Abstract
Inappropriate apoptosis has been implicated in the mechanism of neuronal death in Huntington’s disease (HD). In this study, we report the expression of apoptotic markers in HD caudate nucleus (grades 1–4) and compare this with controls without neurological disease. Terminal transferase-mediated biotinylated-UTP nick end-labeling (TUNEL)-positive cells were detected in both control and HD brains. However, typical apoptotic cells were present only in HD, especially in grade 3 and 4 specimens. Expression of the pro-apoptotic protein Bax was increased in HD brains compared to controls, demonstrating a cytoplasmic expression pattern in predominantly shrunken and dark neurons, which were most frequently seen in grades 2 and 3. Control brains displayed weak perinuclear expression of the anti-apoptotic protein Bcl-2, whereas in HD brains Bcl-2 immunoreactivity was markedly enhanced, especially in severely affected grade 4 brains, and was observed in both healthy neurons and dark neurons. Caspase-3, an executioner protease, was only found in four HD brains of different grades and was not expressed in controls. A strong neuronal and glial expression of poly(ADP-ribose) polymerase (PARP)-immunoreactivity was observed in HD brains. These data strongly suggest the involvement of apoptosis in HD. The exact apoptotic pathway occurring in HD neurodegeneration remains yet unclear. However, the presence of late apoptotic events, such as enhanced PARP expression and many TUNEL-positive cells accompanied with weak caspase-3 immunoreactivity in severely affected HD brains, suggests that caspase-mediated neuronal death only plays a minor role in HD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apoptosis is a form of programmed cell death that results in the orderly and efficient removal of damaged or redundant cells, e.g., occurring after DNA damage or during normal development, immune regulation, and tissue homeostasis [1, 18]. Many factors contribute to the apoptotic cascade that lead to cell destruction, each demonstrating specificity of function, regulation, and pathway involvement [1, 15]. Inappropriate activation of apoptosis has been implicated in the pathogenesis of several chronic age-related neurological disorders, such as Huntington’s disease (HD) [32], by the demonstration of increased DNA degradation and typical apoptotic cells in postmortem HD brains [7, 33, 38]. Furthermore, in vitro studies have provided evidence for a link between apoptosis and the mutant huntingtin protein [10, 13]. In addition, apoptotic cell death has been reported in experimental models of HD, in which the mitochondrial toxin 3-nitropropionic acid induced nuclear DNA fragmentation with an increased expression of apoptosis-related markers in rat striatum [40]. HD is caused by the abnormal expansion of CAG trinucleotide repeats in the N terminus of the huntingtin gene [14], but how this mutation leads to extensive and region-specific neuronal degeneration in HD neostriatum remains unclear.
In recent years, various apoptotic cascades have been characterized in the nervous system, such as the extrinsic and intrinsic pathways of caspase-mediated cell death, as well as caspase-independent apoptosis (Fig. 1). Caspases are cysteine aspartate-specific proteases and constitute a family of intracellular proteins that play an important role in the initiation and execution of apoptotic cell death [1]. The caspase-mediated extrinsic pathway, or the cell-death receptor pathway, is initiated by activation of the Fas receptor after binding to the Fas ligand, whereas the caspase-mediated intrinsic pathway, or the mitochondrial pathway, is regulated by the interaction of members of the Bcl-2 family of mitochondrial proteins, such as Bax and Bcl-2. The intrinsic pathway is triggered by Bax translocation to the mitochondrial outer membrane, which leads to the activation of several caspases. Caspase-3 is an important executioner protease in the apoptotic program and cleaves a broad array of proteins critical for cell survival, including the DNA-repairing enzyme poly(ADP-ribose) polymerase (PARP) into 85- and 28-kDa fragments [8, 23]. The huntingtin protein itself is also a substrate for caspase-3 [10]. Finally, caspase-independent apoptosis is activated by translocation of apoptosis-inducing factor (AIF) to the mitochondria and the nucleus, and produces peripheral chromatin condensation and large-scale DNA strands [8, 23].
