Abstract
The RAS/RAF/MEK/ERK kinase pathway is pivotal in the transduction of mitogenic stimuli from activated growth factor receptors, which regulates cell proliferation, survival, and differentiation. Up-regulation of this pathway due to RAS mutations is found in approximately 30% of human tumors. Recently, activating mutations of B-RAF were identified in a large proportion of human cancers. Gliomas are the most frequent primary central nervous system tumors and the molecular mechanisms that underlie the development and progression of these tumors are far from being completely understood. The purpose of this study was to clarify the incidence of B-RAF mutations and their possible relation with tumor progression in a series of 82 human gliomas, including 49 astrocytic and 33 oligodendroglial tumors. The analysis of B-RAF hotspot regions, exons 11 and 15, showed presence of B-RAF mutations in only 2 out of 34 (6%) glioblastomas, and absence in the remaining histological types. Both mutations were located in the hotspot residue 600 (V600E) at exon 15, which leads to constitutive B-RAF kinase activity. These data suggest that activating mutations of B-RAF are not a frequent event in gliomas; nevertheless, when present they are associated with high-grade malignant lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas are the most frequent primary brain tumors in children and adults [8]. Derived from neuroepithelial cells, gliomas are a heterogeneous group composed of different histological and biological subtypes, including astrocytic, oligodendroglial and mixed oligoastrocytic tumors. These neoplasms are classified in four grades of malignancy according to the World Health Organization (WHO) classification of brain tumors [10]. Glioblastoma (WHO grade IV) is the most malignant and most common adult brain tumor. Despite multimodal treatment, high-grade gliomas (e.g., glioblastoma) are highly invasive and remain a challenge for oncology. The mean survival time of glioblastoma patients is less than 1 year and has changed little over the past three decades [6, 10, 20]. Thus, it is essential to clarify the yet poorly understood molecular mechanisms that underlie the genesis and progression of these tumors for the development of novel therapeutic strategies.
The RAS/RAF/MEK/ERK intracellular signaling cascade can be activated in response to a variety of extracellular stimuli. Growth factor binding to extracellular receptors results in activation of RAS, which in turns induce the stimulation of downstream cellular cascades (RAF/MEK/ERK, PI3K/AKT and RAC/RHO pathways) that control cell proliferation, differentiation and survival [11]. The RAS/RAF/MEK/ERK signaling cascade is frequently activated in human cancers, resulting in uncontrolled tumor growth [5].
Activating RAS mutations are found in approximately 30% of human cancers [5]; however, mutations of RAS have been rarely identified in gliomas [2, 3]. Nevertheless, several lines of evidence suggest that alterations of RAS/RAF/MEK/ERK signaling are involved in the development of gliomas. Using genetically modified normal human astrocytes, Sonoda et al. [19] recently define pathways critical to the development of anaplastic astrocytoma, including RAS/RAF/MEK/ERK pathway activation through mutant H-Ras. In transgenic mouse models studies, overexpression of oncogenic Ras in astrocytes and neural progenitors cells lead to activation of RAS/RAF/MEK/ERK pathway and induction of high-grade astrocytic tumors [4, 9]. Furthermore, Guha et al. [7] showed that the RAS pathway is activated in glioblastomas. It is therefore likely that mechanism other than RAS mutations activate the RAS/RAF/MEK/ERK pathway in gliomas.
Recent studies reported the presence of somatic oncogenic mutations in the B-RAF gene, a member of the RAF family, in a large percentage of human melanomas and less frequently in a wide range of other human cancers such as colorectal, thyroid and lung cancer [3, 13, 14, 18]. The great majority of mutations are located in exon 15, with additional mutations also present in exon 11. These mutations lead to constitutive activation of the RAF/MEK/ERK pathway and are oncogenic in human cell lines [3, 21]. Interestingly, the B-RAF and RAS mutations occur frequently in mutually exclusive fashion in various tumors, suggesting a linear functional relationship for these components in the complex signaling pathways [3, 14, 18]. Davies et al. [3] reported a low frequency (11%) of B-RAF mutation in glioma cell lines, and the absence of B-RAF mutation in a limited series primary glioma tumors.
