Abstract
Background
Ablation of recurrent atrial fibrillation (AF) is common. Studies indicate that AF recurrence is primarily due to pulmonary vein (PV) re-conduction. This retrospective analysis characterized and evaluated recurrent AF patients using focal impulse and rotor mapping (FIRM) plus PV re-isolation, with follow up at 3, 6, 12, and 24 months after the repeat ablation.
Methods and results
Patients (consecutive, n = 100) underwent FIRM-guided ablation followed by conventional PV re-isolation for recurrent AF treatment. All FIRM patients had failed one or more conventional ablation procedures (1.4 ± 0.08) for paroxysmal (14%), persistent (15%), and long-standing persistent (71%) AF. Stable rotors were identified in 97/100 patients: 60% in the right atrium (RA) and 82% in left atrium (LA) (mean 1.5 ± 0.8 and 2.1 ± 1.2 per patient, respectively). No correlation was noted between the previous number of ablations, AF duration, or LA diameter to the number of rotors (R2 = 0.0039, R2 = 0.0017, and R2 = 0.006, respectively). In this limited observation, only 22% of identified rotors were associated with proximity to low voltage areas. The 12- and 24-month arrhythmia free rate was 93% (13/14) and 92% (12/13) for paroxysmal AF, 60% (9/15) and 47% (7/15) for persistent AF, and 70% (48/69) and 64% (43/67) for long-standing persistent AF, respectively, after a single FIRM procedure and re-isolation of the veins.
Conclusions
The data show a benefit for FIRM-guided ablation in recurrent AF at 12 months. No correlation was found between rotors and tissue characterization, AF duration, or previous number of ablations, suggesting that rotors may play an independent role in maintaining recurrent AF after prior failed ablation.
Zusammenfassung
Hintergrund
Rezidive nach vorangegangener Ablation von Vorhofflimmern (VHF) sind häufig. Studien zeigen, dass Vorhofflimmerrezidive in erster Linie auf die erneute Leitungsfähigkeit einer oder mehrerer Pulmonalvenen (PV) zurückzuführen sind. Diese retrospektive Analyse charakterisiert Patienten mit VHF-Rezidiv, die mit fokalem Impuls- und Rotor-Mapping (FIRM) plus PV-Reisolation behandelt wurden. Follow-up-Untersuchungen erfolgten nach 3, 6, 12 und 24 Monaten.
Methoden und Ergebnisse
Insgesamt 100 konsekutive Patienten unterzogen sich einer FIRM-gestützten Ablation, gefolgt von einer konventionellen PV-Reisolation zur Behandlung von rezidivierendem VHF. Alle FIRM-Patienten hatten zuvor eine oder mehrere erfolglose konventionelle Ablationen (1,4 ± 0,08) bei paroxysmalem (14 %), persistierendem (15 %) oder lang persistierendem Vorhofflimmern (71 %) erhalten. Stabile Rotoren wurden bei 97/100 Patienten identifiziert: 60 % im rechten Vorhof (RA) und 82 % im linken Vorhof (LA), durchschnittlich 1,5 ± 0,8 bzw. 2,1 ± 1,2 Rotoren pro Patient. Es wurde keine Korrelation zwischen der vorherigen Anzahl der Ablationen, der Dauer des Vorhofflimmerns oder des LA-Durchmessers und der Anzahl der Rotoren festgestellt (R2 = 0,0039, R2 = 0,0017 bzw. R2 = 0,006). Lediglich 22 % der identifizierten Rotoren befanden sich in der Nähe zu Low-voltage-Arealen. Die Arrhythmiefreiheitsrate nach 12 bzw. 24 Monaten betrug 93 % (13/14) bzw. 92 % (12/13) bei paroxysmalem VHF, 60 % (9/15) bzw. 47 % (7/15) bei persistierendem VHF und 70 % (48/69) bzw. 64 % (43/67) bei lang persistierendem Vorhofflimmern nach einer einzelnen FIRM-gestützten Rotorablation und erneuten PV-Isolation.
Schlussfolgerungen
Unsere Daten zeigen einen Nutzen der FIRM-gestützten Ablation bei rezidivierendem VHF nach 12 Monaten. Es fand sich keine Korrelation zwischen den Rotoren und der Gewebecharakterisierung, der Dauer des VHF oder der vorherigen Anzahl der Ablationen, was darauf hindeutet, dass die Rotoren eine unabhängige Rolle bei der Aufrechterhaltung des rezidivierenden VHF nach einer zuvor fehlgeschlagenen Ablation spielen könnten.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Haissaguerre et al. [1] established the importance of atrial fibrillation (AF) triggers originating from the pulmonary veins. Isolation of the pulmonary veins (PVs) has become the cornerstone of treatment for all stages of AF; however, multiple procedures are often required to significantly reduce AF recurrence [2]. PV reconnection appears to be the main cause of AF recurrence, regardless of the type of energy source used to perform PV isolation [3,4,5]. For persistent and long-standing persistent AF, a stepwise approach was adopted to address the recurrence rate [6]. The need for an improved and easy ablation strategy for non-paroxysmal AF remains, particularly following the STAR-AF 2 trial, which reported a lack of long-term benefit associated with additional substrate ablation [7].
