Abstract
We investigated the effects of methanol on the two rheological properties, dynamic modulus and flow behaviour, for an aqueous solution of hydrophobically ethoxylated urethane (HEUR). When the added methanol constitutes 0–10 mol% of the sample, the gel relaxation time shortens; when it constitutes 20 mol% of the sample, the distribution of relaxation times broadens. Relaxation of the physical gel formed by a transient network is directly related to the lifetime of the crosslink points, i.e. flower micelles. We speculate that methanol addition shortens the relaxation time by changing the hydrophobic interactions in the flower micelles. The changed hydrophobic interactions then affect not only the relaxation time but also the shape of the HEUR-chain molecular associating structures which in turn affects the mechanical spectrum. Under constant shear flow, shear thickening increases with increasing methanol concentration, and the increase in stress under constant shear flow shows unusual behaviour. A possible contributing factor to this behaviour may be the non-cosolvency of methanol with polyethyleneoxide (PEO). At some critical concentration, methanol in PEO aqueous solution becomes a poor solvent, which then affects the properties of the PEO chains in the transient networks of HEUR aqueous solution. The rheological properties of the transient networks clearly affect the properties of both the crosslink points and the chains. In short, methanol addition induces complicated changes in gel mechanical properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Physical gels that exhibit weak interactions are used in foods, cosmetics and other personal-care products, where they are supposed to fracture during use. Particularly, reversible gels, which fracture and reform, are quite interesting from the academic and industrial viewpoint. Hydrophobically ethoxylated urethanes (HEURs) are a type of telechelic polymer that form reversible gels in aqueous solution. The mechanism of the polymers is thought to involve the formation of flower micelles in water, which become crosslink points of the temporary physical network structure (Annable et al. 1992; Francois et al. 1996; Tam et al. 1998).
HEUR aqueous solutions have unique rheological properties. A HEUR solution has a single relaxation time, i.e. it exhibits elastic (rubber-like) properties under rapid deformation, while it flows under slow deformation. This phenomenon makes the HEUR solutions valuable in applications in which mechanical softness is important. The relaxation time of a HEUR solution depends on the length of the HEUR’s middle chain and the size of its hydrophobic residues. Tanaka and Edwards (1992) devised the transient networks theory to explain this behaviour and recently, this theory has been refined to explain the dynamic phenomenon observed in non-linear regions (Tanaka and Koga 2006).
A HEUR having C24 at both ends of a polyethyleneoxide (PEO) called C24HEUR has been developed as a rheology control agent used in cosmetics and personal-care products (Yoshida et al. 2007). Its relaxation time can be tuned to approximately the speed with which the product is applied during use, thereby giving the product a unique ‘hand feel’ by using C24HEUR.
Because some of these industrial products may contain non-aqueous solvents, it is important to investigate how these solvents affect the rheological properties of HEUR aqueous solutions. In particular, water-miscible organic solvents may affect the hydrophobic interactions that occur between the HEUR’s hydrophobes, and thus, affect the system’s relaxation time, i.e. the lifetime of the system’s crosslink points may differ by adding these solvents in the system. Changes in the relaxation time can present a grave problem in the development of industrial products. Water-miscible solvents may also affect the mechanical properties of the system’s middle PEO chains, and thus, of the gel itself. The solvents ethylene glycol (Dai et al. 2003) and glycerol (Kaneda et al. 2009) are already known to change the mechanical properties of the HEUR hydrogel.
Herein, we describe a comprehensive study of the effect of the addition of methanol on HEUR physical gel. Because methanol is one of the simplest organic solvent, studies on methanol may give valuable information for further studies on HEUR physical gels.
Materials and methods
Materials
A HEUR carrying branched alkyl endchains, 2-decyl-tetradecan (C24-HEUR), was synthesized by a method reported elsewhere (Yoshida et al. 2007). Our sample was kindly donated by Adeka Corp. (Japan) and used without further purification. Methanol (reagent grade) was purchased from Wako Pure Chemical (Osaka, Japan). Distilled water was also used.
Figure 1 shows the chemical structure of C24-HEUR. A remarkable feature of the polymer is the size of its hydrophobic moiety. Branched hydrophobic chains of 2-decyl-tetradecan are attached to the main chain of the urethane. The weight-average molecular weight M w of our sample was 27,000; the molecular-weight distribution M w/M n was 2.5. The polymer had been synthesized by the condensation reaction using polyethylene oxide (PEO) chains (M w = ∼10,000); the value of M w/M n indicates that each polymer contained one to three PEO chains.