The detection of morphological features of apoptotic cell death may be difficult in a slowly progressive disorder like HD. To ascertain that apoptosis is involved in HD and to elucidate which apoptotic signaling cascade is predominantly involved in HD pathogenesis, we studied apoptotic features at various time points of the process, since not only the end-products like DNA degradation and apoptotic bodies, but also the expression of several regulatory proteins involved in the cascade, may provide evidence for activation of the apoptotic process. In this study, we focused on the caspase-mediated apoptotic cell death by studying the expression patterns of Bax, Bcl-2, caspase-3 and PARP and the presence of apoptotic cells by using the terminal deoxynucleotidyl transferase-mediated dUTP biotin nick end labeling (TUNEL) method in the caudate nucleus of HD patients and in control brains.
Materials and methods
Human tissue
Caudate samples were obtained at postmortem examination from 18 HD patients (mean age 60.4±14.6 years, mean postmortem delay 20.1 h) and 6 controls with no evidence of neurological disease on neuropathological examination (mean age 72.5±8.7 years, mean postmortem delay 18.0 h). The clinical diagnosis of HD was confirmed neuropathologically, and HD severity was graded according to the classification of Vonsattel et al. [42]. The characteristics of the HD patients and controls are summarized in Table 1.
After removal at autopsy, whole brains were rapidly fixed with 10% paraformaldehyde in phosphate buffer. Tissue samples were then embedded in paraffin, cut into sections of 5 µm and stained with standard neuropathological techniques, including hematoxylin and eosin, cresyl violet, or processed for immunohistochemistry using glial fibrillary acidic protein (GFAP) antibody as a marker of astrocytes.
Immunohistochemistry
Immunohistochemistry was performed on deparaffinized sections of the caudate nucleus using commercially available antisera that were all diluted in 1% BSA/PBS. A polyclonal rabbit antibody directed against GFAP (Dako, Glostrup, Denmark) was used at a dilution of 1:400. Anti-Bax antibody (Zymed laboratories, Uden, The Netherlands) is a mouse monoclonal raised against the N-terminal end of the protein, which reacts specifically with Bax protein of mouse, rat and human origin, dilution 1:50. Polyclonal goat antiserum directed against Bcl-2 (N-19) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used at a dilution of 1:200. Anti-Bcl-2 antibody is directed against the N-terminal end of Bcl-2 of human origin, is specific for mouse, rat and human Bcl-2 and shows no cross-reactivity with other apoptosis-associated proteins. Caspase-3 (1:100) is a rabbit polyclonal antibody from Cell Signaling Technology Inc., Leusden, The Netherlands) and recognizes cleaved caspase-3 at aspartate residue 175. Anti-PARP p85 fragment pAb (Promega Benelux b.v., Leiden, The Netherlands) is a polyclonal rabbit antibody specifically directed against the 85-kDa caspase-cleaved fragment (p85) of human PARP, dilution 1:75.
Prior to Bax, Bcl-2, caspase-3 and PARP immunohistochemistry, deparaffinized sections were subjected to microwave treatment for 10 min in a boiling citrate solution pH 6.0. All sections were incubated for 30 min in 1% H2O2 (Merck, Darmstadt, Germany) in PBS to quench endogenous peroxidase activity and subsequently, unspecific binding was blocked by 30 min incubation with 20% normal goat serum (Bcl-2, caspase-3 and PARP) or 20% normal horse serum (Bax) in PBS. Overnight incubation with the primary antibodies at 4°C was followed by incubation with a secondary biotinylated goat anti-rabbit antibody (Bcl-2, caspase-3 and PARP) or a secondary biotinylated horse anti-mouse antibody (Bax), all used at a dilution of 1:200 in 1% BSA/PBS for 45 min. To visualize antibody binding, a standard avidin-biotin-complex (Vectastain Elite, Vector Labs, Burlingame, CA) was applied to the sections and 3,3’-diaminobenzidine (DAB; Sigma) was used as a chromophore. Finally, the signal of the reaction product was enhanced by incubating sections for 5 min in 0.5% copper sulfate in saline, and sections were counterstained with Mayer’s hematoxylin. Paraffin-embedded rat intestine was used as a positive control for apoptotic markers used in this study.
