-soluble fractions. Triton-X-insoluble pellets were washed, extracted in RIPA buffer (0.1% SDS, 1% NP-40, 0.5% SDS, 5 mM EDTA, 150 mM NaCl, 50 mM Tris Base, pH 8.0) and centrifuged creating the RIPA-soluble fraction. This procedure was repeated with SDS buffer (2% SDS in 50 mM Tris, pH 7.6) and 70% formic acid (FA). FA was evaporated in an Automatic Environmental SpeedVac system (Savant Instruments, Holbrook, NY). The dried pellets were resuspended in sample buffer at 1 ml/g. Protease inhibitors were added to all buffers prior to use. All proteins were resolved by 7.5%/15% sodium dodecyl sulfate-polyacrylamide (SDS) gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Following transfer, membranes were blocked with Tris buffered saline (TBS) containing 5% powdered milk and probed with a mixture of anti-tau monoclonal antibodies (MAbs) T14 (1:3000) [22, 24] and T46 (1:1000) [22, 24], or the anti-alpha-synuclein MAb LB509 (1:1000) [12]. MAbs were detected with horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Santa Cruz Biotechnologies, Santa Cruz, CA) and signals were revealed by an HRP-based chemiluminescent reaction (Pierce, Rockford, IL).
Immunohistochemistry
The tissues were fixed in 10% neutral buffered formalin, paraffin-embedded, and cut into 6 μm thick sections. Immunohistochemistry was preformed as previously described using the ABC method (Vectastatin ABC kit, Vector Laboratories, Burlingame, CA) and 3,3’-diaminobenzidine (DAB) [6, 30, 48]. The following primary antibodies were used: MAbs PHF-1 (1:1000) [14], AT-8 (1:1000) [13, 32], LB509 (1:1000) [12] and the affinity purified polyclonal antibody 17025 (1:3000) [58]. The sections were viewed with a Nikon FXA microscope and images were captured with RS Image software (Roper Scientific Inc, Duluth, GA).
Isoprostane analysis
Brian tissue was homogenized and total lipids were extracted using Folich solution (choloroform/methanol 2:1 vol.). Base hydrolysis was then performed using 15% KOH at 45°C for 1 h and total 8, 12-iso-iPF2α-VI levels were measured as described previously [15, 47]. All samples were coded and analyzed in duplicate in a blinded manner.
Results
Biochemical analysis of tau proteins in Guam PDC brains
Biochemical analysis of nine Guam PDC brains revealed extensive, but highly variable, sarkosyl-insoluble tau pathology throughout various regions of the brain (Fig. 1). In all cases examined, the neocortex, cerebellum and medulla were predominately affected with the frontal and temporal lobes exhibiting the most severe tau pathology (Fig. 1a). Similar to PHF tau proteins (PHFtau) in AD, the biochemical profile of pathological tau in Guam PDC consists of all six isoforms, but, in contrast to the AD brain, wherein white matter tau pathology is virtual absent, all PDC brains examined displayed high levels of insoluble tau in both white and gray matter (Fig. 1b). Notably, this distribution pattern of tau pathology in Guam PDC brains is similar to that of CBD, as shown in Fig. 1b, and as described earlier for CBD and other FTD tauopathies [7, 28, 58, 59]. However, unlike CBD, white matter pathology of Guam PDC consisted of all six tau isoforms.