Arbelo et al. [8] reported the results of a multicenter European trial, with 21% of patients being readmitted for AF recurrences. The majority (18%) required a second procedure during 12-month follow-up. Furthermore, the same study reported that 50% of the ablated AF patients became asymptomatic, indicating an underestimation of the number of recurrences. Those findings, along with that of many other publications, highlight the need for a better individualized procedure that will provide improved long-term outcomes. Recent studies have identified the benefit of rotor ablation in conjunction with PV isolation (PVI) to improve long-term outcome [9,10,11,12,13,14,15,16]. This new technique has been criticized by many who have found the limitation of this technology to be overwhelming [17, 18]. In two recently published single-center studies [19, 20] reporting outcomes for recurrent AF patients, the main findings were that focal impulse and rotor mapping (FIRM) did not increase freedom from atrial arrhythmias in comparison to their controls. A better understanding of the mechanisms of rotors in AF is provided by optical mapping at the cellular level. Animal studies provide evidence for rotor existence [21, 22], suggesting that this is the mechanism responsible for sustained AF. The presence of rotors in isolated human hearts, as revealed by optical mapping, has shown that AF is driven by spatially and temporally stable rotational drivers anchored to micro-anatomic filaments of fibrotic tissue, and that ablation of those rotors terminated the arrhythmia [22].
The authors report the results of FIRM ablation in a recurrent AF patient population, in combination with re-isolation of the conducting PVs. They analyzed the correlation of rotors to AF duration, LA diameter, areas of low voltage, and the number of previous ablations. Based on this analysis, they discuss the possible association between rotor ablation and AF clinical outcome. To the best of their knowledge, this is the first study reporting outcomes at 24-month follow-up relating to rotor ablation in patients with recurrent AF.
Methods
Between June 2014 and September 2017, 100 consecutive patients referred for repeat catheter ablation of paroxysmal, persistent, and long-standing persistent AF (as defined by their initial state prior to their first ablation) were enrolled. The study was approved by the Institutional Review Board (IRB). All participants received a full explanation regarding the study aims and procedures and signed a written informed consent form. Patients with recurrent AF underwent FIRM-guided rotor ablation in both atrial chambers, followed by PV re-isolation.
Procedural details
Recurrence of AF was diagnosed by at least one documented electrocardiogram (ECG), during the 3 months prior to inclusion. All anti-arrhythmic medications were discontinued >five half-lives prior to ablation, except for amiodarone, which was discontinued at least 30 days prior to the procedure. All patients underwent light sedation for the duration of the procedure. Rotor mapping was performed during AF (in 73 patients) or induced AF (27 patients) with a similar induction protocol as previously described [23]. Rotor mapping was conducted by a 64-electrode basket catheter (FIRMap, Abbott, Minneapolis, MN, USA) placed successively in multiple positions for each atrium to record unipolar electrograms. The basket catheter size (50, 60, or 70 mm) was selected based on LA diameter as measured by transesophageal echocardiography (TEE), transthoracic echocardiography (TTE), and/or computerized tomography (CT). In all cases, basket coverage was confirmed by fluoroscopy, electrogram detection on an electrophysiological (EP) recording system, and visualization in a three-dimensional (3D) mapping system (Ensite NavX Precision or Velocity, Abbott) [23]. Rotational activity was identified using the RhythmView system and software (Abbott).
AF propagation maps from unipolar recordings were projected onto two-dimensional (2D) grids referenced to atrial anatomy on electroanatomic shells created using Ensite NavX Precision or Velocity. Radiofrequency (RF) ablation was performed using an irrigated 3.5-mm catheter (Tacticath, Abbott) or an irrigated 4‑mm catheter (Therapy Cool Flex, Abbott) around the identified rotational area. The RF energy was delivered at 30 W with a maximum temperature of 45 °C. In certain LA areas (e.g., posterior wall), ablation energy was reduced to 25 W. Rotors were targeted for ablation based on rotational activity as identified by an algorithm indicating the rotational activity profile (RAP) with a repetitive, spatially stable rotational pattern from the default 4‑s time segment (RhythmView Ver. 4.2 and 5.0; Abbott), or by analysis of the entire 60‑s recording (15 segments of 4 s each) using a stability map (RhythmView Ver. 6.0 and 6.1, Abbott). Rotor mapping and ablation were repeated until complete elimination of rotors was confirmed by re-mapping.
If AF persisted following complete rotor elimination, cardioversion was undertaken to restore sinus rhythm. Conventional ablation for re-isolation of the conducting PVs was then performed for all patients (including the control group). Re-isolation was verified (entrance and exit block) using a circular mapping catheter (Inquiry Optima, Abbott). Finally, additional ablation of relevant atrial tachycardias (AT) was performed as needed.