The polymer solution was prepared as follows. First, methanol aqueous solutions (2–20 mol%) were prepared, then C24HEUR was dispersed in the solutions and mixed gently for at least 24 h at room temperature. The polymer concentration of all of the sample solutions was 1.5 wt.% (w/v). The sample code ‘MX’ denotes that the sample contained X mol% methanol.
Rheology measurements
A strain-controlled ARES rheometer (TA instruments, USA) was used for rheology measurements. Cone plate geometry was used (diameter 50 mm, cone angle 0.0394 rad). The frequency was varied in the range of 0.1 to 100 rad s−1, and the strain was fixed as 0.1 for the dynamic modulus measurement. The shear rate was varied in the range of 0.05 to 500 s−1 for the steady state viscosity measurement. The shear stress growth was also measured under the start-up shear flow in the various shear rates. All measurements were performed at 20°C. Sample temperature was controlled with a circulator attached to the rheometer.
Results and discussion
Influence of methanol on relaxation time
Although HEUR hydrogel is known to exhibit single-mode relaxation (Annable et al. 1992; Larson 1999), we expected that relaxation behaviour might differ if methanol were added to the gel. We thus investigated the mechanical relaxation behaviour of the samples by measuring their dynamic moduli.
For samples having their methanol concentration ranging over 0–10 mol% methanol, the dynamic-modulus profile shows single-mode relaxation that is consistent with a typical single-mode Maxwell model. Therefore, in order to obtain the characteristics of these samples, we analysed the dynamic moduli of M0, M2, M5, M7 and M10 by using the following single-mode Maxwell model:
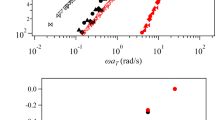
where G′(ω) and G″(ω) are the storage and loss moduli, respectively. G 0 is the equilibrium modulus and τ is the relaxation time, all of these characteristics can be obtained from analysing the measurement results by using Eqs. 1 and 2. Figure 2 shows the storage modulus and loss modulus for M0, M2, M5, M7 and M10. The coordinate systems of these plots are normalized by τ and G 0. The solid lines in Fig. 2 denote the prediction of a Maxwell model. It is clear that the dynamic modulus of the C24HEUR solutions, which methanol content was up to 10 mol% can be described by the Maxwell model. Figure 3 shows the methanol concentration dependency of the Maxwell characteristics the samples containing methanol up to 10 mol%. The relaxation time reduced with the methanol concentration, while G 0 increased with the methanol concentration.
Plot of dynamic modulus against frequency for C24HEUR 1.5 wt% solution containing methanol in range of 0 to 10 mol% (M0, M2, M5, M7 and M10). Coordinate system is normalized with τ and G 0. Circles, squares, triangles, inverse triangles, and diamonds denote the results of M0, M2, M5, M7, and M10, respectively. Open and closed symbols denote storage modulus and loss modulus, respectively. The lines denote the prediction of a Maxwell model (Eqs. 1 and 2)
The relaxation time of HEUR hydrogel related strongly to the lifetime of flower micelles formed from HEUR molecules (Tanaka and Edwards 1992; Tanaka and Koga 2006). Thus, we speculate that the addition of methanol reduces the relaxation time by altering the hydrophobic interactions between the hydrophobic alkyl chains in C24HEUR molecules, and thus, shortening the lifetimes of the elastically effective (i.e. PEO) chains attached to the micelles. In other words, we speculate that methanol changes the free-energy phase shift of the alkyl chain.
Our data show that addition of methanol both reduces the relaxation time and increases G 0, with G 0 increasing slightly with methanol concentration. This behaviour is similar to that induced by the addition of ethylene glycol. According to the classical theory for gel, the modulus of a gel is given simply by the following equation:
where ν is the number of the elastically effective chains in the gel network, k B is the Boltzmann constant and T is temperature. Because our polymer concentration was constant (1.5 wt.%), our observation that G 0 increases with methanol concentration suggests that the number of the elastically effective chains in the gel network increases when methanol is added, perhaps because methanol may reduce the aggregation number of the flower micelles.