Representative Western blots of sarkosyl-insoluble tau proteins in Guam PDC cases. a Dephosphorylated samples from several brain regions of a representative PDC brain were resolved by SDS-PAGE and immunoblotted with a mixture of the tau specific MAbs T14 and T46. b Sarkosyl-insoluble tau isolated from gray and white matter regions of the frontal cortex from four PDC cases. Dephosphorylated samples were resolved by SDS-PAGE and immunoblotted with a mixture of the tau specific MAbs T14 and T46. AD and CBD were used as comparative controls. Insoluble tau from Chamorro patients was composed of all six tau isoforms similar that observed in AD patients. c, d Neurofibrillary pathology in PDC cases is composed of hyperphosphorylated tau. Non-dephosphorylated sarkosyl-insoluble tau isolated from gray and white matter regions of the frontal cortex from three PDC cases were resolved by SDS-PAGE and immunoblotted with phosphorylation independent anti-tau MAbs T14 and T46 (c), or (d) the phosphorylation-dependent antibody PHF-1. Molecular weight standards and recombinant tau isoforms (Rt) are indicated on the left and right of the figure, respectively. Fr frontal lobe, Te temporal lobe, Pa parietal lobe, O occipital lobe, Ce cerebellum, M medulla, G gray matter, W white matter
To further examine the biochemical properties of tau in gray and white matter of Guam PDC brains, we performed Western blot analysis using the phosphorylation- dependent tau antibody PHF-1, which specifically recognizes tau when it is abnormally phosphorylated at Ser-396/Ser-404 (Fig. 1c). Although some variability among cases was observed, sarkosyl-insoluble gray and white matter fractions extracted from the frontal lobes of PDC brains exhibited similar levels of phosphorylation. The profile of highly phosphorylated and insoluble PHF-1 positive tau in both gray and white matter here indicates that all six tau isoforms are abnormal in Guam PDC brains and that these results do not reflect contamination from soluble tau (Fig. 1c).
Biochemical analysis of tau proteins in Guam control brains
We next performed Western blot analysis on sarkosyl-insoluble tau fractions obtained from Guam Chamorro controls of similar/comparable age to the PDC patients. For the purpose of this study, we define a Guam control as an individual of Chamorro dissent without a clinically diagnosed neurodegenerative disorder as described elsewhere [40]. Interestingly, although these individuals do not display any clinical symptoms of PDC, or other tauopathies, they possess elevated levels of pathological tau in several regions throughout the brain (Fig. 2). Similar to the pattern observed in PDC brains, the extent of sarkosyl-insoluble tau in Guam control brains was most severe in the frontal and temporal lobes, with gray matter regions showing higher levels of tau pathology than those observed in white matter regions. Detectable levels of tau pathology were, however, also observed in the cerebellum and medulla, albeit to a much less extent (Fig. 2a). Furthermore, when sarkosyl-insoluble fractions from frontal gray and frontal white matter were probed with PHF-1, only limited levels of PHF-1 positive hyperphosphorylated tau were observed (compare Fig. 2b, c).
Representative Western blot analysis of sarkosyl-insoluble tau proteins in Guam control cases. a Dephosphorylated samples from different brain regions of a representative Guam control brain were resolved by SDS-PAGE and immunoblotted with MAbs T14 and T46. b Sarkosyl-insoluble tau isolated from gray and white matter regions of the frontal cortex from three PDC cases were resolved by SDS-PAGE and immunoblotted with the MAbs T14 and T46, or c the phosphorylation-dependent antibody PHF-1. Molecular weight standards and recombinant tau isoforms (Rt) are indicated on the left and right of the figure, respectively. Fr frontal lobe, Te temporal lobe, Pa parietal lobe, O occipital lobe, Ce cerebellum, M medulla, G gray matter, W white matter
Comparison of tau and alpha-synuclein pathologies in Guam PDC brains
To compare and contrast the patterns of tau and alpha-synuclein pathologies in Guam PDC brains, sequential extractions using buffers of increasing strengths were performed. Upon extraction, soluble (high salt buffer and Triton-X buffer) and insoluble (RIPA buffer, 2% SDS and FA) fractions from Guam control, Guam PDC (early and late stage disease), normal control and AD brains were subjected to Western blot analysis using the MAbs T14/T46 (for tau) and LB509 (for alpha-synuclein) as shown in Figs. 3 and 4, respectively. For this study the term normal control describes age-matched, non-Chamorro patients with no clinical or pathological evidence of a neurodegenerative disease. Soluble high salt and Triton-X fractions isolated from Guam control brains exhibited increased levels of tau, as compared to normal control brains of non-Chamorros (compare Fig. 3a, c). Interestingly, although Guam control patients display no clinical symptoms of PDC, or any other neurodegenerative tauopathy, they possess detectable levels of insoluble tau in multiple gray and whiter matter regions (Fig. 3c). In sequential extractions of early and late PDC brains, insoluble tau pathology was observed in both gray and white matter (compare Fig. 3d, e). This finding is in direct contrast to AD brains wherein there is no or scant insoluble tau in white matter (Fig. 3b, d, e). Although the amount of insoluble tau varied among PDC patients, the severity and insolubility of tau pathology increased with disease progression (Fig. 3d, e). Further, SDS and FA fractions from PDC patients largely contained aggregated forms of tau which appeared as a smear of immunoreactivity when probed with tau specific antibodies (Fig. 3d, e).