Nuclear DNA fragmentation was visualized in deparaffinized sections using the Apoptaq kit (Intergen Company/Oncor Inc., Gaithersburg, MD) containing TdT-mediated dUTP-digoxigenin 3’ nick-end labeling (TUNEL) according to the manufacturer’s recommendations. TUNEL-positive cells were visualized using DAB and 0.5% copper sulfate in saline, after which the sections were counterstained using “Kernechtrot”.
Results were obtained by microscopic evaluation of immunostained sections, in which labeled neurons were determined by virtue of their typical neuronal cell morphology after counterstaining with hematoxylin. Percentages of apoptotic cells and the number of neurons expressing apoptosis-related markers were estimated by counting Bax-, Bcl-2-, caspase-3-, PARP- and TUNEL-positive cells visible per microscopic field (×20 objective). A semi-quantitative method was used to analyze the immunohistochemical results, with indications varying from absent (−, no positive cells) to weak (+, 1–5 positive cells), moderate (++, 5–20 positive cells) and strong (+++, >20 positive cells) in views obtained with a ×20 objective (see Table 1). Two independent observers validated a subset of immunostained sections and identical results were recorded. Digital images were captured using a Sony video camera mounted on a Zeiss Axioscope 2 plus and Adobe Photoshop software.
Results
Histological findings
The caudate nucleus of the HD brains revealed characteristic HD neuropathology and demonstrated neuronal cell loss that varied from moderate to extensive, together with increased GFAP immunoreactivity reflecting the gliotic process (Fig. 2A–C). Apoptotic features, such as nuclear fragmentation and chromatin condensation, were more frequently observed in the hematoxylin and Nissl-stained sections of HD brains than in control brains (see below and Fig. 2C).
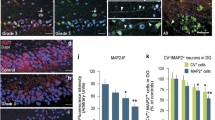
Neuropathological characteristics, expression pattern of apoptosis-related markers and TUNEL labeling in control and in HD brains. A Photomicrograph of a paraffin section of a control, demonstrating a few GFAP-immunostained astrocytes in the caudate nucleus. B Increased expression of GFAP-positive astrocytes in a paraffin section from an HD brain. Note the greater number of neurons in the control brain in comparison to the HD brain showing mainly affected dark neurons (arrowheads). C Cresyl violet-stained section of an HD brain showing neuronal loss, dark neurons and apoptotic features such as nuclear condensation and fragmentation (arrows). D The HD caudate nucleus demonstrates an increased cytoplasmic immunoreactivity for the pro-apoptotic protein Bax in neurons but predominantly in dark neurons (arrowheads). E The perinuclear and diffuse cytoplasmic immunolabeling of the anti-apoptotic protein Bcl-2 in neurons is found in control brain. F HD caudate nucleus demonstrates enhanced expression for Bcl-2: strong Bcl-2 expression is found in neurons and dark neurons (arrowheads). G A few scattered caspase-3-positive cells are found in the HD caudate nucleus and these are not present in control brains. H In control brain, cytoplasmic PARP immunoreactivity is mainly found in neurons, nuclear PARP immunostaining is rare. I In HD brains, PARP expression is markedly increased and a strong granular expression is observed in the cytoplasm and cellular processes of neuronal cells. K PARP immunoreactivity is demonstrated in the cytoplasm and cellular processes of glial cells in HD brains. L Diffusely TUNEL-labeled cells that do not exhibit characteristic apoptotic morphology, are frequently seen in the caudate nucleus of controls. M HD brains with intensively labeled TUNEL-positive cells show DNA fragmentation mainly localized in shrunken nuclei (arrows) and in a few apoptotic bodies (arrowhead). These typical apoptotic cells are mostly detected in the neuropil of the caudate nucleus surrounded by more diffusely TUNEL-labeled cells that do not exhibit characteristic apoptotic morphology (HD Huntington’s disease, GFAP glial fibrillary acidic protein). Bars in A represents 20 µm (also for B, C); in D represents 10 µm (also for E–M)
