Abstract
Marked perivascular clustering (PC), i.e., groups and rows of small round cells along white matter vessels, is seen in temporal lobe epilepsy (TLE) specimens obtained by surgery. This study focuses on the constituting cell types and discusses clinical significance and pathogenesis of PC, which are so far unknown. Based on a series of 59 nonlesional TLE surgical specimens, we characterized PC by immunohistochemistry and correlated the amount of PC with clinical parameters. PC cells were variably positive for galactocerebroside, myelin basic protein and S-100 protein, while glial fibrillary acidic protein, vimentin, nestin and neuronal antigens were not expressed. There was no correlation between the amount of PC and any clinical feature, including age at surgery, age at epilepsy onset, duration of epilepsy, preoperative seizure frequency, childhood febrile convulsions, family history of epilepsy, and postsurgical outcome. Our findings suggest oligodendroglial differentiation of PC, while its primary (dysplastic) versus secondary (reactive) pathogenesis remains unresolved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perivascular clustering (PC) refers to groups and rows of small round cells arranged along white matter vessels. Synonymous terms are “rows of perivascular glia” [5], “perivascular glial clustering” [3], “perivascular satellitosis” [3, 30] and “perivascular glial hyperplasia” [8]. Marked PC has been observed in temporal lobe specimens obtained at surgery for epilepsy [13], and is classified as microdysgenesis by most authors [3, 4, 5, 30]. This study investigates the constituting cell types by immunohistochemistry, and tries to find clues regarding the pathophysiological basis in temporal lobe epilepsy (TLE) by correlations to clinical parameters.
Materials and methods
From a series of 200 consecutive patients surgically treated for medically refractory TLE between 1988 and 2000 at the Epilepsy Center, University of Erlangen, all patients showing focal pathology except Ammon’s horn sclerosis (AHS, defined below) were excluded, e.g., neoplasms, vascular malformations, cortical dysplasia, and (post-) inflammatory, (post-) traumatic or ischemic lesions. The remaining 59 cases entered the study. All patients underwent an anterolateral temporal lobectomy tailored to intraoperative electrocorticography after passing an extensive preoperative evaluation protocol and decision by a multidisciplinary conference. Resections included the anterior portion of the hippocampus (2–3.5 cm) and an individually differing amount of lateral temporal neocortex (ranging from 2 to 7 cm from temporal pole to posterior margin).
Materials were retrieved from the files of the Department of Neuropathology, University of Erlangen. Diagnostic slides were available in hematoxylin-eosin (HE), Nissl, and glial fibrillary acidic protein (GFAP) stains. Perivascular clustering was assessed in standard HE slides per high-power field (HPF, i.e., visual field on ×400 magnification, encompassing 0.29 mm2 white matter area) using the following grading system: marked PC, ≥3 foci consisting of at least 10 cells each/HPF; moderate PC, 1 or 2 foci consisting of at least 10 cells each/HPF, but never 3 foci or more; mild PC, only foci consisting of 5 to 9 cells each/HPF; no PC, no detectable clusters.
Considering terminology, “oligodendroglial clustering” has been reported by studies investigating extrahippocampal magnetic resonance signal changes seen in some TLE cases [10, 18, 19]. In all of these studies, glial clusters were not related to white matter microvessels and should therefore be separated from PC. “Cortical perivascular satellitosis”, referring to a peculiar lesion composed of round cells in dense aggregates in and around cortical vessels [15] is also distinctly different from PC described here.
All available paraffin blocks per case (range 4–12) were evaluated and the maximum PC grade used. All hippocampal specimens were investigated for presence or absence of classical AHS, defined as severe neuronal loss in sectors CA1 and CA3/4 of Ammon’s horn, relative sparing of CA2 and reactive astrogliosis. AHS was only diagnosed when all sectors of Ammon’s horn could be reliably identified. In cases where this condition was not met due to fragmentation or malorientation of the tissue section, the diagnosis of AHS was considered uncertain.
