Abstract
This report concerns the upper motor neuron involvement in 16 autopsy cases of Pick disease with Pick bodies, including 11 cases reported by us previously. Prominent, circumscribed atrophy of the precentral gyrus, conspicuously in the lower portion, was noted in one case. Loss of Betz cells and astrocytosis of the precentral gyrus layer V were encountered in 15 cases (94%) and eight cases (50%), respectively. Appearance of Pick bodies and ballooned neurons in the precentral gyrus layer V was confirmed in seven cases (44%). Degeneration of the pyramidal tract in the medulla oblongata was noted in all 15 cases in which this structure was examined. Pyramidal signs were observed in four (67%) of the six cases that were neurologically sufficiently examined: hyperreflexia in four cases (67%), spasticity in one case (17%). Babinski sign was not encountered in any of the six cases. In all four cases having pyramidal signs, degeneration of the pyramidal tract was observed. In contrast, two cases having degeneration of the pyramidal tract did not develop pyramidal signs. In Pick’s disease with Pick bodies, obvious involvement of the precentral gyrus and pyramidal tract was not previously noticed. Furthermore, we suggest that pyramidal signs in Pick’s disease with Pick bodies have been underestimated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pick’s disease, first described by Arnold Pick [40] in 1892, is a relatively rare degenerative disorder accounting for approximately one percent of all dementia cases [19, 20, 35]. Furthermore, Pick’s disease is a type of frontotemporal dementia (FTD) [15, 22, 32, 47, 48], including corticobasal degeneration (CBD) [41, 51], argyrophilic grain disease (AGD) [53], frontotemporal lobar degeneration with motor neuron disease (FTLD-MND) or amyotrophic lateral sclerosis (ALS) with dementia (ALSD) [55], frontotemporal lobar degeneration with ubiquitin-only-immunoreactive changes (FTLD-U) or motor neuron disease inclusion dementia (MNDID) [44], and dementia lacking distinctive histology (DLDH) [23]. It is well known that Pick’s disease is characterized clinically by a peculiar process of dementia, including the appearance of personality changes, language disturbance, and elements of Klüver–Bucy syndrome in the early stage of the disease, and relative preservation of memory and visuospatial abilities in the early or middle phase of the disease, inconsistent with the clinical features of Alzheimer’s disease, and pathologically by localized atrophy of the frontal and/or temporal lobes with dense gliosis in the subjacent white matter [45]. There has been a lack of consensus regarding the neuropathological diagnostic criteria for Pick’s disease [9, 10, 24, 38, 49, 57]. At present, cases having Pick bodies [1, 3, 36, 39, 58, 60] and ballooned neurons [36, 42], in which the distribution of the circumscribed atrophy are not always consistent with frontotemporal atrophy [8, 25, 54], can be clearly neuropathologically diagnosed as having Pick’s disease [11–13, 26, 31, 43, 50].
It has been believed that the precentral gyrus in Pick’s disease is usually preserved, with the exception of the clinicopathological studies conducted by Tsuchiya et al. [54] in 2001. According to Mansvelt [28], who reviewed 171 autopsy cases of Pick’s disease reported up to 1954, including Pick’s disease with Pick bodies and hereditary Pick’s disease, a macroscopically distinct atrophy of the whole precentral gyrus was encountered in 10 (6%) of the 171 autopsy cases, in which only one case had Pick bodies. In 1957, Lüers and Spatz [27], who compiled a neuropathology of Pick’s disease with and without Pick bodies, noticed that involvement of the precentral gyrus was relatively rare and that in such circumstances the precentral gyrus was involved via the extension of a lesion in the pars opercularis of the inferior frontal gyrus. Jervis [21], who compiled the clinicopathological features of Pick’s disease up to 1971, reported that Pick bodies were found only in one-third of the published cases of Pick’s disease. In the study, Jervis noticed that the precentral gyrus in Pick’s disease was usually normal. According to Seitelberger et al. [46], who in 1983 investigated neuropathologically 29 autopsy cases of Pick’s disease with and without Pick bodies, the precentral gyrus of Pick’s disease was affected only slightly and severe involvement of the precentral gyrus pointed to ALS in combination with Pick’s disease. Furthermore, Constantinidis et al. in 1974 [9], Tissot et al. in 1975 [49] and Constantinidis in 1985 [10] noted that the precentral gyrus was well preserved in ten cases of Pick’s disease with Pick bodies.
The purpose of this report is to describe the clinicopathological features of Pick’s disease with Pick bodies in 16 Japanese autopsy cases, including pyramidal signs and involvement of the primary motor cortex and pyramidal tract, focusing on the presence or absence of astrocytosis, Pick bodies and ballooned neurons, in the fifth layer of the precentral gyrus, in addition to loss of Betz cells; namely, small groupings of fat granule cells in the spaces in which Betz cells were present. Furthermore, we investigated the clinicopathological relation between pyramidal signs and involvement of the pyramidal tract in our six autopsy cases, in which pyramidal signs were investigated. In addition, we addressed in the discussion the pathological heterogeneity in the precentral gyrus among multiple system atrophy (MSA) [52], ALSD [55], CBD [56], and Pick’s disease with Pick bodies, paying attention to the prominent clinicopathological dissociation of the pyramidal signs and lesions of the Betz cells and pyramidal tract in Pick’s disease with Pick bodies, compared with those of MSA, ALSD, and CBD.