In the current study we aimed to evaluate the aforementioned observations in a larger series of primary astrocytic and oligodendroglial tumors. We therefore analyzed 82 cases for the presence of mutations in exon 11 and exon 15, to determine the role of B-RAF mutation in the development and progression of these tumors.
Material and methods
Tissue samples
Eighty-two formalin-fixed and paraffin-embedded glioma tumors were collected at the Department of Pathology of Hospital São João, Porto and Hospital São Marcos, Braga, Portugal. All cases were reviewed by two pathologists (J.M.L. and F.P.) and classified according to the WHO classification of CNS tumors [10]. Forty-nine cases were astrocytic tumors, including 15 diffuse fibrillary astrocytomas (WHO grade II), and 34 glioblastomas (WHO grade IV). Except for one case, all glioblastomas were clinically de novo. Of the 33 oligodendroglial tumors, 15 were oligodendroglioma (WHO grade II) and 18 were anaplastic oligodendroglioma (WHO grade III). Median age and gender of the patients per tumor type is shown in Table 1. None of the patients had a history of familiar cancer clustering.
DNA preparation
Tumor DNA was obtained from paraffin sections as previously described, with some modifications [16]. Briefly, serial 10-μm-thick unstained sections of paraffin blocks were cut, and one hematoxylin and eosin-stained section was taken for identification and selection of the tumor portion. Selected areas contained at least 85% of tumor tissue. Constitutive DNA analysis was not performed due to restriction of non-tumor tissue of the patients. Paraffin was removed by incubation in xylene, followed by ethanol washing, drying and digestion in 100 μl lysis buffer (500 mM TRIS-HCl pH 8.5, 1 mM EDTA pH 8.0) containing proteinase K to a final concentration of 0.5 mg/ml) at 55°C for 48 h. After proteinase K denaturation, DNA samples were stored at −20°C for subsequent molecular analysis.
B-RAF mutation analysis
Screening for exon 11 and 15 mutations was performed by polymerase chain reaction-single strand conformational polymorphism (PCR-SSCP) techniques using genomic DNA. PCR was processed as described elsewhere [18]. Briefly, amplification was carried out in a total volume of 25 μl, comprising 2 μl DNA solution, 1 U Taq DNA polymerase (Platinum-Invitrogen, CA), 1.5 mM MgCl2 (Invitrogen), 0.2 mM of each dNTP (Fermentas, CA), 0.6 μM of both sense and antisense primers, 20 mM TRIS-HCl pH 8.4 (Invitrogen) and 50 mM KCl buffer (Invitrogen). PCR amplification was performed in a Biotech Primus 96 Plus Thermal Cycler, with an initial denaturation step at 95°C for 5 min, then amplified for 38 cycles of denaturation at 95°C for 45 s, annealing (58°C for exon 11, 60°C for exon 15) for 45 s, extension at 72°C for 45 s, and final extension step at 72°C for 10 min. Primers sequence for exon 11 were: 5’-ATAAGGTTTTCTTTTTCTGTTTGGC-3’ (forward) and 5’-ACTTGTCACAATGTCACCACATTAC-3’ (reverse) and as previously reported for exon 15, 5’-TCATAATGCTTGCTCTGATAGGA-3’ (forward) and 5’-GGCCAAAAATTTAATCAGTGGA-3’ (reverse) [3].