Expression pattern of apoptosis-related markers in control and HD brains
Only a few control brains revealed a weak perinuclear immunostaining for both Bax and Bcl-2 proteins in the caudate nucleus, with only a few neurons expressing Bax or Bcl-2 diffusely in the cytoplasm. The overall and diffuse immunostaining was more intense and more evident for Bcl-2 (Fig. 2E). Astrocytes were only weakly positive for Bax and Bcl-2 (not shown). In HD, the caudate nucleus demonstrated an enhanced cytoplasmic immunostaining for both Bax and Bcl-2 in most caudate neurons compared to controls. Interestingly, in HD brains, these apoptosis-related markers were strongly expressed in shrunken and dark neurons, which are most likely compromised by the disease (Fig. 2D, F). Increased Bax immunoreactivity was predominantly found in dark neurons that were moderately labeled (Fig. 2D, arrows), while the average perinuclear Bax staining of neurons in HD was weak and similar as in controls. The up-regulation of pro-apoptotic protein Bax expression was maximal in HD brains grades 2 and 3, presenting most frequently dark neurons. The anti-apoptotic protein Bcl-2 was markedly enhanced in HD brains, with an intense and diffuse cytoplasmic immunoreactivity in dark neurons but was also expressed in healthy neurons (Fig. 2F). This expression pattern was more intense compared to the perinuclear Bcl-2 expression observed in controls. The up-regulation of Bcl-2 expression was maximal in postmortem HD brains grades 3 and 4, reflecting HD patients with disease duration of more than 18 years (see Table 1).
Caspase-3 immunoreactivity was not present in control brains. Only four HD caudate nucleus samples were caspase-3 positive and demonstrated scattered cells with a cytoplasmic granular immunoreactivity located next to a shrunken nucleus (Fig. 2G). The identity of these caspase-3-labeled cells is unclear, although it cannot be excluded that this immunostaining depicts pigment-laden macrophages present in HD brains.
In control brains, PARP immunoreactivity was predominantly found in neurons showing a perinuclear and diffuse cytoplasmic immunostaining, while the weaker perinuclear labeling was also demonstrated in glial cells (Fig. 2H). Nuclear PARP immunoreactivity was rare. In HD brains, the neuronal cytoplasmic PARP expression was strongly increased, and was especially visible as cytoplasmic granules in neurons (Fig. 2I). In addition, HD brains showed PARP immunoreactivity in the cytoplasm and cellular processes of activated astrocytes (Fig. 2K). The markedly enhanced PARP immunostaining in neurons was mainly observed in grade 3 and 4 HD brains, as shown in Table 1.
TUNEL labeling in control and HD brains
TUNEL-positive cells were detected in both control and HD brains. In controls, diffusely labeled cells that lacked apoptotic features (up to 40% of total brain cells) were regarded as non-apoptotic (Fig. 2L), whereas intensely stained TUNEL-positive cells (5–10% of total TUNEL-labeled cells) that demonstrated typical DNA fragmentation in shrunken nuclei and in apoptotic bodies were regarded as specific for apoptotic cell death. Moreover, HD brains showed both more and darker stained TUNEL-positive cells than control brains, and these typical apoptotic cells were markedly present in grade 4 HD brains (Fig. 2M). The identity of the dark and shrunken TUNEL-labeled cells is not entirely clear and possibly includes most brain cell types. However, the location of most darkly stained TUNEL-positive cells in an area of extensive neuronal degeneration in end-stage HD caudate nuclei strongly suggests that these TUNEL-positive cells are predominantly neurons. Furthermore, a minority of HD brains (n=3) and one control brain were TUNEL negative.