Materials and methods
The present investigation was carried out on 16 autopsy cases of Pick’s disease with Pick bodies and ten schizophrenic brains as negative controls. Furthermore, seven MSA, eight ALSD, and ten CBD autopsy cases, previously reported by us [52, 55, 56], were examined as disease controls.
After fixation in formalin, the brains of the 16 cases were sectioned in the coronal plane. The cerebral bisphere and/or hemisphere and/or small blocks, including the frontal, temporal, parietal, and occipital lobes, and the striatum, pallidum, subthalamic nucleus, thalamus, amygdala, and hippocampus, were taken. Additional tissue blocks were taken from the midbrain, including the substantia nigra, brain stem, and cerebellum. The spinal cords were also examined in cases 3, 5, 6, 8, 11, 12, 13, and 15. The brains were embedded in paraffin and cut at a thickness of about 10 μm except for cases 8, 11, 13, and 16, which were being cut at a thickness of about 4 μm. The sections were stained with hematoxylin–eosin (H.E.), and also using the Klüver–Barrera, Holzer, Bodian, methenamine silver, and modified Gallyas-Braak staining methods. Immunocytochemistry was performed using antibodies against monoclonal anti-phosphorylated tau (AT8), human-tau pool 2 (from Dr. H. Mori; Osaka City University), monoclonal ubiquitin (from Dr. H. Mori), polyclonal neuro-filament (200 kDa), and glial fibrillary acidic protein (GFAP).
In all patients, Pick bodies (Fig. 1a) were numerously encountered in the dentate granule cells and pyramidal neurons of the hippocampus, affected regions of the cerebral cortex, prominently in cortical layers II and III, the amygdala, striatum, nucleus basalis of Meynert, and locus ceruleus. A widespread distribution of ballooned neurons (Fig. 1b) was noticed in the involved neocortex, prominently in the fifth layer, parahippocampal gyrus, and amygdala in all 16 patients examined in this study.
The clinical and pathological features of all 16 cases are summarized in Tables 1 and 2.
The clinical information of all cases was from retrospective review of all available hospital records. Pyramidal signs were judged to be present in patients who showed one or more signs of hyperreflexia in the upper and lower extremities, Babinski sign, and spasticity in the extremities.
The degeneration of the primary motor cortex (Fig. 2) was assessed in the lateral intermediate aspect in all cases. Loss of Betz cells (Fig. 3) was judged to be present in cases that showed small groupings of lipofuscin-laden macrophages in a hole, from which Betz cells had presumably disappeared, in the precentral gyrus with the presence of normal and degenerated Betz cells in the absence of an internal granular layer. Astrocytosis of the precentral gyrus layer V (Fig. 4) was considered present in cases showing definite astrocytosis determined using H.E. and Holzer staining or immunohistochemistry using an antibody against GFAP. Presence or absence of Pick bodies and ballooned neurons in the precentral gyrus layer V was also investigated (Fig. 5). Pyramidal tract degeneration (Fig. 6) was also judged as present in cases showing definite loss of myelinated fibers shown by Klüver–Barrera staining, accompanied by gliosis revealed using Holzer staining and immunohistochemistry using an antibody against GFAP. The pertinent data are summarized in Table 3.
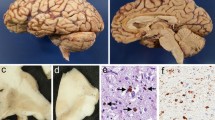
a, b. Case 9. a Dorsal view of the brain after stripping of the leptomeninges shows prominent atrophy of the left frontal lobe, including the precentral gyrus. Arrow indicates the central sulcus. b Lateral view of the left brain discloses prominent atrophy of the precentral gyrus, prominently in the lower portion, and obvious atrophy of the temporal lobe, including the anterior portion of the first temporal gyrus. Arrow indicates the central sulcus
Astrocytosis of the precentral gyrus layer V. a, b Case 1, c–e Case 6. a, c, d Hypertrophic glia (arrow) in the precentral gyrus layer V. b, e Prominent fibrillary gliosis in the precentral gyrus layer V. a, c H.E. stain, Bar 0.02 mm, b Holzer stain, Bar 0.04 mm, d GFAP stain, Bar 0.02 mm, e Holzer stain, Bar 0.02 mm
Presence of Pick bodies and ballooned neurons in the precentral gyrus layer V. a–d Case 6, e, f Case 16. a Pick body in the Betz cell (arrow). b Pick body in the Betz cell (large arrow) and Pick body in the small neuron (small arrow). c Ballooned neuron (large arrow) in the precentral gyrus layer V, containing Betz cell (small arrow). d Ballooned neuron (arrow) in the precentral gyrus layer V. e Ballooned neuron (large arrow) and Pick body (small arrow) in the precentral gyrus layer V. f Ballooned neuron (large arrow) and Pick body (small arrow) in the precentral gyrus layer V. a Bodian stain, Bar 0.02 mm, b, e Tau (AT-8) stain, Bar 0.02 mm, c K.B. stain, Bar 0.02 mm, d H.E. stain, Bar 0.02 mm, f Tau (AT-8) stain, Bar 0.02 mm
Case report
Clinical course and neuropathological findings in case 5
This Japanese patient presented with abnormal behavior when he was 59 years old. Thereafter, he developed excitement, wandering about, and language disturbance. Because of the above-mentioned abnormal behavior and language disturbance, he was often taken into the protective custody of police. At age 60, about 13 months after the disease onset, he was admitted to a psychiatric hospital. After admission, dementia gradually developed and verbal stereotypy was also observed. About 3 months before his death, gastric cancer was discovered following vomiting due to pyloric stenosis. Total gastrectomy was carried out, but he died at the age of 64. The total duration of his disease was 5 years and 3 months, and the clinical diagnosis was Pick’s disease. There was no family history of dementia.