Voltage maps were created during sinus rhythm, either following ablation-mediated restoration to sinus rhythm or cardioversion, prior to re-isolation of the PVs, in most patients. Using a circular mapping catheter, bipolar voltages <0.5 mV considered as low voltage areas were mapped. The low voltage areas were estimated and patients were then divided into three categories: low (less than 25% of chamber surface), medium (above 25% but less than 75%), and high (>75% low voltage area).
Long-term clinical follow-up
Follow-ups were guided by the Heart Rhythm Society (HRS)/European Heart Rhythm Association (EHRA)/European Cardiac Arrhythmia Society (ECAS) consensus documents [2]. An ambulatory monitoring ECG was performed at 3, 6, 12, and 24 months with 48‑h ECG. Recurrence was defined as any ECG recording of AF or AT lasting >30 s.
Endpoints
The primary study effectiveness endpoint for success was defined as freedom from AF at 3, 6, 12, and 24 months after ablation. Previously ineffective antiarrhythmic drugs at the same/lower dose were considered to be treatment failures. A spontaneously self-terminating recurrences without intervention (electrical cardioversion or ablation) was also considered as treatment failure.
Statistical analysis
Frequencies and proportions were used to describe categorical variables, while means and standard deviations were used to describe continuous variables. Pearson correlation coefficients were used to assess the relationship between numbers of rotors and AF duration and number of rotors and LA diameter. Spearman correlation coefficient was used to assess the relationship between previous number of ablations and AF freedom.
Results
Patient characteristics
This study included 100 recurrent AF patients undergoing FIRM-guided rotor ablation. Mean patient age was 62.2 ± 9.2 years; most patients were male (70%). Patients had paroxysmal (14%), persistent (15%), and long-standing persistent (71%) AF. All patients had previously failed at least one or more PVI ablations (mean 1.4 ± 0.8 per patient). AF duration was 4.4 ± 3.4 years (range 1–18 years), and the mean CHA2DS2-VASc score was 2.2 ± 1.4. The control group included 50 patients undergoing re-isolation of PVs only and had similar characteristics to our FIRM group. Patient characteristics are further detailed in Table 1.
Procedural details
Procedural details are summarized in Table 2. Reconnection of at least one pulmonary vein occurred in 99 of 100 patients. A total of 263 reconnections were made in the PVs: 76, right inferior; 64, right superior; 55, left inferior; 58, left superior; and 10, left common. Re-isolation of the PV required on average 44 ± 19 RF applications with an average single ablation time of 19.1 ± 13.6 s.
Total procedure duration averaged 199 ± 41 min, with FIRM-guided ablation taking approximately 61% of that time. Much attention was given to the identification of rotors and their ablation including several remappings for rotor elimination.
The FIRM catheter was selected based on LA diameter, either 50 mm (47%) or 60 mm (51%); a 70-mm basket was used in only two patients (2%). Stable rotors were identified in 97% of patients, with a mean of 2.7 ± 1.4 per patient. Right atrial (RA) rotors were present in 60% (60/100) of patients (mean 1.5 ± 0.8 in patients with RA rotors; range, 1–4), and LA rotors were present in 82% (82/100) of patients (mean 2.1 ± 1.2 in patients with LA rotors; range, 1–5). Rotors were found in both atria of 45 patients.
Rotors were successfully ablated, as confirmed by repeat FIRM mapping. The mean ablation time to terminate each rotor was 352 s (5.9 min). Termination of AF was observed in 6% (6/100) of patients and cycle length prolongation greater than 10% was observed in 17% of patients. No AT was noticed during the procedures. Complications occurred in 6% of patients (6/100): two pericardial effusions during PVI requiring pericardiocentesis, two femoral pseudoaneurysms requiring surgical treatment, one pulmonary embolism, and one atrioventricular block. No complications occurred in relation to the basket catheter.
Long-term clinical outcomes
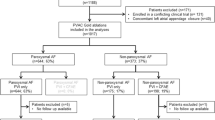
A total of 98 patients completed 12-month and 95 patients completed 24-month follow-up. Fig. 1 displays AF recurrence at 3-, 6‑, 12-, and 24-month follow-up. At 12 months, patients were AF free as follows: paroxysmal group, 92.9% (13/14); persistent group, 60.0% (9/15); and long-standing persistent group, 69.6% (48/69). At 24-month follow-up, the paroxysmal (12/13), persistent (7/15), and long-standing persistent (43/67) groups were 92.3%, 46.7%, and 64.2% free of AF, respectively. Fig. 1 shows that, for all paroxysmal and long-standing persistent AF categories, following the initial fall in AF freedom at 3 months, patients had similar curve decays at 6, 12, and 24 months. Only the persistent AF group continued to fall to <60%.