Table 1 summarizes the TUNEL labeling, together with the results of Bax, Bcl-2, caspase-3 and PARP immunostaining observed in postmortem neostriatal tissue from HD patients and controls.
Discussion
The present study confirms that neostriatal neurodegeneration in HD brains involves an apoptotic mechanism, which is associated with enhanced Bax expression, weak caspase-3 expression, the presence of strong PARP immunoreactivity and many TUNEL-labeled cells exhibiting typical apoptotic morphology in comparison with controls without neurological disease. In a slowly progressive disorder like HD, in which only a few cells at any time would be expected to undergo cell death, the detection of morphological features of apoptosis may be difficult [36]. Another problem is that HD has a duration of about 15 years, leaving even less transient apoptotic neurons to be found in postmortem tissue, when at the time of death most affected neurons are already dead and removed. Because most of the TUNEL-labeled cells were located in an area of extensive neuronal degeneration in end-stage HD brains, it is highly likely that these apoptotic cells were predominantly neurons. Since the TUNEL method has methodological limitations and also detects DNA strand breaks in necrotic cells [3, 11, 41], TUNEL labeling is not sufficient to demonstrate apoptosis in neurons, which also need to be evaluated by the expression of other biochemical markers that are involved in the apoptotic cascade [15, 39]. Therefore, the up-regulation of pro-apoptotic proteins in the HD brains examined, such as Bax, caspase-3 and PARP, in association with the TUNEL labeling provides additional evidence for the hypothesis that specific loss of medium-sized striatal spiny GABAergic neurons in HD results from the inappropriate activation of apoptosis [32]. The altered expression patterns of apoptotic markers in neurons between HD brains and controls that we found in this study are not likely to be due to differences in postmortem delay or fixation times since no obvious correlation was noted between these parameters. There appeared to be a correlation between the different grades of HD severity and enhanced expression levels of apoptosis-related markers. The finding of late apoptotic events in the cascade, such as PARP and TUNEL staining, in the severe grade HD brains could reflect an increased and faster cell death at the end-stage of the neurodegenerative disorder, when compromised cells are at maximum risk of dying, resulting in a maximum occurrence of apoptotic cell death. This is comparable with findings of Kiechle et al. [19], who reported increased expression of caspase-9 and cytochrome c in HD striatal neurons of severe-grade specimens, and suggested that apoptosis may play a greater role in neuronal death at end-stage disease. Furthermore, the grade 4 brains from patients suffering HD for more than 18 years, displayed the highest expression of anti-apoptotic protein Bcl-2, implicating a neuroprotective role in the left-over and still surviving injured neurons at the end-stage of the disease [9, 16].
In line with other studies [12], we observed only a weak perinuclear and cytoplasmic expression for the apoptosis-related proteins Bax and Bcl-2 in controls, while in most HD brains the expression of both Bax and Bcl-2 was increased. This up-regulation of Bax and Bcl-2 proteins was predominantly found in degenerating and affected dark neurons, suggesting a battle for life or death, although anoxic circumstances could also contribute to the injured state of these neurons. However, their maximal expression was at different stages of HD, with Bax implicating the induction of the apoptotic process in grade 2 and 3 HD brains and Bcl-2 implicating a neuroprotective role in the left-over and still surviving injured neurons at the end stage of the disease [8, 16]. Bax and Bcl-2 expression in HD caudate nucleus have not been described before, but Bax and Bcl-2 up-regulation has been shown in Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS) [6, 28, 35, 37]. The increased Bax expression observed in HD brains possibly reflects the formation of functional and pro-apoptotic Bax homodimers, probably preparing neostriatal neurons to die [21, 25, 27, 36]. In affected dark neurons of HD brains, however, the level of Bax protein in the cytoplasm was not yet able to activate the apoptotic process and was probably counteracted by the up-regulation of Bcl-2 [16, 17, 27, 29]. The enhanced anti-apoptotic Bcl-2 expression might be explained as an ongoing survival attempt to protect these mildly injured neurons from death, because it was not or only weakly expressed in control brains.
