Abstract
Catheter ablation has been shown to be an effective treatment for rhythm stabilization in patients with multiple ventricular arrhythmia episodes called electrical storm (ES). These procedures may be complex and are usually only performed in highly specialized and experienced centers. Still the optimum timing for catheter ablation in ES remains unclear.
Early access to perform acute ablation should be considered in patients who are not rhythm stabilized with antiarrhythmic medical treatment. Also patients with hemodynamic compromise (cardiogenic shock) are candidates for an early interventional strategy. In specialized centers it is consensus to perform catheter ablation in these patients as early as eligible especially when considering a high early and late mortality without interventional management. Establishing a structured protocol for treatment and admission to EP centers has helped to further reduce pre-ablation mortality and may optimize treatment of ES. Large scale networking to optimize and structure access to experienced electrophysiology centers is of importance to create a basis for optimizing treatment strategies.
Zusammenfassung
Die Katheterablation bei Patienten mit gehäuften anhaltenden ventrikulären Arrhythmien (elektrischer Sturm, ES) hat sich als effektive Therapie herausgestellt, ein möglichst frühzeitiger Zugang zur Ablation erscheint gerade vor dem Hintergrund der hohen Spätmortalität und Rezidivraten sinnvoll. Der optimale Zeitpunkt für diese komplexe Prozedur ist allerdings noch unklar. Als komplexe Ablationsprozedur wird die Ablation bei ES nur in wenigen erfahrenen Zentren mit hoher Expertise durchgeführt. Initiale Versuche einer frühzeitigen Verlegung dieser Patienten in diese Zentren durch zuweisende Kliniken hat zu einer Verbesserung der Versorgung von Patienten mit ES geführt. Man kann ein frühzeitiges elektives Ablationsvorgehen noch innerhalb des stationären Erstaufenthalts nach Auftreten eines ES nach Rhythmusstabilisierung von einer Akut-Ablation, durchgeführt zur Rhythmusstabilisierung, unterscheiden.
Innerhalb einer Klinik sollte eine Stufenplan zur Festlegung der Behandlungsstrategie erarbeitet werden. Aktuell wird in einem überregionalen Netzwerk von interventionellen elektrophysiologischen Zentren in Bayern ein Etappen-Therapie-Plan entwickelt, der unter anderem auch die Katheterablation als essentiellen Bestandteil beinhaltet. Im Rahmen der einzelnen teilnehmenden Zentren ist eine optimierte Versorgung der ES-Patienten mittels invasiver kardiologischer Therapieoptionen inklusive der interventionellen Elektrophysiologie initiiert. Somit wird versucht, flächendeckend diesen Patienten ein frühzeitiger Zugang zur Katheterablation zu ermöglichen.
Insgesamt erscheint somit ein Netzwerk kooperierender Kliniken zur Optimierung und Strukturierung des Zugang zu erfahrenen elektrophysiologischen Zentren entscheidend als Basis einer optimierten Therapiestrategie.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ablation in patients with electrical storm (ES) has recently been proposed as an effective option to prevent recurrences of ventricular arrhythmia and ES [1–3]. In addition, catheter ablation appears superior to conservative management and may exert prognostic effects in regard to patient survival [4]. Management of ES—as indicated in this issue of the journal—may not only involve intensified or new-onset medical therapy, administration of sedative or anesthetic agents but also invasive treatment options like hemodynamic device-based support, complex coronary interventions or even surgically-based measures.
So far, the optimum time-point for the ablation procedure remains unclear. In a study by Carbucicchio et al. [1] approximately 50 % of patients were ablated within 24 h after onset of ES for rhythm stabilization. In our own experience emergency ablation is rarely necessary because rhythm stabilization using conventional methods may be achieved in nearly all patients [5]. It is of upmost importance though to document any ventricular arrhythmia occurring after hospital admission on 12-lead ECG to enhance efficacy of detecting potential triggering premature ventricular complexes or QRS-morphology during ventricular tachycardia. This is specifically important in the early hospitalization phase when any means to stabilize ventricular electrical stability have not been implemented.
Timing of catheter ablation during ES
Whereas expert consensus is to perform catheter ablation of ES as early as possible no data on the optimum timing does exist. Two different time frames need to be differentiated: (1) Acute and (2) early (elective) ablation. Acute ablation should be considered to interrupt ES if other treatment options have failed to stabilize ventricular rhythm usually with the first 8–24 h after ES onset. Early ablation may be considered as an emergency procedure and only little data on incidence and outcome is available. In contrast early ablation during the initial hospitalization phase aims at permanent substrate modification when initial rhythm stabilization has been achieved.