Follow-up of recurrent atrial fibrillation (AF) patients at 3 and 6 months: Paroxysmal (n = 14), Persistent (n = 15), and Long Persistent (n = 71). At 12 months: n = 14, n = 15, and n = 69, respectively, while at 24 months: n = 13, n = 15, and n = 67, respectively. (Two and five patients were lost to follow-up at 12 and 24 months, respectively)
Characterization of recurrent AF patients
Number of rotors and AF duration
Analysis was performed to determine the correlation between AF duration and the number of identified rotors. Fig. 2 shows the numbers of rotors either in the RA or LA vs the duration of AF in all recurrent patients. Note the very low Pearson correlation coefficient (R2 = 0.0169) and that the number of rotors did not increase with longer AF duration in this cohort of patients.
Rotor proximity to low voltage areas
Low voltage areas were measured at the end of each procedure in sinus rhythm, as described above. The proximity of identified rotors to the low voltage areas was checked in 68 patient maps (nine did not have low voltage areas, and voltage mapping was not performed in the other 23). Much caution was taken during the voltage mapping to delineate the locations of the rotor ablation, so that it would not be included in the analysis of low voltage areas. Fig. 3 presents some examples of this analysis. Note that whether or not low voltage areas were present, rotors were identified prior to voltage mapping. This was taken into consideration when analyzing 5‑mm proximity to previously ablated rotors. However, only 22% of the maps had rotors near the low voltage areas.
Location of rotors in relationship to low voltages areas in the left atria. Only 22% of rotors are in close proximity to low voltage areas (a) the rest are not (b,c). (b) depicts one rotor (orange dots) in proximity, while the other two rotors (green and blue dots) are not. (c) shows rotor identification in an area without any low voltages
Low voltage zone and AF freedom at 12 months
To quantify the contribution of low voltage areas to the clinical outcome, low voltage maps were measured for scar area with a measurable scale, out of the total surface area of the LA. A scale of low, medium, and high was defined, as described in the “Methods” section.
Previously ablated rotor locations were not taken into consideration (ablated area of rotor subtracted from low voltage area). As can been seen in Fig. 4, the highest AF-free rate was observed in the “low” category, while the lowest rate of AF freedom was noted in the “high” low voltage category.
Number of previous ablations and 12-month and 24-month outcomes
Fig. 5 presents the correlation between the number of previous ablations and the number of AF-free patients at 12-month follow-up. The 12-month AF-free rates were 65.2% for Group 1 patients with a previous single ablation (n = 66), 81.5% for Group 2 with two prior ablations (n = 27), and 83.3% for Group 3 with three or more previous ablations (n = 6). Spearman correlation analysis between the previous number of ablations and clinical outcome at 12 months following rotors ablation resulted in a coefficient of rs = −0.5. At 24 months, the numbers were very similar given the comparable outcome results.
Number of previous ablations correlating to 12-month follow-up of patients that were atrial fibrillation (AF)-free and in sinus rhythm (%, actual results within columns). Group 1: One previous ablation; Group 2: two previous ablations; Group 3, three or more previous ablations Spearman correlation coefficient rs = −0.5. Freedom from AF at 24-month follow-up did not change significantly
LA diameter and number of rotors in the LA
LA diameter was measured by the longitudinal axis and associated with the number of rotors in the LA. For analysis, several imaging modalities were used to measure the LA diameter, TTE, TEE, and preacquired CT. Fig. 6 shows a diameter range of 33–69 mm with TTE, 44–65 mm with TEE, and 44–74 mm on CT. The number of rotors ranged between one and five. Pearson correlation (R2 = 0.006) was very low, indicating that the number of rotors is not correlated to LA size in this cohort of patients.
Discussion
The present study reveals several important findings in recurrent AF patients undergoing repeat ablation. Repeat ablation procedures that involve re-isolation of the PVs in addition to rotor mapping and ablation provide a reasonable outcome at 12 and 24 months, but not a statistically different one from the control group at 12 months. LA diameter and AF duration did not correlate with the number of identified rotors in this patient population. The number of rotors did not differ significantly between the recurrent paroxysmal, persistent, or long-standing persistent AF patients. Furthermore, rotor elimination in recurrent AF patients had a similar outcome for both single and multiple prior procedures. Lastly, the proximity of rotors to scar areas was found to be limited, as previously reported [21].