HD brains, and especially the severe grade 4, demonstrated strong immunoreactivity of 85-kDa PARP fragments in the cytoplasm, and was present as granules in neurons, in some nuclei of neurons and in glial cells, suggesting a role for the DNA repair enzyme PARP in HD neurodegeneration. Cookson et al. [5] showed substantial cytoplasmic PARP expression in most neuron types, especially in motor neurons of the spinal cord. Cleaved PARP fragments have been demonstrated in Alzheimer’s disease and Parkinson’s disease [24, 26] and in ALS patients [20]. Because of the dominant cytoplasmic PARP immunoreactivity, it was suggested that PARP up-regulation in HD might be associated with other subcellular components besides the nucleus [5], which indicates additional roles for this enzyme being effective in disease-affected neurons and in activated astrocytes that may contribute to neuronal dysfunction and death in HD.
In our study, scattered caspase-3-positive cells were only found in four HD brains and was not expressed in control brains. Caspase-3 expression has not been shown previously in the caudate nucleus of HD patients. The weak caspase-3 immunostaining most likely depicts pigment-laden macrophages that are present and active in HD brains, clearing dying and disease-affected neurons away. In Parkinson’s disease, an increased immunoreactivity of caspase-3 has been reported in a small proportion of the substantia nigra [37]. In transgenic mice models of HD, transcriptional up-regulation of caspase-1 and caspase-3 has been reported [4, 30], and activation of caspase-8, caspase-9 and release of cytochrome c has been demonstrated in human striatal tissue of HD patients [19, 34]. Usually, caspases are the major executioners of the apoptotic pathway [2] but the low caspase-3 immunoreactivity observed in our study may indicate that the caspase-mediated apoptotic cell death was not intensively involved in HD. Moreover, it was reported that caspase-3 and other caspases may be more activated in acute neuronal cell death, such as in brain damage after ischemia or trauma [23], whereas in chronic neurodegenerative diseases, the chronic and sublethal activation of caspases appears to mediate cell dysfunction, which precedes cell death [22, 31]. In the present study we could not confirm the latter; we focused on the intrinsic caspase-mediated apoptotic cell death that may contribute to delayed loss of neurons [23], as expected to occur in the slowly progressing neurodegenerative disorder HD.
It remains unclear which apoptotic pathway predominantly occurs in HD. The findings of increased PARP expression and TUNEL labeling, which are both markers of the end-stage of the apoptotic process, accompanied by weak caspase-3 immunoreactivity in severely affected HD brains, might favor a role for the non-caspase-mediated neuronal apoptotic pathway being involved in HD pathogenesis. However, it seems that caspase-mediated neuronal death is not always accompanied by the morphological changes that are typical of apoptosis in other tissues [24]. This might explain differences in expression of apoptotic markers in nervous tissue compared to other tissues and may suggest that other markers and pathways are induced in HD. The precise pathway by which neurons in the HD caudate nucleus die requires further investigation, but this study shows that up-regulation of Bax and PARP are important factors in the apoptotic process. It is possible that at different stages of the disease particular apoptotic proteins are important to prepare and induce cell death at the beginning of the disease (Bax) or to execute increased cell death (PARP) at end-stage HD. Knowledge about the pathway by which neurons die in HD may help the development of therapies that are aimed at reducing cell degeneration and death in HD.