For samples containing >10 mol% methanol, the profile differs. Particularly, the dynamic modulus profile of M20 follows a power law in the at high-frequency region and shows a new relaxation mode in the middle-frequency region (Fig. 4). We speculate that such power-law behaviour is caused by a broad distribution of relaxation times. Our reasoning is as follows: telechelic polymer—the class that includes HEUR—are known to exhibit various types of molecular assemblies, including simple bridges, super bridges, simple loops, super loops and dangles. If a sample profile shows a single Maxwell mode in its mechanical spectrum, presumably just a single bridge type is dominant. However, if it shows a broadened profile, presumably various types of coexisting molecular assemblies are present, each with a different relaxation time. Although the details of molecular assembly are unknown, the addition of some critical concentration of methanol clearly affects the association of telechelic polymers. Although no results are shown here, the sample, which contains methanol above 20 mol%, became a low viscosity fluid. It is considered that too much methanol may destroy the molecular association.
Influence of methanol on steady-flow behaviour
It is reported that a HEUR aqueous solution exhibits shear thickening at flow rates approximately equal to the relaxation speed and shear thinning at shear rates higher than the relaxation speed (Annable et al. 1992). Shear thickening is thought to be related to the non-linear expansion effect of the effective (i.e. PEO) chains in a transient network (Indei et al. 2005; Ma and Cooper 2001).
We investigated the shear-thickening behaviour of viscosity under constant shear flow to investigate the effect of methanol on effective chains. We determined the steady-state viscosity at various shear rates in the range 0.05–500 s−1 for samples containing methanol in range of 0 to 10 mol%. Because methanol concentration affects the relaxation time and shear moduli, we analysed our data in terms of normalized viscosity η/η init and the Weissenberg number \(\left( {Wi=\tau \dot{{\gamma }}} \right)\), where η init is the viscosity measured at 0.05 s−1 and τ is obtained from measurements of dynamic moduli (Fig. 4). Such a normalized coordinate system enables the comparison of the shear thickening behaviour at different methanol concentrations.
Figure 5 shows flow curves (normalized viscosity η/η init vs Wi) for the measured samples. The normalized viscosity increases gradually at first, then at Wi < 1, it decreases abruptly. The intensity of shear thickening, which is the peak of η/η init, depends on methanol concentration and becomes quite significant (Fig. 6); moreover, the viscosity peak shifts to shorter time (lower shear rate) with increasing methanol concentration. Although shear-thickening behaviour is affected by methanol concentration, the shear-thinning behaviour of the samples at flow rates above the critical shear rate are quite similar, and the flow curves of the samples essentially overlap beyond Wi = 1. This phenomenon is expected and we speculate that it is caused by shear-banding transitions.
Flow curves for C24 HEUR 1.5 wt.% solutions containing methanol in range of 0 to 10 mol% (M0, M2, M5, M7, and M10). Normalized viscosity and shear rate are employed in the coordinate system. Viscosity is normalized with η init which is the apparent viscosity at 0.05 s−1. Shear rate is shown as Weissenberg number \(\left( {Wi=\tau \dot{{\gamma }}} \right)\). The values of τ for these samples were estimated by a Maxwell model (Eqs. 1 and 2)
A number of experimental reports suggest that surfactant micellar solutions (Berret 2005; Lerouge and Berret 2010) and block copolymer solutions (Berret and Serero 2001; Le Meins and Tassin 2001; Manneville et al. 2007) undergo such shear-banding transitions. The transition is usually associated with a new mesoscopic organization of the system called shear-induced supramolecular architecture. The rheological characteristics of shear-banding transition are as follows. The measured σ(γ) is composed of two stable branches, one of high viscosity (viscous) and one of low viscosity (fluid), separated by a stress plateau (area of constant stress). From a macroscopic view, flow becomes unstable and evolves towards a banded state where the viscous and fluid phases coexist at constant stress (Cates et al. 1993). The associated polymer solution reportedly undergoes phase separation under shear flow as the shape of the molecular assembly changes. Therefore, we presume that the abrupt shear-thinning behaviour evident in Fig. 5 is because of shear-banding transitions.
Figure 7 shows flow curves (normalized shear stress σ/G 0 vs Wi) for the measured samples. The values of G 0 were obtained from dynamic measurements (Fig. 3). The curves show clear non-monotonic behaviour, particularly at the stress plateau around σ/G 0 = 1. The presence of a shear stress plateau indicates that shear stress does not always increase with increasing shear rate, but rather that plastic flow or slippage occurs in samples. Moreover, the stress plateaus of all of the samples essentially overlap, indicating the existence of a mesoscopic supramolecular structure, at least in the border region between the separated phases. This behaviour is similar to that observed in Fig. 6, i.e. after the shear-banding transition.