The weight of the brain was 1,080 g. A macroscopic examination revealed atrophy of the bilateral frontal and temporal lobes. Histological examination showed neuronal loss with astrocytosis, status spongiosus, Pick bodies, and ballooned neurons in the affected cerebral cortex. Neuronal loss was also observed in the hippocampus, subiculum, and parahippocampal gyrus. The amygdala was not examined. Moderate neuronal loss was evident in the caudate nucleus and putamen. Slight neuronal loss was observed in the pallidum. Neuronal loss was not seen in the thalamus, substantia nigra, locus ceruleus, pontine nucleus, Purkinje cell, dorsal motor nucleus of the vagus, inferior olive, hypoglossal nucleus, or anterior horn of the spinal cord.
Clinical course and neuropathological findings in case 9
The patient was a Japanese man aged 76 years at the time of death. He developed abnormal behavior, including collection of garbage, wandering about at night, and throwing lighted cigarettes into a garbage dump at age 71. At age 74, about 3 years after the onset of the disease, he was admitted to a psychiatric hospital. At admission, verbal stereotypy was observed. At age 75, he developed cholecystitis due to gallstones. Thereafter, he was bedridden, and died at age 76. The total duration of the disease was 4 years 8 months, and the clinical diagnosis was senile dementia.
The weight of the brain was 970 g. A macroscopic examination disclosed prominent, circumscribed atrophy in the left precentral gyrus, conspicuously in the lower portion, and in the temporal lobes, including the anterior one-third of the first temporal gyrus (Fig. 2a, b). Furthermore, atrophy of the amygdala and caudate nucleus was obvious. Histological examination revealed neuronal loss with astrocytosis, status spongiosus, Pick bodies, and ballooned neurons in the cerebral cortex, prominently in the frontal lobes, including the precentral gyrus, and temporal lobes. Neuronal loss was also found in the hippocampus and subiculum. Severe neuronal loss was evident in the amygdala, prominently in the basolateral (BL) group, with slight neuronal loss in the caudate nucleus, putamen, and pallidum. Neuronal loss was not evident in the thalamus, substantia nigra, locus ceruleus, pontine nucleus, dentate nucleus, Purkinje cell, hypoglossal nucleus, dorsal motor nucleus of the vagus, or inferior olive.
Clinical course and neuropathological findings in case 11
This Japanese patient presented with memory disturbance when he was 76 years old. At age 77, he became quick-tempered. At this stage, he was clinically diagnosed with Pick’s disease. At age 81, dementia became obvious. He died at age 83. The total duration of his disease was about 7 years.
The weight of the brain was 950 g, with the weight of the cerebrum being 800 g, and that of the brainstem and cerebellum 150 g (18.8%), respectively. Macroscopic examination revealed atrophy of the frontotemporal lobes, prominently on the right. Histological examination disclosed neuronal loss with astrocytosis, status spongiosus, Pick bodies, and ballooned neurons in the affected cerebral cortex. Neuronal loss was also observed in the hippocampus, subiculum, and parahippocampal gyrus. The amygdala was not examined. Moderate neuronal loss was evident in the caudate nucleus and putamen. Slight neuronal loss was observed in the pallidum. Neuronal loss was noted in the substantia nigra, but was not observed in the thalamus, locus ceruleus, pontine nucleus, dentate nucleus, Purkinje cell, facial nucleus, hypoglossal nucleus, dorsal motor nucleus of the vagus, inferior olive, or anterior horn of the spinal cord.
Clinical course and neuropathological findings in case 15
The patient was a Japanese man aged 72 years at the time of death. He developed personality changes, including a lack of concern about road accidents caused by the patient himself and neglecting his occupation, at age 58. At age 61, about three years after the onset of the disease, he was admitted to a hospital. At admission, severe dementia, perseveration, heterophagia, bulimia, action stereotypy, echolalia, and “stehende Redensarten”, were noted. At age 65, he became bedridden, and at this stage an oral tendency was noticed. At age 69, gastrostomy was conducted. At age 72, he died of repeated aspiration pneumonia. The total duration of the disease was 14 years, and the clinical diagnosis was Pick’s disease. There was no family history of dementia, Parkinsonism, or ALS.
The weight of the brain was 770 g. Macroscopic examination revealed prominent atrophy of the frontal and temporal lobes. Histological examination disclosed neuronal loss with astrocytosis, status spongiosus, Pick bodies, and ballooned neurons in the affected cortex. Neuronal loss was also observed in the hippocampus, subiculum, and parahippocampal gyrus. Severe neuronal loss was evident in the amygdala, prominently in the BL group, with moderate neuronal loss in the caudate nucleus, putamen, and thalamus. Slight neuronal loss was noted in the pallidum and locus ceruleus. Neuronal loss was also noted in the substantia nigra. Neuronal loss was not evident in the pontine nucleus, dentate nucleus, Purkinje cell, hypoglossal nucleus, dorsal motor nucleus, or inferior olive.