For immunohistochemical characterization of cells constituting PC, 5-μm-thick paraffin sections obtained from the cases with marked PC were stained for the following antigens (antibody species, dilutions, microwave pretreatment and sources given in brackets): S-100 protein (rabbit, 1:12,000, Dako, Glostrup, Denmark), myelin basic protein (MBP, rabbit, 1:1,000, microwave, Dako), galactocerebroside (rabbit, 1:10, DPC Biermann, Bad Nauheim, Germany), GFAP (rabbit, 1:4,000, Dako), neuron-specific enolase (NSE, mouse, 1:200, Dako), neuronal nuclear antigen (NeuN, mouse, 1:100, microwave, Chemicon, Temecula, CA), synaptophysin (rabbit, 1:2, Dako), vimentin (mouse, 1:100, Dako), nestin (rabbit, 1:200, microwave, Chemicon) and CD68 (mouse, 1:50 [26]). Detection was performed with the Chem Mate Link Biotinylated Secondary Antibody system (Dako, Hamburg, Germany) and diaminobenzidine as chromogen using a Tech Mate Horizon automated staining apparatus (Dako, Germany).
Clinical data was obtained for each patient by review of all clinical charts available at the documentation archives, Epilepsy Center, University of Erlangen. Data included information about the patients’ gender, age at onset of habitual seizures, age at operation, frequency of preoperative complex-partial and generalized seizures, positive family seizure history (first or second degree relative with definite epilepsy), history of febrile convulsions or other significant initial precipitating injuries (IPI) according to Mathern et al. [17], age at IPI, and most recent postoperative outcome category classified according to Engel [11].
Statistics were performed using χ2, Mann-Whitney U, Kruskal-Wallis, and ANOVA tests using SPSS 10.0 for Windows software.
Results
Clinical data
Of the 59 patients, 34 were male and 25 female; the mean ± SD age at the time of surgery was 35.2±9.9 years. Resections were performed on the right in 39 cases and on the left in 20 cases. Mean age at onset of habitual seizures was 13.0±9.4 years, epilepsy had lasted 22.2±11.4 years at the time of surgery. All but 1 patient experienced complex partial seizures during the preoperative period at maximum frequencies ranging from 1 per month to 50 per month. In 88.1% of patients, secondarily generalized seizures occurred, which presented as single to rare annual events in the majority of cases (69.5%). Histories of childhood febrile convulsions were reported by 18 patients (30.5%). Another 11 patients reported other IPIs, including meningitis (8 patients) or a non-fever-related seizure event (3 patients). In total, 49.2% of patients were positive for an IPI. All but two IPIs had occurred within the first 5 years of life, the exceptional cases reporting a history of meningitis at age 17 and age 12. Of 29 IPIs, 19 occurred within the first 2 years of life (65.5%). The latency between age at IPI and onset of habitual seizures ranged from no latency to 37 years (mean 7.8 years, median 5.0). A positive family history for epilepsy was noted in 13 patients (22.0%). Information regarding the patients’ most recent postoperative outcome classified according to Engel was available for all but 1 patient: 35 patients ranked as class I (seizure-free since surgery), 15 as class II, 5 as class III and 3 as class IV. The mean follow-up period was 2 years, with 46 patients observed for at least 1 year.
Histological and immunohistochemical findings
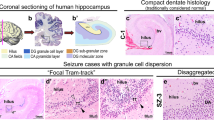
PC was observed in all 59 cases, with 33 (55.9%) rated as mild, 12 (20.3%) as moderate and 14 (23.7%) as marked. AHS was diagnosed in 24 cases (40.7%), while it had to be considered uncertain in 32 cases. Three specimens did not display the typical pattern of neuronal loss, so that AHS was designated absent. The 24 AHS cases exhibited the same distribution of PC grades as the whole group (Table 1). Immunohistochemically, most cells constituting PC expressed S-100 protein, and a variable fraction of the cells were also weakly positive for the oligodendroglial proteins MBP and galactocerebroside (Fig. 1). This staining pattern corresponded to that of typical white matter oligodendrocytes. The cells were negative for GFAP, vimentin, nestin, neuronal antigens (synaptophysin, NeuN, NSE) and CD68.