Shear stress growth behaviour under the constant shear flow for C24HEUR 1.5 wt.% solution containing methanol in range of 0 to 10 mol% (M0, M2, M5, M7, and M10). Normalized shear stress and shear rate are employed in the coordinate system. Shear stress is normalized with the equilibrium shear modulus (G 0). Shear rate is shown as Weissenberg number \(\left( {Wi=\tau \dot{{\gamma }}} \right)\). The values of G 0 and τ for these samples were estimated by a Maxwell model (Eqs. 1 and 2)
Stress growth under start-up shear flow
Because the linear rheological properties of C24HEUR aqueous solution can be described by a single-mode Maxwell model, viscosity increase under constant shear flow can be described as follows.
However, as mentioned above, the viscosity of C24HEUR aqueous solution varies with shear rate. Thus, an important property of complex fluids used in industrial products is their transient stress behaviour under constant shear flow. Such properties are expected to affect the product’s hand feel, which is particularly important for such products (e.g. cosmetics and personal-care products (Nakamura et al. 2009))
In a transient network, stress under a particular level of strain is determined by the balance between the tension between the end of the active chain and the lifetime dissociation rate of the transient crosslink point. The C24HEUR aqueous solution reportedly exhibits an unusual increase or upturn in stress at high shear rates (Koga et al. 2009a, b). The cause of this phenomenon is thought to be a non-linear stretching effect of the PEO chains of C24HEUR. To investigate the effect of methanol on this upturn, we investigated the viscosity (stress) growth curves at constant shear flow for samples. The upturn peak shifts with the relaxation time, so we again introduced the concept of normalized shear rate (Weissenberg number). The upturn peak for C24HEUR aqueous solution reportedly appears normally at a deformation speed that is higher than the relaxation speed; therefore, we observed stress response for samples at Wi = 0.1, 1.0, and 10.
Figure 8 shows viscosity growth curves for M0, M5 and M10 (Fig. 8a–c, respectively). The solid lines denote the linear elastic response calculated by the following equation.
The dotted lines denote the viscoelastic response predicted by the Maxwell model, as described in Eq. 4. As mentioned above, an upturn in stress is clearly evident for M0 at high enough shear rate (Wi = 10), although the viscosity response was monotonic and well described by a Maxwell model at low shear rate (Wi = 0.1). It is interesting that the viscosity growth behaviour was different each other for these samples at Wi = 1.0. For M0, shear thinning was already observed at Wi = 1.0. However, the steady state viscosities of the samples containing methanol (M5 and M10) were almost equal to the prediction of a Maxwell model at Wi = 1.0 (Fig. 8b and c). This phenomenon is consistent with the results of the steady state viscosity (Fig. 5).
Although an upturn in stress was observed in each samples at Wi = 10, the intensity of upturn in stress reduced with increasing the methanol concentration. To estimate the intensity of the upturn in stress, we introduced the stress-upturn index S
where t peak is the time at which the stress-upturn peak occurs in the viscosity growth curve. Figure 9 shows the methanol concentration dependency of the stress upturn index. This figure shows that the intensity of the stress upturn under constant shear flow decreases with increasing methanol concentration. As mentioned above, the mechanical response of the HEUR aqueous solution is thought to be governed by the balance between the tension of the effective (i.e. PEO) chains in a transient network and the lifetime of the crosslink point. Because methanol weakens the crosslink points, effective chains never experience redundant tension. This may be the cause of the disappearance of the stress upturn on the addition of methanol.
Variation of stress upturn index (S) vs methanol concentration. The value of stress upturn indices were estimated by using Eq. 6
Conclusion
The effect of methanol addition on the rheological properties of C24HEUR aqueous solution is quite complicated.
Methanol addition shortens the relaxation time, consistent with the known behaviour of other water-miscible solvents, presumably because of its effect on hydrophobic interactions.
In addition, methanol addition weakens the aggregation of hydrophobic residues in the cores of flower micelles, shortening the lifetime of the transient crosslink points. Hence, the relaxation time of the transient network shortens. The effect on crosslink points of the transient network also may be related to the observed change in the stress upturn under constant shear flow. How methanol addition affects the mechanical properties of PEO chains, particularly non-linear stretching, is still unknown, but a possible mechanism is that it causes crosslink points to become brittle, and thus, prevents the build-up of unusual tension under the relatively high-speed shear flow.