In reports on ES ablation the mean time interval between onset of ES and catheter ablation is in between 3 and 14 days and most ablations are performed on an early elective basis [1, 5–8]. But mortality within the first week of ES onset may be as high as 80 % specifically when no interventional rhythm stabilization is performed [9].
Only in highly specialized centers focusing on ventricular arrhythmia treatment urgent (acute) ablation is performed even earlier (within 24 h of ES onset) in approximately 50 % of patients [1]. This may be too late for some patients as indicated in a report on a network like organization of cooperating cardiology departments where an approximated 15 % of patients died before being considered for catheter ablation [5]. In our own experience acute ablation within the first hours after ES onset is rarely needed if the complete armentarium of conventional non-ablation options for rhythm stabilization are applied, although some patients (in between 20 % up to 50 % of patients) may be in cardiogenic shock as a result of multiple ventricular arrhythmia episodes. In these patients ablation within the first 24 h should be considered [1, 6].
Local organization of invasive ES treatment in a cooperating network
In our institution we have attempted to organize the availability of interventional ES treatment options in cooperation with different local cardiology departments/clinics. Cooperating centers have the opportunity to admit patients with ES any time on a 24 h/7 days a week basis. At our institution a prespecified “best medical practice” pathway is established and a staged treatment protocol is available.
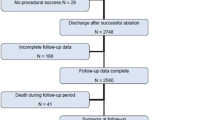
Any patient with ES that is enrolled within this network is documented in order to specify the pre-center incidence and treatment and potentially acute mortality of ES. At our center 12-lead continuous ECG monitoring in a dedicated unit is available to document the specificities of the clinical ventricular arrhythmia and concise treatment is possible. In this treatment protocol catheter ablation is considered usually after rhythm stabilization but may also be performed in the early unstable phase to achieve interventional abatement of ventricular arrhythmia (see ◉ Fig. 1). A cooperative unit of interventional cardiologists, electrophysiologists and also cardiac surgeons is available to discuss and optimize availability of treatment.
It is important to stress that the tertiary electrophysiology center in such a network should be capable of performing complex ventricular arrhythmia ablation procedures that may also involve epicardial VT ablation or ablation with the aid of hemodynamic invasive support.
Whereas in the initial phase of establishing such a network 14 % of patients died prior to ES ablation we were able to further optimize admission policies and within 2012 no patient who was admitted into this network died during the initial hospitalization phase. In 2012 23 patients were admitted from 12 cooperating centers and all patients (total of 29 including patients initially admitted to our center) were initially conservatively rhythm stabilized. Invasive electrophysiology was able to ablate the clinical arrhythmia in all but 2 patients (93 %). During a median follow-up of 9 months 28 % of patients had recurrent ventricular arrhythmia including 4 patients (14 %) with recurrent electrical storm. All of the patients with recurrent ES had non-ischemic cardiomyopathy and underwent a second epicardial ablation procedure and were free of any ventricular arrhythmia afterwards (cumulated overall short-term ventricular arrhythmia freedom 86 % after multiple ablation sessions and redo-procedures). Overall 7 out of 9 patients with non-ischemic cardiomyopathy and ES underwent epicardial mapping and ablation. During a median survival follow-up of 12 months 2 patients (6 %) died (1 non-cardiac and 1 unknown cause).
Data from this local ES network indicates that early admission of ES-patients to invasive treatment and a structured treatment protocol may be beneficial. Ablation of ES is complex and should be performed in centers with the capability of a wide range of interventional and electrophysiological interventions.
Regional organization of ES ablation
In order to further optimize ES ablation availability over-regional structures are warranted. For the first time 13 electrophysiology centers have committed on registering any ES-patient admitted to these centers attempting to document exact treatment for any of these patients including follow-up in a first step of the ES NET BAVARIA (see ◉ Fig. 2). In a second step data will be analyzed to identify and optimize a staged treatment protocol. The optimum timing for ES ablation can also be identified. A third step will then test the identified treatment protocol to all patients admitted to these dedicated centers. By this way an over-regional concept to optimize therapy for ES-patients may be achieved (population of Bavaria 12.5 million inhabitants, 46 clinic-based cardiology departments).
In conclusion, analyzing compiled data from many experienced centers will help to further enhance the knowledge on acute mortality, overall mortality, treatment strategies and prognostic value of different treatment strategies. Optimizing availability of interventional ES treatment will help to generalize the good outcome of catheter ablation proven for highly specialized and dedicated centers.