The PCR product was then analyzed by SSCP: 18 μl PCR product was mixed with 18 μl loading dye (98% formamide and 10 mM EDTA, pH 8.0), denatured at 94°C for 10 min and quenched on ice. Of the mixture, 12 μl were loaded onto 0.8× MDE gel (Cambrex, Rockland, ME) at 180 V, for 16 h, at 4°C. The gels were silver stained. PCR products showing mobility shifts were then purified using MicroSpinTM S-300 HR columns (Pharmacia Biotech, Uppsala), submitted to a cycle sequencing reaction using an ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit (Perkin-Elmer) and analyzed with an ABI PRISM 3100 Genetic Analyzer. Samples presenting altered bands were amplified and sequenced twice.
Results
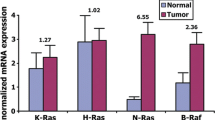
The mutation screening of B-RAF exon 15 by PCR-SSCP (Fig. 1), followed by direct sequencing showed the presence of the hotspot V600E mutation (formerly referred as codon 599) [12] in 2 out of 34 glioblastomas, corresponding to a mutation frequency of 6% (Fig. 2, Table 1). In both cases there was no non-tumoral tissue available for the screening of B-RAF mutations. The tumor in one of the cases recurred, and the V600E mutation was also present in the second glioblastoma biopsy. Clinically, both mutated glioblastomas were de novo and occurred in young adult patients (41 and 46 years old). All the other astrocytic tumors and the 33 oligodendroglial tumors were negative for exon 15. The mutation analysis of B-RAF exon 11 showed the absence of mutations in the astrocytic and oligodendroglial tumors analyzed (Table 1).
Discussion
The RAF kinase family consist of three members: A-, B- and C-RAF (also known as RAF-1), with regulatory properties in RAS signal transduction cascade that regulates cell proliferation and differentiation in response to growth factors, cytokines and hormones [17]. Activated RAS induce phosphorylation and activation of RAF serine/threonine kinases, triggering sequential phosphorylation and activation of MEK and ERK [11]. Although the oncogenic potential of RAF (particularly of C-RAF in in vitro models) has been known for a long time [15], it is only recently that a large-scale screen for genes mutated in cancer has reported the presence of somatic B-RAF mutations in human tumors, including melanomas, colorectal, lung and thyroid cancers [3, 13, 14, 18]. The most frequent mutation is a single transversion in exon 15 (T1799A), which accounts for 90% of B-RAF mutations in human cancers, and leads to a substitution of valine by glutamic acid at position 600 (V600E) in the B-RAF kinase activation domain [3]. A second hotspot region for B-RAF mutation was found in exon 11, and frequently involves a substitution of a glycine to an alanine at residue 469 (G469A) due to change of a guanine to a cytosine at position 1406 (G1406C) [3]. The primary consequence of both (V600E and G469A) mutant proteins is a higher kinase activity, which through distinct mechanisms stimulates ERK activity independently of RAS status [21], inducing in vitro transformation of human NIH3T3 cell lines [3].
The results of the present study show that B-RAF mutations are not a frequent event in gliomas, in concurrence with a previous report [3]. We observed the presence of B-RAF mutations in 6% of glioblastomas and the absence of such mutations in the other histological types. At variance with melanoma, where B-RAF mutations are an early event [13], in gliomas the presence of mutations exclusively in high-grade lesions suggests that they may represent a late event in the gliomagenesis. In line with previous studies, the mutations identified were in the hotspot V600E, indicating that RAF/MEK/ERK cascade is constitutively activated in these tumors. Although we have only analyzed two exons of the B-RAF gene, hitherto no other activating mutations have been identified outside of these hotspot exons [3, 21]. Due to the low number of B-RAF mutated cases, we could not assess the putative association of B-RAF activation with clinical and pathological feature of gliomas.
The RAS/RAF/MEK/ERK pathway mediates several hallmarks of cancer. The low frequency of B-RAF mutations observed in this and a previous study [3], and the reported absence of RAS and C-RAF mutations in gliomas [2, 3], is consistent with other alterations possibly occurring upstream the RAS/RAF pathway in gliomas. The amplification and/or overexpression of growth factor receptors such as EGFR and PDGFR are frequently encountered in gliomas [22].