Immunohistochemical characterization. Cells comprising perivascular clusters (arrows) express S-100 protein (A) and myelin basic protein (B), while glial fibrillary acidic protein (C), nestin (D), neuronal nuclear antigen (E) and CD68 (F) are restricted to astrocytes (C), vascular cells (D), heterotopic white matter neurons (E) and microglia (F), respectively
Correlations between PC and clinical features
PC grading results were not differentially distributed according to side of surgery or sex (χ2 test). No significant association between PC grades and age at surgery, age at epilepsy onset, duration of epilepsy or preoperative seizure frequencies was found (ANOVA and Kruskal-Wallis tests). Presence or absence of positive family history, childhood febrile convulsions or positive IPI history were not associated with PC grades (χ2 test). Also, no relation to type of IPI was found. Mean age at the time of IPI or mean latencies from IPI to epilepsy onset were not different between PC grade categories (ANOVA). No correlation between PC grading and postsurgical outcome category was revealed (Kruskal-Wallis test).
Discussion
We have investigated occurrence, immunohistochemical characteristics and clinical correlates of PC in temporal lobe white matter from patients surgically treated for medically refractory nonlesional TLE.
By applying a semiquantitative grading system, we found that all TLE specimens contained PC. A percentage of approximately 20% marked PC corresponds to the results of our earlier studies [13, 14], while the majority of cases displayed lower degrees of PC (mild PC: 55.9%, moderate PC: 20.3%). Thus, PC turned out as a regular finding in temporal lobe white matter from nonlesional TLE cases. The amount of PC in TLE caused by other pathologies, e.g., tumors, has not been studied so far. No study on PC has been performed in areas other than temporal lobe from TLE patients or using tissue from other epilepsy syndromes. Whether marked PC exists in conditions other than epilepsy is unclear. One study reporting PC from different cerebral diseases [4] did not provide information about the prevalence of epilepsy in their patient groups, leaving open the question whether PC in that series related to the disease or a possibly unrecognized epileptic condition.
Data from normal temporal lobe tissue is scarce. In one study, marked PC was not found in normal autopsy brains [13]. Another study, not applying grading, reported PC as being very rare. At maximum, 3 of 160 (i.e., 1.9%) visual fields examined in each cerebral lobe from autopsy controls showed PC, while occipital tissue was completely negative [4]. However, the exact prevalence of PC in control tissue is not known yet.
To characterize the cellular components of PC, which are so far unknown, several immunohistochemical markers were applied. Variable staining for S-100 protein, galactocerebroside and MBP was seen in both PC and oligodendrocytes of the surrounding white matter, suggesting an oligodendroglial phenotype. While the histological appearance of small round cells with occasional clear cytoplasm is compatible with a neuronal/neurocytic nature, absence of the neuronal markers NeuN, NSE and synaptophysin virtually excludes advanced neuronal differentiation. Perivascular microglia and astrocytes were ruled out by negativity for CD68 and GFAP, respectively. Finally, neural progenitor cells were considered, because they may reside in adult white matter and in perivascular areas [22, 23], and because they may undergo proliferation following experimental seizures and in hippocampal tissue from TLE patients [24, 25]. Negativity for vimentin and nestin in our study argues against neural progenitor cells, because they usually express these intermediate filament proteins [6]. However, it cannot be excluded that PC cells have originated from perivascular stem cells undergoing local proliferation and oligodendroglial differentiation.
We conclude that PC is likely built up by oligodendrocytes. A small fraction of oligodendroglia is located in perivascular areas under physiological conditions [2] and oligodendroglial extensions to vascular walls resembling astroglial end-feet have been described [9]. However, little is known about the functional role of these “perivascular oligodendrocytes”. Further studies are required to resolve the precise origin and function of this particular cellular population in the epileptic and normal brain.