Conclusions
Electrical storm patients may benefit from optimized rhythm stabilization strategies within a network of cooperating cardiac centers. The optimum timing for catheter ablation in a staged protocol of treatment for patients with ES still needs to be defined. Acute access to perform acute ablation should be considered in patients who are not rhythm stabilized with antiarrhythmic medical treatment. Also, patients with hemodynamic compromise (cardiogenic shock) are candidates for an early interventional strategy. In specialized centers it is consensus to perform catheter ablation in these patients as early as possible, especially when considering a high early and late mortality without interventional management. Establishing a structured protocol for treatment and admission to EP centers has helped to further reduce pre-ablation mortality and may optimize treatment of ES. Networks to optimize and structure access to experienced electrophysiology centers are of importance to create a basis for optimizing treatment strategies.
Fazit für die Praxis
-
Bei Patienten mit elektrischem Sturm kann die Katheterablation akut zur Rhythmusstabilisierung anderweitig nicht stabilisierbarer Patienten eingesetzt werden
-
Strukturierte Therapiestrategien können helfen, die Therapie von Patienten mit ES zu optimieren
-
Eine Kooperation von Kliniken ohne interventionelle Kardiologie/interventionelle Elektrophysiologie mit erfahrenen Zentren kann die Behandlung weiter verbessern
-
Ein möglichst frühzeitiger Zugang dieser Patienten zur Ablation kann so ermöglicht werden
-
Aufgrund der geringen Anzahl dieser Patienten sind überregionale Netzwerke zur Etablierung einer optimierten Therapiestrategie sinnvoll
References
Carbucicchio C, Santamaria M, Trevisi N, Maccabelli G, Giraldi F, Fassini G, Riva S, Moltrasio M, Cireddu M, Veglia F, Bella Della P (2008) Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long-term outcomes in a prospective single-center study. Circulation 117:462–469
Exner DV, Pinski SL, Wyse DG, Renfroe EG, Follmann D, Gold M, Beckman KJ, Coromilas J, Lancaster S, Hallstrom AP, AVID Investigators (2001) Antiarrhythmics versus implantable defibrillators. Electrical storm presages nonsudden death: the antiarrhythmics versus implantable defibrillators (AVID) trial. Circulation 103:2066–2071
Deneke T, Lemke B, Mügge A, Shin D-I, Grewe PH, Horlitz M, Balta O, Bösche LI, Lawo T (2011) Catheter ablation of electrical storm. Expert Rev Cardiovasc Ther 9:1051–1058. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=21878049&retmode=ref&cmd=prlinks
Izquierdo M, Ruiz-Granell R, Ferrero A, Martínez A, Sánchez-Gomez J, Bonanad C, Mascarell B, Morell S, García-Civera R (2012) Ablation or conservative management of electrical storm due to monomorphic ventricular tachycardia: differences in outcome. Europace 14(12):1734–17349
Deneke T, Shin D-I, Lawo T, Bösche LI, Balta O, Anders H, Bünz K, Horlitz M, Grewe PH, Lemke B, Mügge A (2011) Catheter ablation of electrical storm in a collaborative hospital network. Am J Cardiol 108:233–239
Kozeluhova M, Peichl P, Cihak R, Wichterle D, Vancura V, Bytesnik J, Kautzner J (2010) Catheter ablation of electrical storm in patients with structural heart disease. Europace 13:109–113
Kolettis TM, Naka KK, Katsouras CS (2005) Radiofrequency catheter ablation for electrical storm in a patient with dilated cardiomyopathy. Hellenic J Cardiol 46:366–369
Marrouche NF, Verma A, Wazni O, Schweikert RA, Martin DO, Saliba W, Kilicaslan F, Cummings JE, Burkhardt DJ, Bhargava M, Bash D, Brachmann J, Guenther J, Hao S, Beheiry S, Rossillo A, Raviele A, Themistoclakis S, Natale A (2004) Mode of initiation and ablation of ventricular fibrillation storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol 43:1715–1720
Nademanee K, Taylor R, Bailey WE, Rieders DE, Kosar EM (2000) Treating electrical storm: sympathetic blockade versus advanced cardiac life support-guided therapy. Circulation 102:742–747
Compliance with ethical guidelines
Conflict of Interest
T. Deneke, P. Müller, J. Krug, K. Nentwich, D. Shin, P. Grewe, A. Mügge, A. Schade state that there are no conflicts of interest.
The accompanying manuscript does not include studies on humans or animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deneke, T., Müller, P., Krug, J. et al. Catheter ablation in patients with electrical storm: benefit of a network of cooperating clinics. Herzschr Elektrophys 25, 105–108 (2014). https://doi.org/10.1007/s00399-014-0306-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00399-014-0306-x