References
Ashe P, Berry M (2003) Apoptotic signaling cascades. Prog Neuropsychopharmacol Biol Psychiatry 27:199–214
Brouillet E, Conde F, Beal MF, Hantraye P (1999) Replicating Huntington’s disease phenotype in experimental animals. Prog Neurobiol 59:427–468
Charriaut-Marlangue C, Ben-Ari Y (1995) A cautionary note on the use of the TUNEL stain to determine apoptosis. Neuroreport 7:61–64
Chen M, Ona V, Li M, Ferrante R, Fink K, Zhu S, Bian J, Guo L, Farrell L, Hersch S, Hobbs W, Vonsattel J, Cha J, Friedlander R (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6:797–801
Cookson M, Ince P, Usher P, Shaw P (1999) Poly(ADP-ribose) polymerase is found in both the nucleus and cytoplasm of human CNS neurons. Brain Res 834:182–185
Cotman, C, Anderson, A (1995) A potential role for apoptosis in neurodegeneration and Alzheimer’s disease. Mol Neurobiol 10:19–45
Dragunow M, Faull RL, Lawlor P, Beilharz EJ, Singleton K, Walker EB, Mee E (1995) In situ evidence for DNA fragmentation in Huntington’s disease striatum and Alzheimer’s disease temporal lobes. Neuroreport 6:1053–1057
Ferrer I, Planas A (2003) Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol 62:329–339
Ferrer I, Lopez E, Blanco R, Rivera R, Ballabriga J, Pozas E, Marti E (1998) Bcl-2, Bax, and Bcl-x expression in the CA1 area of the hippocampus following transient forebrain ischemia in the adult gerbil. Exp Brain Res 121:167–173
Goldberg Y, Nicholson D, Rasper D, Kalchman M, Koide H, Graham R, Bromm M, Kazemi-Esfarjani P, Thornberry N, Vaillancourt J, Hayden M (1996) Cleavage of huntingtin by apopain, a proapoptotic cysteine protease, is modulated by the polyglutamine tract. Nat Genet 13:442–449
Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R (1995) In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology 21:1465–1468
Hara A, Hirose Y, Wang A, Yoshimi N, Tanaka T, Mori H (1996) Localization of Bax and Bcl-2 proteins, regulators of programmed cell death, in the human central nervous system. Virchows Arch 429:249–253
Hickey M, Chesselet M (2003) Apoptosis in Huntington’s disease. Prog Neuropsychopharmacol Biol Psychiatry 27:255–265
Huntington’s Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72:971–983
Huppertz B, Frank H, Kaufmann P (1999) The apoptosis cascade-morphological and immunohistochemical methods for its visualization. Anat Embryol (Berl) 200:1–18
Isenmann S, Stoll G, Schroeter M, Krajewski S, Reed J, Bahr M (1998) Differential regulation of Bax, Bcl-2, and Bcl-X proteins in focal cortical ischemia in the rat. Brain Pathol 8:49–62
Kaneda K, Kashii S, Kurosawa T, Kaneko S, Akaike A, Honda Y, Minami M, Satoh M (1999) Apoptotic DNA fragmentation and upregulation of Bax induced by transient ischemia of the rat retina. Brain Res 815:11–20
Kerr JF, Wyllie AH, Curie AR (1972) Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Kiechle T, Dedeoglu A, Kubilus J, Kowall NW, Beal MF, Friedlander R, Hersch SM, Ferrante RJ (2002) Cytochrome C and caspase-9 expression in Huntington’s disease. Neuromolecular Med 1:183–195
Kim S, Henkel J, Beers D, Sengun I, Simpson E, Goodman J, Engelhardt J, Siklos L, Appel S (2003) PARP expression is increased in astrocytes but decreased in motor neurons in the spinal cord of sporadic ALS patients. J Neuropathol Exp Neurol 62:88–103
Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang H, Reed J (1994) Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol 145:1323–1336
Li M, Ona VO, Chen M, Kaul M, Tenneti L, Zhang X, Stieg PE, Lipton SA, Friedlander RM (2000) Functional role and therapeutic implications of neuronal caspase-1 and - 3 in a mouse model of traumatic spinal cord injury. Neuroscience 99:333–342
Love S (2003) Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry 27:267–282