Western blots of dephosphorylated soluble and insoluble tau from sequential extractions of normal control, AD, Guam control and Guam PDC brains. a Soluble high salt (HS), Triton-X100 (Tx) and RIPA (R) fractions and insoluble SDS (S) and FA soluble fractions from normal control brains were separated by SDS-PAGE and immunoblotted with MAbs T14 and T46. b The same fractions as in (a) from AD brains were probed by the same Western blot methods as above using a mixture of T14 and T46. c The same fractions from Guam control brains were probed by the same western blot methods using T14 and T46 as described above. d, e The same fractions from early (d) and late (e) Guam PDC brains were analyzed as described above using T14 and T46. Note that the soluble extracts contain all six adult brain tau isoforms and the insoluble extracts from PDC brains also include all six tau isoforms similar to AD
Western blot analysis of dephosphorylated soluble and insoluble alpha-synuclein from sequential extraction of normal control, AD, Guam control and Guam PDC brains. a Soluble high salt (HS), Triton-X100 (Tx) and RIPA (R) fractions and insoluble SDS (S) and FA soluble fractions from normal control brains were separated by SDS-PAGE and immunoblotted with the anti-alpha-synuclein MAb LB509. b The same FA fractions as in (a) from AD brains were analyzed by Western blot in the same manner using MAb LB509. c The same fractions from Guam control brains were analyzed by Western blot with LB509 as above. d, e The same fractions from early (d) and late (e) Guam PDC brains were analyzed as above with LB509
Because alpha-synuclien pathology has previously been reported to occur in the substantia nigra, cerebellum and amygdala of Guam PDC patients [6, 50, 55], we examined the profile of alpha-synuclein pathology in normal non-Chamorro control, Guam Chamorro control and Guam PDC brains (Fig. 4). Sequential extractions revealed no detectable insoluble alpha-synuclein pathology in normal, Guam control or early stage PDC brains (Fig. 4a, c, d), however, limited and regional specific insoluble alpha-synuclein pathology was observed in the late stage PDC patients (Fig. 4e).
Immunohistochemical examination of tau pathology
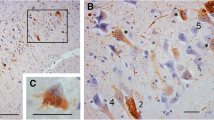
Immunohistochemical analysis of tau pathology was performed on tissue sections obtained from frontal and temporal lobes of four Guam PDC and four Guam control brains. Using a panel of epitope specific tau antibodies, we observed the presence of tau-positive inclusions in both the gray and white matter in all PDC Guam cases examined (Figs. 5, 6). These morphologically variable tau lesions included thread-like pathology (Fig. 5a) as well as both neuronal and glial inclusions, detected by phospho-dependent AT-8 and PHF-1 tau antibodies and phospho-independent (affinity purified 17025) tau antibodies (Fig. 5b–e). Interestingly, the severity of pathology observed by immunohistochemistry in all Guam PDC brains examined was considerably less, especially in white matter regions (Fig. 6), as compared to that documented by Western blot analysis (compare Fig. 1 with Fig. 6). Further, Guam controls, which exhibited an abnormally high level of tau pathology when examined biochemically, showed scant tau neuropathology by immunohistochemistry in the regions examined here (Fig. 7). Taken together, these results suggest that Western blot analysis may reveal a larger burden of pathological tau than immunohistochemical methods and microscopy in Guam PDC and Guam control brains.