Results
Clinical features
The main clinical information on the 16 patients (11 males, 5 females) is summarized in Table 1. The patients had no hereditary burden. The age at onset of symptoms was from the fifth to eighth decade of their lives (average 61 years 1 months), except for case 2 and case 14, in which the clinical features were not known. The duration of the disease in 14 cases ranged from 18 months in case 4 to 19 years in case 3, with the mean duration of the 14 cases being 8 years and 1 month. Seven patients presented with abnormal behavior and personality changes, compatible with the clinical features of Pick’s disease as defined today, as the initial sign (cases 5, 8, 9, 10, 13, 15, and 16). Memory disturbance, inconsistent with the clinical signs in Pick’s disease and FTD, was noted in three patients as the initial sign (cases 7, 11, and 12). Two patients initially developed speech apraxia (cases 1 and 6). Delusion of persecution was noted as the initial sign in case 3. As the initial sign, a depressive state without dementia was noticed in case 4. Dementia was observed in 13 of 14 patients in whom clinical information was available. A clinical diagnosis of Pick’s disease and Pick-Alzheimer’s disease was recorded in the seven cases.
Pathological features
The neuropathological data are summarized in Table 2. Brain weights at autopsy ranged from 1,350 to 710 g (average 1,011 g). Prominent, circumscribed atrophy in the left precentral gyrus, conspicuously in the lower portion, was observed in case 9 (Fig. 2a, b). In the cerebral cortex of all 16 cases, neuronal loss with Pick bodies and ballooned neurons was mainly encountered in the frontal and temporal lobes. In all 14 cases in which the amygdala was examined, the basolateral group of the amygdala revealed severe or moderate neuronal loss. In all 16 cases, the caudate nucleus and putamen showed moderate or slight lesions. In the pallidum, slight lesions were evident in all 16 cases examined in this study. Neuronal loss in the thalamus was noted in 12 (cases 1, 2, 3, 4, 6, 7, 8., 10, 12, 13, 15, and 16) of 16 cases. Neuronal loss in the substantia nigra was noted in 5 (cases 8, 11, 13, 15, and 16) of 15 cases, in which the substantia nigra was investigated.
Clinicopathological relation between pyramidal signs and involvement of the precentral gyrus and pyramidal tract
Pyramidal signs and involvement of the precentral gyrus and pyramidal tract are summarized in Table 3. Pyramidal signs were noted in four cases (cases 1, 7, 8, and 12). Loss of Betz cells was observed in 15 of 16 cases, with the exception of case 7. Loss of Betz cells was noted in all disease control cases including seven MSA cases, eight ALSD cases, and ten CBD cases. In addition, loss of Betz cells was not confirmed in all ten shizophrenic brains as negative controls. Furthermore, astrocytosis of the precentral gyrus layer V, detected by HE, Holzer, and GFAP staining, was obvious in eight (cases 1, 2, 5, 6, 8, 9, 13, and 16). Pick bodies and ballooned neurons in the precentral gyrus layer V were encountered in seven cases (cases 1, 2, 5, 6, 9, 15, and 16). Degeneration of the pyramidal tract was found in all 15 cases in which this structure was examined, and the distal portion (medulla oblongata) was more affected than the proximal portion (midbrain).
Discussion
It has been believed that in Pick’s disease, pyramidal signs are rare. For example, Constantinidis et al. [9] Tissot et al. [49] and Constantinidis [10], who divided 32 autopsy cases of Pick’s disease into three groups, namely 10 cases in group A having Pick bodies and ballooned neurons, 10 cases in group B having only ballooned neurons without Pick bodies, and 12 cases in group C without Pick bodies or ballooned neurons, reported that in group A, pyramidal signs were not observed, but that in group B, compatible with CBD at present [56], extrapyramidal and pyramidal signs were very often noted. In 1998, Markesbery [29], who reviewed the clinicopathological features of Pick’s disease, on the basis of 18 Pick’s disease cases from an unpublished autopsy series of 557 elderly demented subjects, noticed that in Pick’s disease, upper motor neuron signs such as spasticity and extensor plantar responses, were not frequent findings, indicating the preservation of the precentral gyrus.
In contrast, there were a few autopsy cases of Pick’s disease with Pick bodies, in which upper motor neuron signs were clearly elucidated in the clinical stages. In 1979, McGeachie et al. [30], described an autopsy case of Pick’s disease with Pick bodies. The female patient presented with depression at 60 years of age, followed by abnormal behavior and memory disturbance. Thereafter, a marked reduction in language output with indifference to her environment developed. Neurological examination at age 68, about 4 months before her death, revealed marked spasticity, brisk deep tendon reflex, and bilateral Babinski signs. The patient died of lung cancer about 9 years after the disease onset and her brain weighed 925 g. Severe atrophy affected the entire frontal lobe with the exception of the precentral gyrus. The middle and inferior gyri of the temporal lobe were markedly involved. Histological examination disclosed not only severe neuronal loss in the atrophic cortex, but also Pick bodies in the hippocampus and subiculum. Neither loss of Betz cells nor pyramidal tract involvement in the case were mentioned. Furthermore, in 1981, Cambier et al. [8], who first disclosed the presence of an autopsy case of parietal form of Pick’s disease with Pick bodies, noted the presence of pyramidal signs in the middle stage of the disease about 5 years after the disease onset. They reported that in this case, the tendon reflex of the right upper extremity was exaggerated, in addition to unilateral, parietal, focal neurological signs. The patient died nine years after the disease onset, and the brain weight was 1,240 g. Macroscopical examination showed mainly left circumscribed cortical atrophy in the parietal lesions. Histological examination revealed not only Pick bodies, but also ballooned neurons. In addition, in 1994, Lang et al. [25], reported a second patient with autopsy-proven parietal predominant Pick’s disease with Pick bodies. In their case, the deep tendon reflexes were generally brisk, with the plantar responses being flexor, about one year after the disease onset. Reviewing the literature concerning pyramidal signs, including hyperreflexia, Babinski sign, and spasticity in Pick’s disease with Pick bodies cases, it becomes clear that pyramidal signs have been rarely documented. In this regard, our data showing that four cases of Pick’s disease with Pick bodies (cases 1, 7, 8, and 12) presented with pyramidal signs in the clinical course, deserves attention.