Based on the low frequency of B-RAF mutations observed, we concluded that B-RAF activation is not a major event in glioma pathogenesis. Nevertheless, due to the lack of effective treatment for glioblastoma, it is possible that those tumors with B-RAF mutations can be candidate for a therapy involving RAF-pathway inhibitors [1].
References
Bollag G, Freeman S, Lyons JF, Post LE (2003) Raf pathway inhibitors in oncology. Curr Opin Investig Drugs 4:1436–1441
Bos JL (1989) ras oncogenes in human cancer: a review. Cancer Res 49:4682–4689
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al (2002) Mutations of the BRAF gene in human cancer. Nature 417:949–954
Ding H, Roncari L, Shannon P, Wu X, Lau N, Karaskova J, Gutmann DH, Squire JA, Nagy A, Guha A (2001) Astrocyte-specific expression of activated p21-ras results in malignant astrocytoma formation in a transgenic mouse model of human gliomas. Cancer Res 61:3826–3836
Downward J (2003) Targeting RAS signaling pathways in cancer therapy. Nat Rev Cancer 3:11–22
Forsyth PA, Cairncross JG (1995) Treatment of malignant glioma in adults. Curr Opin Neurol 8:414–418
Guha A, Feldkamp MM, Lau N, Boss G, Pawson A (1997) Proliferation of human malignant astrocytomas is dependent on Ras activation. Oncogene 15:2755–2765
Gurney JG, Kadan-Lottick N (2001) Brain and other central nervous system tumors: rates, trends, and epidemiology. Curr Opin Oncol 13:160–166
Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN (2000) Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet 25:55–57
Kleihues P, Cavenee WK (2000) Pathology and genetics of tumours of the nervous system, 2nd edn. IARC Press, Lyon
Kolch W (2000) Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J 351:289–305
Kumar R, Angelini S, Czene K, Sauroja I, Hahka-Kemppinen M, Pyhonen S, Hemminki K (2003) BRAF mutations in metastatic melanomas: a possible association with clinical outcome. Clin Cancer Res 9:3362–3368
Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, et al (2003) High frequency of BRAF mutations in nevi. Nat Genet 33:19–20
Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE (2002) Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature 418:934
Rapp UR, Goldsborough MD, Mark GE, Bonner TI, Groffen J, Reynolds FH Jr, Stephenson JR (1983) Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci USA 80:4218–4222
Reis RM, Konu-Lebleblicioglu D, Lopes JM, Kleihues P, Ohgaki H (2000) Genetic profile of gliosarcomas. Am J Pathol 156:425–432
Robinson MJ, Cobb MH (1997) Mitogen-activated protein kinase pathways. Curr Opin Cell Biol 9:180–186
Soares P, Trovisco V, Rocha AS, Lima J, Castro P, Preto A, Maximo V, Botelho T, Seruca R, Sobrinho-Simoes M (2003) BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene 22:4578–4580
Sonoda Y, Ozawa T, Hirose Y, Aldape KD, McMahon M, Berger MS, Pieper RO (2001) Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res 61:4956–4960
Surawicz TS, Davis F, Freels S, Laws ER, Menck HR (1998) Brain tumor survival: results from the National Cancer Data Base. J Neurooncol 40:151–160
Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R (2004) Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116:855–867
Zhu Y, Parada LF (2002) The molecular and genetic basis of neurological tumours. Nat Rev Cancer 2:616–626
Author information
Authors and Affiliations
Corresponding author
Additional information
The first two authors contributed equally to the present study
Rights and permissions
About this article
Cite this article
Basto, D., Trovisco, V., Lopes, J.M. et al. Mutation analysis of B-RAF gene in human gliomas. Acta Neuropathol 109, 207–210 (2005). https://doi.org/10.1007/s00401-004-0936-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0936-x