Characteristic brain lesions of Guam PDC. a Dystrophic neurites or thread pathology in neocortex (tau immunostain, PHF-1). b NFT in frontal lobe (tau immunostain, AT-8). c NFTs in temporal lobe (tau immunostain, PHF-1). d Astrocytic cytoplasmic inclusion in neocortex (tau immunostain, PHF-1). e Oligodendroglial inclusion (coiled body) in white matter (tau immunostain, AT-8); Scale Bar; 10 μm
Immunohistochemical examination of tau pathology in Guam PDC brains. Significantly less tau pathology was detected by immunohistochemistry as compared to biochemical analysis. a, b Gray and white matter regions from the frontal cortex of Guam PDC brains stained with tau specific, affinity purified, antibody 17025. c, d Gray and white matter regions from the frontal cortex of Guam PDC brains stained with the phosphorylation-dependent tau antibody AT8. e, f Gray and white matter regions from the frontal cortex of Guam PDC brains stained with the phosphorylation-dependent tau antibody PHF-1
Immunohistochemical examination of tau pathology in Guam control brains. Significantly less tau pathology was detected by immunohistochemistry as compared to biochemical analysis. a, b Gray and white matter regions from the frontal cortex of Guam control brains stained with tau specific, affinity purified, antibody 17025. c, d Gray and white matter regions from the frontal cortex of Guam control brains stained with the phosphorylation-dependent tau antibody AT8. e, f Gray and white matter regions from the frontal cortex of Guam control brains stained with the phosphorylation-dependent tau antibody PHF-1
Isoprostane levels in patients with Guam PDC
Due to the high content of polyunsaturated fatty acids in the CNS, oxidative damage linked to mechanisms of neurodegeneration can result in lipid peroxidation, as reflected by increased brain, cerebrospinal fluid, plasma and urine levels of 8, 12-iso-iPF2α-VI isoprostane, but while levels of 8, 12-iso-iPF2α-VI rise early in the onset of AD, enigmatically, this does not appear to be the case in FTD including FTD tauopathies [15, 45–47]. Thus, to investigate the role of oxidative stress in Guam PDC we examined brain levels of 8, 12-iso-iPF2α-VI isoprostane in gray and white matter of the frontal and temporal lobes of five Guam control brains and nine Guam PDC brains (Fig. 8a, b). In contrast to AD, where 8, 12-iso-iPF2α-VI isoprostane levels are markedly increased, no statistical differences were observed between the two experimental groups (Fig. 8b). For example, 8, 12-iso-iPF2α-VI levels (pg/ml of tissue) in the frontal gray matter, the frontal white matter, temporal gray matter and temporal white matter of Guam control brains were 86.40±24.96, 100.80±23.53, 93.60±27.19 and 109.25±10.73, respectively, while 8, 12-iso-iPF2α-VI levels (pg/ml of tissue) in the frontal gray matter, the frontal white matter, temporal gray matter and temporal white matter of PDC Guam brains were 88.43±25.83, 98.86±15.34, 81.25±15.15 and 89.20±10.27, respectively.
Levels of 8, 12-iso-iPF2α-VI isoprostane (IPF2α-VI) are not elevated in PDC brain homogenates. a Raw data of all brain regions from Guam control and Guam PDC cases examined. b Average 8, 12-iso-iPF2α-VI levels in various neocortical regions from Guam control (black bars) and Guam PDC (gray bars) brain homogenates. The data represent the average of at least five cases performed in duplicate ± S.E.M. Fg frontal gray, Fw frontal white, Tg temporal gray, Tw temporal white
Discussion
Using biochemical and immunohistochemical approaches, we examined the distribution and extent of pathological tau in multiple brain regions of 17 Chamorro individuals (nine PDC and eight Guam controls) and observed widespread accumulations of insoluble, hyperphosphorylated tau similar to AD PHFtau throughout the gray and white matter. Western blot analysis showed tau pathology in the neocortex, cerebellum and medulla, all brains regions documented to undergo severe neuron loss during the course of PDC progression. The presence of such pathology was further confirmed by immunohistological studies which identified multiple types of tau-positive neuronal and glial inclusions. Biochemical examination of alpha-synuclien pathology showed considerable less insoluble pathology, as compared to tau. However, the limited detection of alpha-synuclein pathology in this study is most likely due to regional variability, as accumulation of alpha-synuclien in Lewy bodies and Lewy neurites in the amygdala and, to a lesser extent, the substantia nigra and locus ceruleus of Guam PDC patients has been well documented [6, 19, 50, 55].
A high degree of tau pathology in the hippocampus and entorhinal cortex was also widely detected in the brains of Guam controls [40]. Interestingly, although these Chamorro individuals showed no evidence of neurological disease as reported previously [40], moderate NFTs were present in these brain regions that are similarly affected in PDC. The underlying etiology of these filamentous tau inclusions, which exceed levels associated with normal aging and of that reported in other populations, is currently not understood. It has been suggested that such individuals represent an early, pre-symptomatic stage of the disease and if they had lived longer they would have developed clinically diagnosed features of PDC [1, 39, 40]. However, it remains unclear whether the NFTs identified in Guam controls represent a preclinical stage of the disease or background age-associated pathology of the Chamarro population.