It has been believed that in Pick’s disease, including Pick’s disease with Pick bodies, the precentral gyrus are relatively preserved. For example, in 1998 Binetti et al. [6], who reviewed a pathological series of 35 patients with Pick’s disease, including 23 Pick’s disease with Pick bodies autopsy cases, in Massachusetts General Hospital, described that in Pick’s disease the motor strip, caudal dorsal temporal lobe, and occipital lobe were relatively well preserved, in contrast with the definite and circumscribed atrophy in the frontotemporal region. In 1998, Dickson [11] elucidated that the cardinal pathological features of Pick’s disease are circumscribed cortical atrophy, most often affecting the frontal and temporal poles and argyrophilic, round intraneuronal inclusions (Pick bodies), and reported that areas spared included the posterior part of the superior temporal gyrus and the pre- and postcentral gyri. Furthermore, in 2001, Vonsattel et al. [59], who reexamined the postmortem series of 35 autopsy cases in Massachusetts General Hospital, noticed that the average weight of Pick’s disease brains were as follows: Women, 955±174 g; Men 1,085±172 g. Furthermore, Vonsattel et al. noted that in gross examination of Pick’s disease brains there was relative preservation of the caudal third of the superior temporal gyrus, precentral gyrus, and occipital lobe. Moreover, in 2002, Mirra and Hyman [33], who reviewed the neuropathology of ageing and dementia, noted that most neuropathologists would see approximately one case of Pick’s disease for every 50–100 cases of Alzheimer’s disease that they encountered, but that they would readily make the diagnosis in typical cases of frontotemporal atrophy with relative sparing of the posterior portion of the superior temporal gyrus and relative preservation of the precentral and postcentral gyri with microscopic evidence of Pick bodies and ballooned cells. In 2003, Bergeron et al. [5], noted that in Pick’s disease with Pick bodies the precentral gyrus and the posterior two-thirds of the superior temporal gyrus were consistently relatively preserved. From the literature concerning the involvement of the precentral gyrus and pyramidal tract in Pick’s disease with Pick bodies autopsy cases, it is obvious that there are few reports showing loss of Betz cells in the precentral gyrus and involvement of the pyramidal tract of Pick’s disease with Pick bodies autopsy cases. Thus, our data, showing that loss of Betz cells was observed in 15 (94%) of 16 cases, with astrocytosis of the precentral gyrus layer V being encountered in the eight cases (50%), and Pick bodies and ballooned neurons in the precentral gyrus layer V being observed in the seven cases (44%), respectively, are important. Furthermore, degeneration of the pyramidal tract in the medulla oblongata was observed in all 15 cases in which the pyramidal tract was investigated.
In the present study, all 15 cases examined, including a case (case 4) having very short clinical duration (1 year 6 months), had pathological changes in the pyramidal tract. Furthermore, among the six cases (cases 1, 4, 6, 7, 8 and 12) examined neurologically, four cases (cases 1, 7, 8 and 12) developed pyramidal signs in the middle to later stage (4 to 8 years after the disease onset). But two cases (cases 4 and 6), of which neurological examination was conducted in the early stage (1 year 6 months, 3 years, respectively), did not develop pyramidal signs. Furthermore, case 6 did not present with pyramidal signs until his death. These findings suggests that the pyramidal tract in Pick’s disease with Pick bodies may be involved in the early stage, but that pyramidal signs are not always detected in the early stage of the disease course.
In 2000, Tsuchiya et al. [52] investigated pyramidal signs, including spasticity, hyperreflexia, and Babinski sign, and the involvement of the precentral gyrus, in seven Japanese autopsy cases of MSA. In the study, pyramidal signs were observed in six (86%) of the seven MSA autopsy cases. Hyperreflexia and Babinski sign were evident in five (71%) patients, and five (71%), respectively, but spasticity was observed in only one patient. Loss of Betz cells in the precentral gyrus was noticed in all seven MSA autopsy cases. Astrocytosis in the layer V was noted in five (71%) of the seven MSA cases. Involvement of the pyramidal tract in the spinal cord and medulla oblongata was observed in all seven MSA autopsy cases, but there was no involvement of the pyramidal tract in the midbrain in any of the six autopsy cases in which this structure was examined.
Subsequently, in 2002, Tsuchiya et al. [55] explored the pyramidal signs, including hyperreflexia, Babinski sign, and spasticity, as well as the involvement of the precentral gyrus and pyramidal tract, in eight autopsy cases of ALSD. Pyramidal signs were observed in seven (88%) of the eight autopsy cases. Hyperreflexia and Babinski sign were evident in seven (88%) and three (38%) patients, respectively. Loss of Betz cells in the precentral gyrus was evident in all seven autopsy cases in which this structure was examined. In contrast, astrocytosis of the precentral gyrus layer V was noticed in only three cases. Involvement of the pyramidal tract in the medulla oblongata was observed in all eight ALSD autopsy cases, but no involvement of the pyramidal tract in the midbrain was encountered in any of the eight autopsy cases.