Love S, Barber R, Wilcock G (1999) Increased poly(ADP-ribosyl)ation of nuclear proteins in Alzheimer’s disease. Brain 122:247–253
MacGibbon G, Lawlor P, Sirimanne E, Walton M, Connor B, Young D, Williams C, Gluckman P, Faull R, Hughes P, Dragunow M (1997) Bax expression in mammalian neurons undergoing apoptosis, and in Alzheimer’s disease hippocampus. Brain Res 750:223–234
Mandir A, Przedborski S, Jackson-Lewis V, Wang Z, Simbulan-Rosenthal C, Smulson M, Hoffman B, Guastella D, Dawson V, Dawson T (1999) Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc Natl Acad Sci USA 96:5774–5779
Merry D, Korsmeyer S (1997) Bcl-2 gene family in the nervous system. Annu Rev Neurosci 20:245–267
Mogi M, Harada M, Kondo T, Mizuno Y, Narabayashi H, Riederer P, Nagatsu T (1996) Bcl-2 protein is increased in the brain from parkinsonian patients. Neurosci Lett 215:137–139
Nakasu S, Nakajima M, Nakazawa T, Nakasu Y, Handa J (1998) Alteration of bcl-2 and bax expression in embolized meningiomas. Brain Tumor Pathol 15:13–17
Ona V, Li M, Vonsattel J, Andrews L, Khan S, Chung W, Frey A, Menon A, Li X, Stieg P, Yuan J, Penney J, Young A, Cha J, Friedlander R (1999) Inhibition of caspase-1 slows disease progression in a mouse model of Huntington’s disease. Nature 399:263–267
Pasinelli P, Houseweart M, Brown RJ, Cleveland D (2000) Caspase-1 and −3 are sequentially activated in motor neuron death in Cu,Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 97:13901–13096
Petersen A, Mani K, Brundin P (1999) Recent advances on the pathogenesis of Huntington’s disease. Exp Neurol 157:1–18
Portera-Cailliau C, Hedreen JC, Price DL, Koliatsos VE (1995) Evidence for apoptotic cell death in Huntington disease and excitotoxic animal models. J Neurosci 15:3775–3787
Sanchez I, Xu CJ, Juo P, Kakizaka A, Blenis J, Yuan J (1999) Caspase-8 is required for cell death induced by expanded polyglutamine repeats. Neuron 22:623–633
Satou T, Cummings B, Cotman C (1995) Immunoreactivity for Bcl-2 protein within neurons in the Alzheimer’s disease brain increases with disease severity. Brain Res 697:35–43
Sugimoto T, Xiao C, Ichikawa H (1998) Neonatal primary neuronal death induced by capsaicin and axotomy involves an apoptotic mechanism. Brain Res 807:147–154
Tatton W, Chalmers-Redman R, Brown D, Tatton N (2003) Apoptosis in Parkinson’s disease: signals for neuronal degradation. Ann Neurol 53:S61–70
Thomas LB, Gates DJ, Richfield EK, O’Brien TF, Schweitzer JB, Steindler DA (1995) DNA end labeling (TUNEL) in Huntington’s disease and other neuropathological conditions. Exp Neurol 133:265–272
Uysal H, Cevik I, Soylemezoglu F, Elibol B, Ozdemir Y, Evrenkaya T, Saygi S, Dalkara T (2003) Is the cell death in mesial temporal sclerosis apoptotic? Epilepsia 44:778–784
Vis JC, Verbeek MM, Waal RM de, Donkelaar HJ ten, Kremer B (2001) The mitochondrial toxin 3-nitropropionic acid induces differential expression patterns of apoptosis-related markers in rat striatum. Neuropathol Appl Neurobiol 27:68–76
Vogel P, Dux E, Wiessner C (1997) Evidence of apoptosis in primary neuronal cultures after heat shock. Brain Res 764:205–213
Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP, Jr (1985) Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol 44:559–577
Acknowledgements
We thank the Department of Neurology of Leiden University Medical Center, Leiden, the Netherlands, for donation of brain tissue used in this study. This study was supported by a grant from the Netherlands Organization for Scientific Research (NWO-MW).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vis, J.C., Schipper, E., de Boer-van Huizen, R.T. et al. Expression pattern of apoptosis-related markers in Huntington’s disease. Acta Neuropathol 109, 321–328 (2005). https://doi.org/10.1007/s00401-004-0957-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0957-5