Guam PDC is a progressive neurodegenerative disease characterized neuropathologically by the prominent intracellular accumulation of hyperphosphorylated tau proteins in neurons and glia [17, 18] in the near absence of extracellular senile plaques in most cases [11], although Aβ-rich plaques similar to those seen in AD have been detected in a small percentage of Guam PDC brains [49]. Although NFTs found in PDC brains are biochemically and ultrastructurally similar to NFTs present in classical AD [3, 30] there are noticeable differences between the tau pathologies observed in PDC and AD including the distribution and brain cells affected by these lesions. For example, there is abundant glial tau pathology in PDC while this is absent or scant in AD. Consistent with this, the biochemical analyses here revealed a high level of insoluble tau in white matter regions of PDC brains, in contrast to AD. Tau-positive astrocytic plaques, astrocytic inclusions and oligodendrocyte coiled bodies also have been observed in PDC brains using immunohistochemical techniques [17, 18]. In PDC NFTs are preferentially localized to layers II and III of the neocortex, while neocortical pathology in AD is predominate in layers V and VI [20]. Furthermore, NFTs have also been observed in the spinal cords of PDC patients, an occurrence which has been noted in PSP patients [2, 52], but rarely or not at all in AD patients. Finally, neurophil threads, which are frequently encountered in AD, are inconsistently observed in Guam PDC [21]. Such differences in the distribution of NFTs may account for the observed differences in the presentation of clinical symptoms between the two diseases. For example, memory and cognitive impairments overlap in PDC and AD, probably because of involvement by NFTs of overlapping areas of limbic and neocortex. However, the prominent motor symptoms of PDC reflect the NFT burden affecting the nigrostriatal circuitry [20].
Interestingly, the severity of pathology detected by immunohistochemistry in the both PDC and Guam control brains was considerably less as compared to that documented by Western blot analysis. The exact correlation between tau insolubility and its mechanistic relationship to inclusion formation are currently not well understood. However, similar accumulation of insoluble white matter tau without accompanied immunohistochemical detectable tau was also observed in 23 cases of progressive supranuclear palsy, another tauopathy (V. Zhukareva et al., submitted).
Approximately 45% of FTDs contain prominent tau abnormalities while alpha-synuclein pathologies are less abundant in FTDs and show a distribution that often is restricted to the amygdala [44]. Thus, while the profile of tau pathologies in Guam PDC is similar in many aspects to that in AD, the differences in the tau pathologies of PDC and AD together with the findings described here for alpha-synuclein pathologies and 8, 12-iso-iPF2α-VI isoprostane levels suggest that the neuropathological profile of tau, alpha-synuclein and 8, 12-iso-iPF2α-VI isoprostane in Guam PDC tauopathy more closely resembles that for FTD tauopathies. Taken together, these and other findings support the hypothesis that although distinct pathogenic mechanisms are likely to underlie Guam PDC and related tauopathies among Chamorros, these mechanisms may share more in common with FTD tauopathies than with AD, the most prevalent tauopathy.