Long-term clinical outcome
AF is a challenging arrhythmia and multiple catheter-based strategies have evolved. However, there is currently no general acceptance of a single method. The present study shows that adding rotor ablation to PV re-isolation has a long-term AF-free outcome of approximately 65%. In a recent report by Barakat et al. [3], the 1‑year outcome of repeat ablation with and without antiarrhythmic drugs was inferior, with only 41.5% and 14.5% of patients being arrhythmia-free, respectively. The authors’ outcome results are in agreement with most single-center experiences in ablating de-novo persistent AF patients guided by FIRM mapping. In those studies, long-term AF-free outcome ranged from 70% to 80% [9,10,11,12,13,14,15,16]. Conversely, in a recent single-center study of long-term outcome in a limited number of patients (n = 21), the authors report a much lower success rate in recurrent AF patients treated with FIRM and PVI (33.3%) [19]. Henley et al. [20] completed 16-month follow-up on recurrent AF patients, re-ablated guided by FIRM and PV re-isolation. They report freedom from AF and atrial tachycardias in paroxysmal patients (n = 18) at 83.3% and 40.7% for the persistent patients (n = 27). Henley et al.’s results do not differ from the authors’ results in the paroxysmal patients, but are much lower in the persistent group. Another recurrent AF patient study treated by cryoballoon technology and re-ablated with RF reported outcomes of 55% at 2‑year follow-up [4]. The multicenter study conducted in 10 European countries with 72 centers reported a very low outcome, with AT as one of the main reasons for recurrence. Only 30.2% of persistent AF patients were arrhythmia-free at 12-month follow-up [8]. Pappone et al. [24] reported another mapping and ablating technique aiming at individualized treatment for persistent AF patients, called repetitive regular activities (RRas). They reported a 73.2% arrhythmia-free rate in patients ablated with this technique. Careful interpretation suggests similarities in the findings between rotors and RRAs (short CL [cycle length], organized activity, and a limited amount of areas of interest). While the acceptance of rotor ablation is still controversial [17, 18], several groups are trying to identify the alleged drivers or triggers sustaining AF [24, 25]. Although durable and sustained isolation of PVs are desirable, Winkle et al. [26] recommend that additional repeat ablation should be performed in all AF patients to increase long-term success rates. The authors hypothesize that additional ablation of drivers/rotors/triggers may be needed to develop an individualized ablation strategy in AF patients.
Rotors and substrate characterization
Several studies in human isolated hearts [22, 27] and several clinical studies [28, 29] have attempted to describe the correlation of rotors to atrial substrates. In explanted isolated human hearts, the ability to utilize voltage sensitive dyes and powerful non-clinical magnetic resonance imaging (MRI; 9T) suggested that rotors (reentry) were anchored to micro-filaments of fibrotic tissue [22]. In the present study, the voltage mapping showed rotors within 5 mm distance from low voltage areas in only 22% of the cases. The differences between intramural scars revealed by high-intensity MRI, which is not clinically available, versus endocardial voltage mapping may explain the relative lack of spatial correlation between rotors and substrate. Examining rotors identified from the body surface, Cochet et al. reported a good correlation with late gadolinium enhanced (LGE) MRI (1.5T) [28]. In their study, the number of re-entrant regions during AF related to the extent zone of LGE findings, with re-entry occurring at the LGE zone borders. Notably, the authors did not perform LGE, and it is unclear whether their low voltage regions would have been abnormal on LGE. Nevertheless, it remains unclear why body surface mapping (of the epicardium) may differ from endocardial mapping. The DECAAF study [30] concluded that atrial tissue fibrosis measured by LGE independently associates with recurrent arrhythmia after AF ablation, in agreement with other findings of poor correlation of low voltage zones to AF type [29, 31]. Given the lack of a gold standard for LGE, these findings require validation.
Another interesting topic of in the present study was the low number of identified rotors (2.7 per patient) in comparison to other studies reporting on average more than five rotors (re-entrant activity) per patient [9, 28]. The authors’ recurrent AF patients had undergone at least one previous ablation, targeting the PVs. It has been well described that about 40% of rotors/reentrant activities are identified near the PVs [28, 32]. While performing the initial PV ablation, the chance of eliminating those rotors is high and may reduce the number of identified rotors in recurring patients, according to the present study. Furthermore, Fig. 2 displays the relationship between AF duration in those recurrent AF patients and the number of rotors found. A high correlation of more rotors in the persistent population vs paroxysmal patients has been previously described [33]. However, as stated above, the previous ablation may have eliminated the rotors around the PVs, obliterating the relationship between rotors and AF duration. A similar explanation may be provided for the findings of LA size and rotors in the present study. The LA diameter varied between 33 and 74 mm and was uncorrelated to number of identified rotors. The effect of repeat ablation on LA diameter was investigated by Montserrat et al. [34], who reported that reduction in LA size after initial ablation did not change with repeated procedures. However, additional ablation lines in the LA in repeated procedures may reduce LA size even more [35].
The correlation between the previous number of ablations and clinical outcome in our study was also low. This suggests that targeting the correct sources (rotors) at repeat ablation will increase the probability of the patient remaining arrhythmia-free. This is very much in line with the findings of Ammar et al. [35], who suggested that initial substrate modification, as well as substrate modification during a repeat procedure, do not lead to significantly better results. In other words, nonspecific ablation of substrate does not benefit patients even in repeat procedures.
Clinical implication of rotor ablation in recurrent AF patients
Many studies of recurrent AF report ATs as a leading cause of recurrence [35, 36]. These findings [35] raise the need for a better understanding of the mechanism of such ATs. Those ATs are not always mapped in recurring patients, and may potentially be confused with focal non-PV triggers or micro-reentries. Rotational activity and rotors are not identified by sequential mapping and may be documented in such cases as ATs or repetitive local electrograms. Regardless of the definition, ablation of individualized areas in recurring AF patients may provide improved long-term outcome.