In principle, PC may either represent a dysplastic lesion or it may be a secondary alteration related to epilepsy. In our study, unraveling the pathophysiological basis of PC by means of clinicopathological correlations turned out to be a difficult task, because no significant correlations were revealed. Most previous reports have considered PC as sign of microdysgenesis, implicating a malformative nature [3, 4, 5, 30]. For TLE, this interpretation would match with the maldevelopmental hypothesis of AHS, where a dysplastic nidus for temporal lobe injury by focal insults (e.g., an IPI) has often been postulated [7]. It is conceivable that PC could reflect a focal defect of the blood-brain barrier (BBB), a condition allowing proconvulsive agents to enter the CNS and leading to a focal seizure [29]. In support of a dysplastic pathogenesis, a recent study [4] reported a strong correlation between PC and neuronal migration disorders (NMD): comparing autopsy tissue from several CNS disease groups, PC appeared more frequent in NMD (289 of 950 visual fields, 30%), being rarely found in neurovascular (9 of 400 visual fields, 2.3%) or neurodegenerative diseases (32 of 1400 visual fields, 2.3%) [4]. However, this finding could merely reflect the different prevalence of epilepsy within the disease groups, which is high among NMD [28]. Our data did not show correlations definitely supporting a genetical or maturational susceptibility, i.e., links to positive family history, history of IPI, or an early age at epilepsy onset.
The alternative hypothesis of PC being secondary to epilepsy is also not supported by our data, since direct correlations with both duration of epilepsy and seizure frequencies were absent. Nevertheless, induction by factors other than seizure numbers cannot be excluded. First, the striking perivascular pattern of PC may be related to seizure-related vascular changes. Ultrastructural microvascular alterations, like thickening of the basal lamina and pericyte degeneration, have been found in chronic epileptogenic foci [16].Pronounced alterations of cerebral blood flow occur during seizures and vary remarkably in intensity [27]. Ictal changes occur at the BBB with transient disruption of barrier function [12] and affection of perivascular glia [1]. Second, cells in close relation to cerebral microvessels, e.g., pericytes and glia, form a complex network with CNS endothelium [20], and mediate immunological and inflammatory signals [32]. Thus, perivascular areas seem to represent an interface between central nervous and immune systems [21]. Since immunological signal cascades are activated and modulated by seizures [31], PC could be triggered by some unknown immunological signal. Third, PC could represent a pharmacologically induced tissue change related to anticonvulsant drugs coming via the bloodstream.
In conclusion, the cells constituting PC exhibit oligodendroglial differentiation. Their role in epilepsy needs further investigation. The striking perivascular assembly could point to a pathophysiological basis related to the cerebral vasculature. The question whether PC is a primary (dysplastic) or a secondary (reactive) feature is yet unresolved.
References
Abbott NJ, Khan EU, Rollinson CM, Reichel A, Janigro D, Dombrowski SM, Dobbie MS, Begley DJ (2002) Drug resistance in epilepsy: the role of the blood brain barrier. Novartis Found Symp 243:38–47
Ambrosi G, Virgintino D, Benagiano V, Maiorano E, Bertossi M, Roncali L (1995) Glial cells and blood-brain barrier in human cortex. Ital J Anat Embryol 100 Suppl 1:177–184
Arai N (1999) Cortical dysplasia in surgical pathology of intractable epilepsy. Neuropathology 19:229–232
Arai N, Umitsu R, Komori T, Hayashi M, Kurata K, Nagata J, Tamagawa K, Mizutani T, Oda M, Morimatsu Y (2003) Peculiar form of cerebral microdysgenesis characterized by white matter neurons with perineuronal and perivascular glial satellitosis: a study using a variety of human autopsied brains. Pathol Int 53:345–352
Armstrong DD (1993) The neuropathology of temporal lobe epilepsy. J Neuropathol Exp Neurol 52:433–443
Blümcke I, Schewe JC, Normann S, Brüstle O, Schramm J, Elger CE, Wiestler OD (2001) Increase of nestin-immunoreactive precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus 11:311–321