References
Anderson FH, Richardson EP Jr, Okazaki H, Brody JA (1979) Neurofibrillary degeneration on Guam: frequency in Chamorros and non Chamorros with no known neurological disease. Brain 102:65–77
Behrman S, Carroll JD, Janota I, Matthews WB (1969) Progressive supranuclear palsy. Clinico-pathological study of four cases. Brain 92:663–678
Buee-Scherrer V, Buee L, Hof PR, Leveugle B, Gilles C, Loerzel AJ, Perl DP, Delacourte A (1995) Neurofibrillary degeneration in amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam. Immunochemical characterization of tau proteins. Am J Pathol 146:924–932
Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR (2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33:95–130
Esclaire F, Kisby G, Spencer P, Milne J, Lesort M, Hugon J (1999) The Guam cycad toxin methylazoxymethanol damages neuronal DNA and modulates tau mRNA expression and excitotoxicity. Exp Neurol 155:11–21
Forman MS, Schmidt ML, Kasturi S, Perl DP, Lee VM-Y, Trojanowski JQ (2002) Tau and alpha-synuclein pathology in amygdala of parkinsonism-dementia complex patients of Guam. Am J Pathol 160:1725–1731
Forman MS, Zhukareva V, Bergeron C, Chin SS, Grossman M, Clark C, Lee VM-Y, Trojanowski JQ (2002) Signature tau neuropathology in gray and white matter of corticobasal degeneration. Am J Pathol 160:2045–2053
Forman MS, Trojanowski JQ, Lee VM-Y (2004) Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med 10:1055–1063
Galasko D, Salmon DP, Craig UK, Thal LJ, Schellenberg G, Wiederholt W (2002) Clinical features and changing patterns of neurodegenerative disorders on Guam, 1997–2000. Neurology 58:90–97
Garruto RM, Fukatsu R, Yanagihara R, Gajdusek DC, Hook G, Fiori CE (1984) Imaging of calcium and aluminum in neurofibrillary tangle-bearing neurons in parkinsonism-dementia of Guam. Proc Natl Acad Sci USA 81:1875–1879
Gentleman SM, Perl D, Allsop D, Clinton J, Royston MC, Roberts GW (1991) Beta (A4)-amyloid protein and parkinsonian dementia complex of Guam. Lancet 337:55–56
Giasson BI, Jakes R, Goedert M, Duda JE, Leight S, Trojanowski JQ, Lee VM-Y (2000) A panel of epitope-specific antibodies detects protein domains distributed throughout human alpha-synuclein in Lewy bodies of Parkinson’s disease. J Neurosci Res 59:528–533
Goedert M, Jakes R, Vanmechelen E (1995) Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett 189:167–169
Greenberg SG, Davies P (1990) A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA 87:5827–5831
Grossman M, Farmer J, Leight S, Work M, Moore P, Van Deerlin V, Pratico D, Clark CM, Coslett HB, Chatterjee A, Gee J, Trojanowski JQ, Lee VM-Y (2005) Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer’s disease. Ann Neurol 57:721–729
Hirano A, Llena J (1986) Neurological features of parkinsonism-dementia complex on Guam: reappraisal and comparative study with Alzheimer’s disease and Parkinson’s disease. Prog Neuropathol 6:17–31
Hirano A, Kurland LT, Krooth RS, Lessell S (1961) Parkinsonism-dementia complex, an endemic disease on the island of Guam. I. Clinical features. Brain 84:642–661
Hirano A, Malamud N, Ku Rland LT (1961) Parkinsonism-dementia complex, an endemic disease on the island of Guam. II. Pathological features. Brain 84:662–679
Hirano A, Malamud N, Elizan TS, Kurland LT (1966) Amyotrophic lateral sclerosis and parkinsonism-dementia complex on Guam. Further pathologic studies. Arch Neurol 15:35–51
Hof PR, Perl DP, Loerzel AJ, Morrison JH (1991) Neurofibrillary tangle distribution in the cerebral cortex of parkinsonism-dementia cases from Guam: differences with Alzheimer’s disease. Brain Res 564:306–313
Hof PR, Perl DP, Loerzel AJ, Steele JC, Morrison JH (1994) Amyotrophic lateral sclerosis and parkinsonism-dementia from Guam: differences in neurofibrillary tangle distribution and density in the hippocampal formation and neocortex. Brain Res 650:107–116
Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, Morris JC, Wilhelmsen KC, Schellenberg GD, Trojanowski JQ, Lee VM-Y (1998) Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 282:1914–1917