Limitations
This was a single-center retrospective, non-randomized study in a limited recurrent AF population. Rotor identification was limited to basket electrode coverage of the mapped chamber, as well as individual contact of the electrodes to the atrial tissue, although multiple basket positions were used per atrium. Nevertheless, such limitations may have reduced the number of identified rotors affecting the analysis and the outcome. An additional limitation in acute termination to sinus rhythm (only 6% in this study) may be explained by the inability to precisely locate the core of the rotational activity. A low voltage substrate map was created using multiple sequential acquisitions at each location during sinus rhythm not representing the true substrate during arrhythmia. Arrhythmia recurrence may have been underestimated by 48‑h ECG Holter monitoring, despite compliance with recommended guidelines [2]. However, the authors’ confidence in the results are encouraged by other studies reporting similar outcomes after ablating the rotors during the initial procedure.
Conclusions
The data here show similar benefit of FIRM-guided ablation for recurrent AF at 12 months. No correlation was found between rotors and tissue characterization, AF duration, or previous number of ablations, suggesting that rotors may play an independent role in maintaining recurrent AF after prior failed ablation.
Larger multicenter randomized studies will be required to confirm the findings. A prospective randomized controlled trial of FIRM-guided ablation in redo procedures is underway: ClinicalTrials.gov (NCT02799043).
References
Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clemente J (1998) Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. n Engl J Med 339(10):659–666. https://doi.org/10.1056/NEJM199809033391003
Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D (2017) 2017HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 14:528–606. https://doi.org/10.1093/europace/eus027
Barakat AF, Wazni OM, Saliba WI, Yzeiraj E, Amuthan R, Rehman KA, Tarakji KG, Bassiouny M, Baranowski B, Tchou P, Bhargava M, Dresing T, Callahan T, Cantillon DD, Kanj M, Chung M, Lindsay BD, Hussein AA (2018) Repeat ablation or medical management alone for recurrent arrhythmias after ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol 29:551–558. https://doi.org/10.1111/jce.13434
Conte G, Chierchia JB, Sieira J, Levinstein M, Casado-Arroyo R, De Asmundis C, Sarkozy A, Rodriguez-Manero M, Di Giovanni G, Baltogiannis G, Wauters K, Brugada P (2013) Repeat procedure using radiofrequency energy for recurrence of atrial fibrillation after initial cryoballoon ablation: a 2-year follow-up. Europace 15:1421–1425. https://doi.org/10.1093/europace/eut098
Lin D, Santangeli P, Zado ES, Bala R, Hutchinson MD, Riley MP, Franel DS, Garcia F, Dixit S, Callans D, Marchilinski FE (2015) Electrophysiologic findings and long-term outcomes in patients undergoing third or more catheter ablation procedures for atrial fibrillation. J Cardiovasc Electrophysiol 26:371–377. https://doi.org/10.1111/jce.12603
Schreiber D, Rostock T, Frohlich M, Sultan A, Servatius H, Hoffmann BA, Luker J, Berner I, Schaffer B, Wegscheider K, Lezius S, Willems S, Steven D (2015) Five-year follow-up after catheter ablation of persistent atrial fibrillation using the stepwise approach and prognostic factors for success. Circ Arrhythm Electrophysiol 8:308–317. https://doi.org/10.1161/CIRCEP.114.001672
Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P, STAR AF II Investigators (2015) Approaches to catheter ablation for persistent atrial fibrillation. n Engl J Med 372:1812–1822. https://doi.org/10.1056/NEJMoa1408288
Arbelo E, Brugada J, Hindricks G, Maggioni AP, Tavazzi L, Vardas P, Laroche C, Anselme F, Inama G, Jais P, Kalarus Z, Kautzner J, Lewalter T, Mairesse GH, Perez-Villacastin J, Riahi S, Taborsky M, Theodorakis G, Trines SA, Atrial Fibrillation Ablation Pilot Study Investigators (2014) The atrial fibrillation ablation pilot study: A European survey on methodology and results of catheter ablation for atrial fibrillation conducted by the Eur Heart Rhythm Association. Eur Heart J 35:1466–1478. https://doi.org/10.1093/eurheartj/ehu001
Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM (2012) Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 60:628–636. https://doi.org/10.1016/j.jacc.2012.05.022
Tomassoni G, Duggal S, Muir M, Hutchins L, Turner K, McLoney AM, Hesselson A (2015) Long-term follow-up of FIRM-guided ablation of atrial fibrillation: a single-center experience. J Innov Cardiac Rhythm Manag 6:2145–2151