Furthermore, in 2005, Tsuchiya et al. [56] elucidated the clinicopathological dissociation of pyramidal signs and severe involvement of the precentral gyrus in ten autopsy cases of CBD. Pyramidal signs were observed in six (60%) of the ten cases, with hyperreflexia and spasticity being observed in six (60%), and three (30%) patients, respectively. Loss of Betz cells associated with prominent astrocytosis in the precentral gyrus layer V was observed in all ten cases. On the basis of their data showing that pyramidal signs were observed in six (60%) of the ten CBD autopsy cases, and that prominent astrocytosis of the precentral gyrus layer V and loss of Betz cells were obvious in all ten CBD autopsy cases, Tsuchiya et al. pointed out that the importance of pyramidal signs in CBD has been underestimated.
Some limitations in this study must be discussed. The possibility that the lesions presented in Fig. 3, namely loss of Betz cells in the primary motor cortex in Pick’s disease with Pick bodies, are results from loss of other neurons except for Betz cells, is not completely excluded. However, the lesions were found only in the primary motor cortex, supporting that they reflect loss of Betz cells. In addition, two previous studies reported that the pyramidal tract was well preserved in Pick’s disease with Pick bodies [17, 37]. There are several possible reasons for the difference between the results presented here and those in the previous study by Ikeda et al. [17], in which seven cases of Pick’s disease with Pick bodies were examined. First, since the sample size in the quantitative study was small, the changes could not be detected. Second, since mean disease duration in the cases presented here is longer than that in the previous study by Ikeda et al. (9.2 years vs. 5.8 years). Third, in our present study, the pathological change of the pyramidal tract was determined only by subjective judgment using K.B. Holzer, and GFAP methods, but not a quantitative assessment. Further detailed studies with more sample size are needed to warrant our findings. Although a study by Odawara et al. [37] also noted no pyramidal tract degeneration in three Pick’s disease with Pick bodies autopsy cases, they did not describe the detailed methods of determination concerning the pyramidal tract involvement.
On the basis of our data showing that pyramidal signs were observed in only four cases (67%) of the six Pick’s disease with Pick bodies autopsy cases, in which the pyramidal signs were investigated, and that loss of Betz cells, astrocytosis of the precentral gyrus layer V, and presence of Pick bodies and ballooned neurons in the precentral gyrus layer V, were encountered in 15 (94%), 8 (50%), and 7 (44%), of the 16 autopsy cases, respectively, we believe that the importance of pyramidal signs in Pick’s disease with Pick bodies has been underestimated. Furthermore, degeneration of the pyramidal tract in the medulla oblongata was observed in all 15 cases in which this structure was examined and this also deserves attention.
References
Alzheimer A (1911) Über eigenartige Krankheitfälle des späteren Alters. Z Ges Neurol Psychiatr 4:356–385
Arima K (1989) Involvement of subcortical nuclei and brainstem in Pick’s disease: a topographical study of Pick bodies. Neuropathology 9:105–115
Arima K, Oyanagi S, Kosaka K, Matsushita M (1987) Distribution of Pick bodies in the central nervous system of the Pick’s disease with a special reference to their association with neuronal loss (in Japanese with English abstract). Psychiatr Neurol Jpn 89:43–72
Arima K, Akashi T (1990) Involvement of the locus coeruleus in Pick’s disease with or without Pick body formation. Acta Neuropathol 79:629–633
Bergeron C, Morris HR, Rossor M (2003) Pick’s disease. In: Dickson DW (eds) Neurodegeneration: the molecular pathology of dementia and movement disorders. ISN Neuropath Press, Basel pp 124–131
Binetti G, Growdon JH, Vonsattel J-PG (1998) Pick’s disease. In: Growdon JH, Rossor MN (eds) The Dementias, vol 19, Blue books of practical neurology. Butterworth–Heinemann, Boston pp 7–44
Broe M, Hodges JR, Schofield E, Shepherd CE, Kril JJ, Halliday GM (2003) Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology 60:1005–1011
Cambier J, Masson M, Dairou R, Henin D (1981) Étude anatomo-clinique d’une forme pariétale de maladie de Pick. Rev Neurol (Paris) 137:33–38
Constantinidis J, Richard J, Tissot R (1974) Pick’s disease. Histological and clinical correlations. Eur Neurol 11:208–217
Constantinidis J (1985) Pick dementia: anatomoclinical correlations and pathophysiological considerations. In: Rose FC (eds) Interdisciplinary Topics Geront., vol 19, Modern approaches to the Dementias Part I: Etiology and pathophysiology. Karger, Basal pp 72–97