Hudson AJ, Rice GP (1990) Similarities of guamanian ALS/PD to post-encephalitic parkinsonism/ALS: possible viral cause. Can J Neurol Sci 17:427–433
Kosik KS, Orecchio LD, Binder L, Trojanowski JQ, Lee VM-Y, Lee G (1988) Epitopes that span the tau molecule are shared with paired helical filaments. Neuron 1:817–825
Kurland LT, Mulder DW (1954) Epidemiologic investigations of amyotrophic lateral sclerosis. I. Preliminary report on geographic distribution and special reference to the Mariana Islands, including clinical and pathologic observations. Neurology 4:438–448
Kurland LT, Mulder DW (1955) Epidemiologic investigations of amyotrophic lateral sclerosis. 2. Familial aggregations indicative of dominant inheritance I. Neurology 5:182–196
Lee VM-Y, Balin BJ, Otvos L Jr, Trojanowski JQ (1991) A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science 251:675–678
Lee VM-Y, Goedert M, Trojanowski JQ (2001) Neurodegenerative tauopathies. Annu Rev Neurosci 24:1121–1159
Lee VM-Y, Giasson BI, Trojanowski JQ (2004) More than just two peas in a pod: common amyloidogenic properties of tau and alpha-synuclein in neurodegenerative diseases. Trends Neurosci 27:129–134
Mawal-Dewan M, Schmidt ML, Balin B, Perl DP, Lee VM-Y, Trojanowski JQ (1996) Identification of phosphorylation sites in PHF-TAU from patients with Guam amyotrophic lateral sclerosis/parkinsonism-dementia complex. J Neuropathol Exp Neurol 55:1051–1059
McGeer PL, Schwab C, McGeer EG, Haddock RL, Steele JC (1997) Familial nature and continuing morbidity of the amyotrophic lateral sclerosis-parkinsonism dementia complex of Guam. Neurology 49:400–409
Mercken M, Vandermeeren M, Lubke U, Six J, Boons J, Van de Voorde A, Martin JJ, Gheuens J (1992) Monoclonal antibodies with selective specificity for Alzheimer Tau are directed against phosphatase-sensitive epitopes. Acta Neuropathol (Berl) 84:265–272
Morris HR, Steele JC, Crook R, Wavrant-De Vrieze F, Onstead-Cardinale L, Gwinn-Hardy K, Wood NW, Farrer M, Lees AJ, McGeer PL, Siddique T, Hardy J, Perez-Tur J (2004) Genome-wide analysis of the parkinsonism-dementia complex of Guam. Arch Neurol 61:1889–1897
Murakami N (1999) Parkinsonism-dementia complex on Guam - overview of clinical aspects. J Neurol 246(Suppl 2):II16–II18
Nakano I, Hirano A (1983) Neuron loss in the nucleus basalis of Meynert in parkinsonism-dementia complex of Guam. Ann Neurol 13:87–91
Oyanagi K, Wada M (1999) Neuropathology of parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam: an update. J Neurol 246(Suppl 2):II19–II27
Oyanagi K, Makifuchi T, Ohtoh T, Chen KM, Gajdusek DC, Chase TN, Ikuta F (1994) The neostriatum and nucleus accumbens in parkinsonism-dementia complex of Guam: a pathological comparison with Alzheimer’s disease and progressive supranuclear palsy. Acta Neuropathol (Berl) 88:122–128
Oyanagi K, Makifuchi T, Ohtoh T, Ikuta F, Chen KM, Chase TN, Gajdusek DC (1994) Topographic investigation of brain atrophy in parkinsonism-dementia complex of Guam: a comparison with Alzheimer’s disease and progressive supranuclear palsy. Neurodegeneration 3:301–304
Perl DP, Hof PR, Steele JC, Purohit DP, Peterson R, Belli D (1995) Changes in the outbreak of ALS/parkinsonism-dementia complex: neuropathologic studies of asympyomatic Chamorros. J Neuropathol Exp Neurol 54:416
Perl DP, Hof PR, Purohit DP, Loerzel AJ, Kakulas BA (2003) Hippocampal and entorhinal cortex neurofibrillary tangle formation in Guamanian Chamorros free of overt neurologic dysfunction. J Neuropathol Exp Neurol 62:381–388
Plato CC, Galasko D, Garruto RM, Plato M, Gamst A, Craig UK, Torres JM, Wiederholt W (2002) ALS and PDC of Guam: forty-year follow-up. Neurology 58:765–773
Plato CC, Garruto RM, Galasko D, Craig UK, Plato M, Gamst A, Torres JM, Wiederholt W (2003) Amyotrophic lateral sclerosis and parkinsonism-dementia complex of Guam: changing incidence rates during the past 60 years. Am J Epidemiol 157:149–157