Blümcke I, Wiestler OD (2002) Ammon’s horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol 12:199–211
Burger PC, Scheithauer BW, Vogel FS (2002) Surgical pathology of the nervous system and ist coverings, 4th edn. Churchill Livingston, New York, pp 397–398
Carpenter MB, Sutin J (1983) Human neuroanatomy, 8th edn. Williams & Wilkins, Baltimore
Choi D, Na DG, Byun HS, Suh YL, Kim SE, Ro DW, Chung IG, Hong SC, Hong SB (1999) White-matter change in mesial temporal sclerosis: correlation of MRI with PET, pathology, and clinical features. Epilepsia 40:1634–1641
Engel J Jr (1993) Surgical treatment of the epilepsies. Raven Press, New York, p 615
Janigro D (1999) Blood-brain barrier, ion homeostasis and epilepsy: possible implications towards the understanding of ketogenic diet mechanisms. Epilepsy Res 37:223–232
Kasper BS, Stefan H, Buchfelder M, Paulus W (1999) Temporal lobe microdysgenesis in epilepsy versus control brains. J Neuropathol Exp Neurol 58:22–28
Kasper BS, Stefan H, Paulus W (2003) Microdysgenesis in mesial temporal lobe epilepsy: a clinicopathological study. Ann Neurol 54:501–506
Komori T, Arai N, Shimizu H, Yagishita A, Mizutani T, Oda M (2002) Cortical perivascular satellitosis in intractable epilepsy; a form of cortical dysplasia? Acta Neuropathol 104:149–154
Liwnicz BH, Leach JL, Yeh HS, Privitera M (1990) Pericyte degeneration and thickening of basement membranes of cerebral microvessels in complex partial seizures: electron microscopic study of surgically removed tissue. Neurosurgery 26:409–420
Mathern GW, Pretorius JK, Babb TL (1995) Influence of the type of initial precipitating injury and at what age it occurs on course and outcome in patients with temporal lobe seizures. J Neurosurg 82:220–227
Meiners LC, vanGils A, Jansen GH, deKort G, Witkamp TD, Ramos LMP, Valk J, Debets RMC, vanHuffelen AC, vanVeelen CWM, Mali WP (1994) Temporal lobe epilepsy: the various appearances of histologically proven mesial temporal sclerosis. AJNR 15:1547–1555
Mitchell LA, Jackson GD, Kalnins RM, Saling MM, Fitt GJ, Ashpole RD, Berkovic SF (1999) Anterior temporal abnormality in temporal lobe epilepsy. A quantitative MRI and histopathological study. Neurology 52:327–336
Neuwelt EA, Abbott NJ, Drewes L, Smith QR, Couraud PO, Chiocca EA, Audus KL, Greig NH, Doolittle ND (1999) Cerebrovascular biology and the various neural barriers: challenges and future directions. Neurosurgery 44:604–609
Pachter JS, Vries HE de, Fabry Z (2003) The blood-brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol 62:593–604
Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH (1999) Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci 19:8487–8497
Palmer TD, Willhoite AR, Gage FH (2000) Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425:479–494
Parent JM (2002) The role of seizure-induced neurogenesis in epileptogenesis and brain repair. Epilepsy Res 50:179–189
Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH (1997) Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 17:3727–3738
Paulus W, Roggendorf W, Kirchner T (1992) Ki-M1P as a marker for microglia and brain macrophages in routinely processed human tissues. Acta Neuropathol 84:538–544
Pereira A, Ferrandon A, Nehlig A (2002) Local cerebral blood flow during lithium-pilocarpin seizures in the developing and adult rat: role of coupling between blood flow and metabolism in the genesis of neuronal damage. J Cereb Blood Flow Metab 22:196–205
Porter BE, Brooks-Kayal A, Golden JA (2002) Disorders of cortical development and epilepsy. Arch Neurol 59:361–365
Remler MP, Marcussen WH (1984) The blood-brain barrier and the systemic convulsant model of epilepsy. Epilepsia 25:574–577
Scaravilli F (ed) (1997) Neuropathology of epilepsy. World Scientific, Singapore, p 206
Vezzani A, Moneta D, Richichi C, Aliprandi M, Burrows SJ, Ravizza T, Perego C, De Simoni MG (2002) Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia 43 Suppl 5:30–35
Williams K, Alvares X, Lackner AA (2001) Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia 36:156–164
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasper, B.S., Paulus, W. Perivascular clustering in temporal lobe epilepsy: oligodendroglial cells of unknown function. Acta Neuropathol 108, 471–475 (2004). https://doi.org/10.1007/s00401-004-0914-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0914-3