Dickson DW (1998) Pick’s disease: a modern approach. Brain Pathol 8:339–354
Esiri MM, Hyman BT, Beyreuther K, Masters CL (1997) Pick’s disease. In: Graham DI, Lantos PL (eds) Greenfield’s neuropathology, sixth edition. vol II. Arnold, London, pp 192–198
Hauw JJ, Duyckaerts C, Seilhean D, Camilleri S, Sazdovitch V, Rancurel G (1996) The neuropathologic diagnostic criteria of frontal lobe dementia revisited. A study of ten consecutive cases. J Neurol Transm [suppl] 47:47–59
Hayashi S, Wakabayashi K, Iwanaga K, Kakita A, Seki K, Tanaka M, Okuizumi K, Onodera O, Tanaka H, Tsuji S, Takahashi H (1998) Pick’s disease: selective occurrence of apolipoprotein E-immunoreactive Pick bodies in the limbic system. Acta Neuropathol 95:1–4
Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, Kril JJ, Halliday GM (2004) Clinicopathological correlates in frontotemporal dementia. Ann Neurol 56:399–406
Ichimiya Y, Ikeda K, Hanawa S, Kosaka K (1984) An autopsy case of Binswanger’s disease with many Pick bodies (in Japanese). Geriatr Psychiatry 11:706–712
Ikeda K, Akiyama H, Arai T, Ueno H, Tsuchiya K, Kosaka K (2002) Morphometrical reappraisal of motor neuron system of Pick’s disease and amyotrophic lateral sclerosis with dementia. Acta Neuropathol 104:21–28
Ikeda M, Ikeda K, Endoh M, Haga C, Mizutani Y (1994) An autopsied case of slowly progressive Pick’s disease (in Japanese with English abstract). Clin Psychiatry 36:1167–1171
Jellinger K, Danielczyk W, Fischer P, Gabriel E (1990) Clinicopathological analysis of dementia disorders in the elderly. J Neurol Sci 95:239–258
Jellinger K (1996) Structural basis of dementia in neurodegenerative disorders. J Neural Transm [Suppl] 47:1–29
Jervis GA (1971) Pick’s disease. In: Minckler J (eds) Pathology of the nervous system, vol. 2. McGraw-Hill, New York, pp 1395–1404
Kersaitis C, Halliday GM, Kril JJ (2004) Regional and cellular pathology in frontotemporal dementia: relationship to stage of disease in cases with and without Pick bodies. Acta Neuropathol 108:515–523
Knopman DS, Mastri AR, Frey WHII, Sung JH, Rustan T (1990) Dementia lacking distinctive histologic features: a common non-Alzheimer degenerative dementia. Neurology 40:251–256
Kosaka K, Ikeda K, Kobayashi K, Mehraein P (1991) Striatopallidonigral degeneration in Pick’s disease: a clinicopathological study of 41 cases. J Neurol 238:151–160
Lang AE, Bergeron C, Pollanen MS, Ashby P (1994) Parietal Pick’s disease mimicking cortical-basal ganglionic degeneration. Neurology 44:1436–1440
Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS, McKee A, Dickson D, Bancher C, Tabaton M, Jellinger K, Anderson DW (1996) Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 55:97–105
Lüers Th, Spatz H (1957) Picksche Krankheit (Progressive umschriebene Großhirnatrophie). In: Lubarsch O, Henke F, Rössle R (eds) Handbuch der speziellen pathologischen Anatomie und Histologie, vol XIII/1A. Springer, Berlin Heidelberg New York pp 614–715
Mansvelt J van (1954) Pick’s disease. A syndrome of lobar cerebral atrophy, its clinico-anatomical and histopathological types. Dissertation, Utrecht, the Netherlands
Markesbery WR (1998) Pick’s disease. In: Markesbery WR (eds) Neuropathology of dementing disorders. Arnold, London, pp 142–157
McGeachie RE, Fleming JO, Sharer LR, Hyman RA (1979) Diagnosis of Pick’s disease by computed tomography. J Comput Assist Tomogr 3:113–115
Mendez MF, Selwood A, Mastri AR, Frey WHII (1993) Pick’s disease versus Alzheimer’sdisease: a comparison of clinical characteristics. Neurology 43:289–292
Mendez MF, Cummings JL (2003) Frontotemporal dementia and the asymmetric cortical atrophies. In: Mendez MF, Cummings JL (eds) Dementia. A clinical approach. 3rd edn. Butterworth-Heinemann, Philadelphia, pp 179–233
Mirra SS, Hyman BT (2002) Pick’s disease. In: Graham DI, Lantos PL (eds) Greenfield’s neuropathology, seventh edn, vol II. Arnold, London pp 227–230
Mori F, Hayashi S, Yamagishi S, Yoshimoto M, Yagihashi S, Takahashi H, Wakabayashi K (2002) Pick’s disease: α-and β-synuclein-immunoreactive Pick bodies in the dentate gyrus. Acta Neuropathol 104:455–461
Morris JH (1997) Pick’s disease. In: Esiri MM, Morris JH (eds) The neuropathology of dementia. Cambridge University Press, Cambridge, pp 204–218
Murayama S, Mori H, Ihara Y, Tomonaga M (1990) Immunocytochemical and ultrastructural studies of Pick’s disease. Ann Neurol 27:394–405
Odawara T, Iseki E, Kanai A, Arai T, Katsuragi T, Hino H, Furukawa Y, Kato M, Yamamoto T, Kosaka K (2003) Clinicopathological study of two subtypes of Pick’s disease in Japan. Dement Geriatr Cogn Disord 15:19–25