Poorkaj P, Tsuang D, Wijsman E, Steinbart E, Garruto RM, Craig UK, Chapman NH, Anderson L, Bird TD, Plato CC, Perl DP, Weiderholt W, Galasko D, Schellenberg GD (2001) TAU as a susceptibility gene for amyotropic lateral sclerosis-parkinsonism dementia complex of Guam. Arch Neurol 58:1871–1878
Popescu A, Lippa CF, Lee VM-Y, Trojanowski JQ (2004) Lewy bodies in the amygdala: increase of alpha-synuclein aggregates in neurodegenerative diseases with tau-based inclusions. Arch Neurol 61:1915–1919
Pratico D, Sung S (2004) Lipid peroxidation and oxidative imbalance: early functional events in Alzheimer’s disease. J Alzheimers Dis 6:171–175
Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM-Y (2001) Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci 21:4183–4187
Pratico D, Clark CM, Liun F, Rokach J, Lee VM-Y, Trojanowski JQ (2002) Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol 59:972–976
Schmidt ML, Carden MJ, Lee VM-Y, Trojanowski JQ (1987) Phosphate dependent and independent neurofilament epitopes in the axonal swellings of patients with motor neuron disease and controls. Lab Invest 56:282–294
Schmidt ML, Lee VM-Y, Saido T, Perl D, Schuck T, Iwatsubo T, Trojanowski JQ (1998) Amyloid plaques in Guam amyotrophic lateral sclerosis/parkinsonism-dementia complex contain species of A beta similar to those found in the amyloid plaques of Alzheimer’s disease and pathological aging. Acta Neuropathol (Berl) 95:117–122
Sebeo J, Hof PR, Perl DP (2004) Occurrence of alpha-synuclein pathology in the cerebellum of Guamanian patients with parkinsonism-dementia complex. Acta Neuropathol (Berl) 107:497–503
Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, Robertson RC (1987) Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science 237:517–522
Steele JC, Richardson JC, Olszewski J (1964) Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 10:333–359
Trojanowski JQ, Ishihara T, Higuchi M, Yoshiyama Y, Hong M, Zhang B, Forman MS, Zhukareva V, Lee VM-Y (2002) Amyotrophic lateral sclerosis/parkinsonism dementia complex: transgenic mice provide insights into mechanisms underlying a common tauopathy in an ethnic minority on Guam. Exp Neurol 176:1–11
Wiederholt WC (1999) Neuroepidemiologic research initiatives on Guam: past and present. Neuroepidemiology 18:279–291
Yamazaki M, Arai Y, Baba M, Iwatsubo T, Mori O, Katayama Y, Oyanagi K (2000) Alpha-synuclein inclusions in amygdala in the brains of patients with the parkinsonism-dementia complex of Guam. J Neuropathol Exp Neurol 59:585–591
Yanagihara R, Garruto RM, Gajdusek DC, Tomita A, Uchikawa T, Konagaya Y, Chen KM, Sobue I, Plato CC, Gibbs CJ Jr (1984) Calcium and vitamin D metabolism in Guamanian Chamorros with amyotrophic lateral sclerosis and parkinsonism-dementia. Ann Neurol 15:42–48
Yoshiyama Y, Lee VM-Y, Trojanowski JQ (2001) Frontotemporal dementia and tauopathy. Curr Neurol Neurosci Rep 1:413–421
Zhukareva V, Mann D, Pickering-Brown S, Uryu K, Shuck T, Shah K, Grossman M, Miller BL, Hulette CM, Feinstein SC, Trojanowski JQ, Lee VM-Y (2002) Sporadic Pick’s disease: a tauopathy characterized by a spectrum of pathological tau isoforms in gray and white matter. Ann Neurol 51:730–739
Zhukareva V, Trojanowski JQ, Lee VM-Y (2004) Assessment of pathological tau proteins in frontotemporal dementias: qualitative and quantitative approaches. Am J Geriatr Psychiatry 12:136–145
Acknowledgements
The authors acknowledge support for their research from the NIH (AG14382) and thank the Chamorro families of the patients studied here for making this research possible. JQT is the William Maul Measy-Truman G. Schnabel Jr. M.D. Professor of Geriatric Medicine and Gerontology. VM-YL is the John H. Ware 3rd Chair of Alzheimer’s Disease Research at the University of Pennsylvania.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Winton, M.J., Joyce, S., Zhukareva, V. et al. Characterization of tau pathologies in gray and white matter of Guam parkinsonism-dementia complex. Acta Neuropathol 111, 401–412 (2006). https://doi.org/10.1007/s00401-006-0053-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0053-0