Rashid H, Sweeney A (2015) Approaches for focal impulse and rotor mapping in complex patients: a US private practice experience. J Innov Cardiac Rhythm Manag 6:2193–2198
Sommer P, Kircher S, Rolf S, John S, Arya A, Dinov B, Richter S, Bollmann A, Hindricks G (2016) Successful catheter ablation of recurrent longstanding persistent atrial fibrillation with rotor elimination as the procedural endpoint: a case series. J Cardiovasc Electrophysiol 27:274–280. https://doi.org/10.1111/jce.12874
Miller JM, Karla V, Das MK, Jain R, Garlie J, Brewster J, Dandamudi G (2017) Clinical benefit of ablating localized sources for human atrial fibrillation—The Indiana University FIRM registry. J Am Coll Cardiol 69:1247–1256. https://doi.org/10.1016/j.jacc.2016.11.079
Tilz RR, Lin T, Rillig A, Heeger CH, Scholz A, Wohluth P, Bucur T, Metzner A, Mathew S, Wissner E, Ouyang F, Kuck KH (2017) Focal impulse and rotor modulation for the treatment of atrial fibrillation: locations and 1 year outcomes of human rotors identified using a 64-electrode basket catheter. J Cardiovasc Electrophysiol 28:367–374. https://doi.org/10.1111/jce.13157
Kis Z, Theuns DA, Bhagwandien R, Wijchers S, Yap SC, Szili-Torok T (2017) Type and rate of atrial fibrillation termination due to rotational activity ablation combined with pulmonary vein isolation. J Cardiovasc Electrophysiol 28:862–869. https://doi.org/10.1111/jce.13240
Brachmann J, Hummel JD, Wilber DJ, Sarver AE, Rapkin J, Shpun S, Szili-Torok T (2019) Prosepctive randomization comparison of rotor ablation vs conventional ablation for treatment of persistent atrial fibrillation—The REAFFIRM Trial. LBCT01-01. Presented at: Heart Rhythm Society Annual Scientific Sessions; May 8–11, 2019; San Francisco. https://www.abstractsonline.com/pp8/#!/5753/presentation/31210. Accessed 8 Oct 2020
Hummel JD (2016) Atrial mapping with basket catheters—a basket case? JACC Clin Electrophysiol 2:66–68. https://doi.org/10.1016/j.jacep.2015.12.006
Mohanty S, Mohanty P, Triveti C, Gianni C, Rocca DDG, Di Biase L, Natale A (2018) Long-term outcome of pulmonary vein isolation with and without focal impulse and rotor modulation mapping insights from a meta-analysis. Circ Arrhythm Electrophysiol 11:e5789. https://doi.org/10.1161/CIRCEP.117.005789
Peigh G, Wasswrlauf J, Kaplan RM, Amaral AP, Trivedi A, Chicos AB, Arora R, Kim S, Lin A, Verma N, Knight BP, Passman RS (2020) Repeat pulmonary vein isolation with or without FIRM-guided ablation for recurrent atrial fibrillation ablation with pulmonary vein reconnection. J Cardiovasc Electrophysiol 31:1031–1037. https://doi.org/10.1111/jce.14426
Henley P, Foreman JR, Padanilam BJ, Nair GV, Olson JA, Joshi S, Aziz Z, Foster T, Prystowsky EN (2019) Single-center experience of the FIRM technique to ablate paroxysmal and persistent atrial fibrillation. J Cardiovasc Electrophysiol 30:493–502. https://doi.org/10.1111/jce.13832
Vaquero M, Calvo D, Jalife J (2008) Cardiac fibrillation: from ion channels to rotors in the human heart. Heart Rhythm 5:872–879. https://doi.org/10.1016/j.hrthm.2008.02.034
Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, Simonetti OP, Higgins R, Killic A, Mohler PJ, Janssen P, Weiss R, Hummel JD, Fedorov VV (2015) Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J 36:2390–2401. https://doi.org/10.1093/eurheartj/ehv233
Spitzer SG, Karoly L, Rammler C, Scharfe F, Weinmann T, Zieschank M, Langbein A (2017) Treatment of recurrent nonparoxysmal atrial fibrillation using focal impulse and rotor mapping (FIRM)-guided rotor ablation: early recurrence and long-term outcomes. J Cardiovasc Electrophysiol 28:31–38. https://doi.org/10.1111/jce.13110
Pappone C, Ciconte G, Vicedomini G, Mangual JO, Li W, Conti M, Giannelli L, Lipartiti F, McSpadden L, Ryu K, Guazzi M, Menicanti L, Santinelli V (2018) Clinical outcome of electrophysiologically guided ablation for nonparoxysmal atrial fibrillation using a novel real-time 3‑dimensional mapping technique results from a prospective randomized trial. Circ Arrhythm Electrophysiol 11:e5904. https://doi.org/10.1161/CIRCEP.117.005904
Salinet J, Schlindwein FS, Stafford P, Almeida TP, Li X, Vanheusden FJ, Guillem MS, Ng GA (2017) Propagation of meandering rotors surrounded by areas of high dominant frequency in persistent atrial fibrillation. Heart Rhythm 14:1269–1278. https://doi.org/10.1016/j.hrthm.2017.04.031
Winkle RA, Mead RH, Engel G, Patrawala RA (2011) Long-term results of atrial fibrillation ablation: the importance of all initial ablation failures undergoing a repeat ablation. Am Heart J 162:193–200. https://doi.org/10.1016/j.ahj.2011.04.013