Onari K, Spatz H (1926) Anatomische Beiträge zur Lehre von der Pickschen umschriebenen Großhirnrinden-Atrophie (“Picksche Krankheit”). Z Ges Neurol Psychiatr 101:470–511
Oyanagi S, Tanaka M, Omori T, Matsushita M, Ishii T (1979) Regular arrangements of tubular structures in Pick bodies formed in an autopsied case of Pick’s disease (in Japanese with English abstract). Adv Neurol Sci 23:441–451
Pick A (1892) Ueber die Beziehungen der senilen Hirnatrophie zur Aphasie. Prag Med Wochenschr 17:165–167
Rebeiz JJ, Kolodny EH, Richardson EP Jr (1968) Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol 18:20–33
Richter H (1918) Eine besondere Art von Stirnhirnschwund mit Verblödung. Z Ges Neurol Psychiatr 38:127–160
Rossor MN (1999) Differential diagnosis of frontotemporal dementia: Pick’s disease. Dement Geriatr Cogn Disord 10(Suppl 1):43–45
Rossor MN, Revesz T, Lantos PL, Warrington EK (2000) Semantic dementia with ubiquitin-positive tau-negative inclusion bodies. Brain 123:267–276
Schneider C (1927) Über Picksche Krankheit. Mschr Psychiatr Neurol 65:230–275
Seitelberger F, Gross H, Pilz P (1983) Pick’s disease: a neuropathologic study. In: Hirano A, Miyoshi K (eds) Neuropsychiatric disorders in the elderly. Igaku-Shoin, Tokyo pp 87–117
Snowden JS, Neary D, Mann DMA (1996) Fronto-temporal lobar degeneration: front-temporal dementia, progressive aphasia, semantic dementia. Clinical Neurology and Neurosurgery Monographs. Churchill Livingstone, New York
The Lund, Manchester Groups (1994) Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry 57:416–418
Tissot R, Constantinidis J, Richard J (1975) La maladie de Pick. Masson, Paris
Tolnay M, Probst A (2002) Frontotempolar lobar degeneration–tau as a pied piper? Neurogenetics 4:63–75
Tsuchiya K, Ikeda K, Uchihara T, Oda T, Shimada H (1997) Distribution of cerebral cortical lesions in corticobasal degeneration: a clinicopathological study of five autopsy cases in Japan. Acta Neuropathol 94:416–428
Tsuchiya K, Ozawa E, Haga C, Watabiki S, Ikeda M, Sano M, Ooe K, Taki K, Ikeda K (2000) Constant involvement of the Betz cells and pyramidal tract in multiple system atrophy: a clinicopathological study of seven autopsy cases. Acta Neuropathol 99:628–636
Tsuchiya K, Mitani K, Arai T, Yamada S, Komiya T, Esaki Y, Haga C, Yamanouchi H, Ikeda K (2001) Argyrophilic grain disease mimicking temporal Pick’s disease: a clinical, radiological, and pathological study of an autopsy case with a clinical course of 15 years. Acta Neuropathol 102:195–199
Tsuchiya K, Ikeda M, Hasegawa K, Fukui T, Kuroiwa T, Haga C, Oyanagi S, Nakano I, Matsushita M, Yagishita S, Ikeda K (2001) Distribution of cerebral cortical lesions in Pick’s disease with Pick bodies: a clinicopathological study of six autopsy cases showing unusual clinical presentations. Acta Neuropathol 102:553–571
Tsuchiya K, Ikeda K, Mimura M, Takahashi M, Miyazaki H, Anno M, Shiotsu H, Akabane H, Niizato K, Uchihara T, Tominaga I, Nakano I (2002) Constant involvement of the Betz cells and pyramidal tract in amyotrophic lateral sclerosis with dementia: a clinicopathological study of eight autopsy cases. Acta Neuropathol 104:249–259
Tsuchiya K, Murayama S, Mitani K, Oda T, Arima K, Mimura M, Nagura H, Haga C, Akiyama H, Yamanouchi H, Mizusawa H (2005) Constant and severe involvement of Betz cells in corticobasal degeneration is not consistent with pyramidal signs: a clinicopathological study of ten autopsy cases. Acta Neuropathol 109:353–366
Uchihara T, Ikeda K, Tsuchiya K (2003) Pick body disease and Pick syndrome. Neuropathology 23:318–326
Uchihara T, Tsuchiya K, Nakamura A, Akiyama H (2005) Silver staining profiles distinguish Pick bodies from neurofibrillary tangles of Alzheimer type: comparison between Gallyas and Compbell–Switzer methods. Acta Neuropathol 109:483–489
Vonsattel JPG, Binetti G, Hedley-Whyte ET, Growdon JH (2001) Pick disease. In: Duckett S and De La Torre JC (eds) Pathology of the aging human nervous system, 2nd edn. Oxford, Oxford, pp 190–206
Yokota O, Ishizu H, Terada S, Tsuchiya K, Haraguchi T, Nose S, Kawai K, Ikeda K, Kuroda S (2002) Preservation of nigral neurons in Pick’s disease with Pick bodies: a clinicopathological and morphometric study of five autopsy cases. J Neurol Sci 194:41–48
Acknowledgements
We wish to express our gratitude to Dr. O. Yokota (Tokyo Institute of Psychiatry) for his valuable advice. We also wish to thank Mr. Y. Shoda and Ms E. Matsui for assisting us in photography.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsuchiya, K., Piao, YS., Oda, T. et al. Pathological Heterogeneity of the Precentral Gyrus in Pick’s Disease: A Study of 16 Autopsy Cases. Acta Neuropathol 112, 29–42 (2006). https://doi.org/10.1007/s00401-005-0028-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-005-0028-6