Fedorov VV, Hansen BJ (2018) A secret marriage between fibrosis and atrial fibrillation drivers. JACC Clin Electrophysiol 4(1):30–32. https://doi.org/10.1016/j.jacep.2017.09.176
Cochet H, Dubois R, Yamashita S, Al Jefairi N, Berte B, Sellal JM, Hooks D, Frontera A, Amraoui S, Zemoura A, Denis A, Derval N, Sacher F, Corneloup O, Latrabe V, Clement-Guinaudeau S, Relan J, Zahid S, Boyle PM, Trayanova NA, Bernus O, Montaudon M, Laurent F, Hocini M, Haissaguerre M, Jais P (2018) Relationship between fibrosis detected on late gadolinium-enhanced cardiac magnetic resonance and re-entrant activity assessed with electrocardiographic imaging in human persistent atrial Fibrillation. JACC Clin Electrophysiol 4(1):17–29. https://doi.org/10.1016/j.jacep.2017.07.019
Zaman JAB, Baykaner T, Clopton P, Swarup V, Kowal RC, Daubert JP, Day JD, Hummel J, Schricker AA, Krummen DE, Mansour M, Tomassoni GF, Wheelan KR, Vishwanathan M, Park S, Wang PJ, Narayan SM, Miller JM (2017) Recurrent post-ablation paroxysmal atrial fibrillation shares substrates with persistent atrial fibrillation an 11-center study. JACC Clin Electrophysiol 3:393–402. https://doi.org/10.1016/j.jacep.2016.10.006
Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duyschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J (2014) Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 311:498–506. https://doi.org/10.1001/jama.2014.3
Nery PB, Al Dawood W, Nair GM, Redpath CJ, Sadek MM, Chen L, Green MS, Wells G, Birnie DH (2018) Characterization of low voltage areas in patients with atrial fibrillation: insights from high-density intracardiac mapping. Can J Cardiol. https://doi.org/10.1016/j.cjca.2018.04.008
Miller JM, Kowal RC, Swarup V, Kaubert JP, Daoud EG, Day JD, Ellenbogen KA, Hummel JD, Baykaner T, Krummen DE, Narayan SM, Reddy V, Shivkumar K, Steinberg JS, Wheelan KR (2014) Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol 25:921–929. https://doi.org/10.1111/jce
Narayan SM, Krummen DE, Rappel WJ (2012) Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J Cardiovasc Electrophysiol 23(5):447–454. https://doi.org/10.1111/j.1540-8167.2012.02332.x
Montserrat S, Sitges M, Calvo N, Silva E, Tamborero D, Vidal B, Berruezo A, Bernado C, Mont L, Brugada J (2011) Effect of repeated radiofrequency catheter ablation on left atrial function for the treatment of atrial fibrillation. am J Cardiol 108:1741–1746. https://doi.org/10.1016/j.amjcard.2011.07.041
Ammar S, Hessling G, Reents T, Fichtner S, Wu J, Zhu P, Kathan S, Estner LH, Jilek C, Kolb C, Haller B, Deisenhofer I (2011) Arrhythmia type after persistent atrial fibrillation ablation predicts success of the repeat procedure. Circ Arrhythm Electrophysiol 4:609–614. https://doi.org/10.1161/CIRCEP.111.963256
Ammar-Busch S, Kaess BM, Bruhm A, Reents T, Bourier F, Buiatti A, Semmler V, Telishevska M, Kottmaier M, Hessling G, Deisenhofer I (2015) Atrial tachycardias following persistent atrial fibrillation ablation: predictors of recurrence after the repeat ablation. J Cardiovasc Electrophysiol 26:1315–1320. https://doi.org/10.1111/jce.12817
Acknowledgements
The authors are grateful to Shlomo Shpun, D.Sc., for help with drafting an earlier version of the manuscript and for very helpful comments and support in finalizing the manuscript.
Author information
Authors and Affiliations
Contributions
SGS devised the project and the main conceptual ideas. AL worked out the technical details. CR and MZ analyzed the data. SGS, LK, and AL contributed to the interpretation of the results. SGS and AL took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Corresponding author
Ethics declarations
Conflict of interest
S.G. Spitzer has received speaker fees and support from Medtronic, St. Jude Medical, Abbott, and Topera, and participated in clinical trials for Abbott and Medtronic. L. Károlyi has received speaker fees from Abbott and participated in clinical trials for Abbott and Medtronic. A. Langbein has received speaker fees and travel support from Medtronic, Abbott, and Boston Scientific, and participated in clinical trials for Abbott and Medtronic. C. Rämmler and M. Zieschank declare that they have no competing interests.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Spitzer, S.G., Károlyi, L., Rämmler, C. et al. Retrospective analysis of FIRM-guided ablation in patients with recurrent atrial fibrillation: a single-center study. Herzschr Elektrophys 31, 417–425 (2020). https://doi.org/10.1007/s00399-020-00724-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00399-020-00724-5