Finally, methanol addition affects shear-flow behaviour by reinforcing shear thickening. The effect of methanol on PEO (the effective chain of the transient network) is interesting. Methanol is known to exhibit a non-cosolvency effect on PEO in aqueous solutions (Kaneda and Vincent 2004; Shankar et al. 2009). When adding methanol concentrations of approximately 30 mol%, methanol reportedly causes the PEO chains to shrink, but at methanol concentrations <20 mol%, it functions as a good solvent for PEO. This phenomenon is related to cooperative hydration/dehydration of the water-soluble polymer.
The effective concentration of methanol that induces the non-cosolvency differs from the concentration observed in this report. However, if hydration behaviour changes with PEO modification, i.e. by end-capping of its hydrophobes, the effective concentration of methanol that induces non-cosolvency possibly changes as well. Therefore, the non-cosolvency effect is one of the possible causes of the unexpected phenomenon observed in this study. For a precise explanation of the unexpected behaviour of C24HEUR in methanol aqueous solution, we must now investigate the characteristics of hydrophobically modified PEO in methanol aqueous solution.
References
Annable T, Buscall R, Ettelaie R, Whittestone D (1992) The rheology of solutions of associating polymers: comparison of experimental behavior with transient network theory. J Rheol 37(4):695–726
Berret JF (2005) Rheology of wormlike micelles: equilibrium properties and shear-banding transition. In: Molecular gels, Elsevier, Dordrecht
Berret JF, Serero Y (2001) Evidence of shear-induced fluid fracture in telechelic polymer networks. Phys Rev Lett 87(4):048303
Cates ME, McLeish TCB, Marrucci G (1993) The rheology of entangled polymers at very high shear rates. Europhys Lett 21(4):451–456
Dai S, Sio ST, Tam KC Jenkins RD (2003) Rheology and aggregation behavior of hydrophobically modified urethane ethoxylate in ethylene glycol–water mixtures. Macromolecules 36:6260–6266
Francois J, Maitre S, Rawiso M, Sarazin D, Beinert G, Isel F (1996) Neutron and X-ray scattering studies of model hydrophobically end-capped poly(ethylene oxide) aqueous solutions at rest and under shear. Colloids Surf A 112:251–265
Indei T, Koga T, Tanaka F (2005) Theory of shear-thickening in transient networks of associating polymers. Macromol Rapid Commun 26:701–706
Kaneda I, Vincent B (2004) Swelling behavior of PMMA-gPEO microgel particles by organic solvents. J Colloid Interface Sci 274:49–54
Kaneda I, Koga T, Tanaka F (2009) Time-dependent flow properties of transient hydrogel with temporal network junctions. Prog Colloid Polym Sci 136:31–38
Koga T, Tanaka F, Kaneda I (2009a) Stress growth in transient polymer networks under startup shear flow. Prog Colloid Polym Sci 136:39–46
Koga T, Tanaka F, Kaneda I, Winnik FM (2009b) Stress buildup under strat-up flows in self-assembled transient networks of telechelic associating polymers. Langmuir 25(15):8626–8638
Larson RG (1999) The structure and rheology of complex fluids. Oxford University Press, New York, pp 248–260
Le Meins J-F, Tassin J-F (2001) Shear-induced phase separation in an associating polymer solution. Macrololecules 34:2641–2647
Lerouge S, Berret J-F (2010) Shear-induced transitions and instabilities in surfactant wormlike micelles. Adv Polym Sci 230:1–71
Ma SX, Cooper SL (2001) Shear thickening in aqueous solutions of hydrocarbon end-capped poly(ethylene oxide). Macrololecules 34:3294–3301
Manneville S, Colin A, Waton G, Schosseler F (2007) Wall slip, shear banding, and instability in the flow of triblock copolymer micellar solution. Phys Rev E 75(6):061502
Nakamura A, Sogabe A, Machida A, Kaneda I (2009) Novel attempt for quantitative sensory evaluation of cosmetics using the Nutting parameters. Nihon Reoroji Gakkaishi 37(5):247–251
Shankar R, Klossner RR, Weaver JT, Koga T, van Zanten JH, Krause WE, Colina CM, Tanaka F, Spontak RJ (2009) Competitive hydrogen-bonding in polymer solution with mixed solvents. Soft Matter 5:1–5
Tam KC, Jenkins RD, Winnik MA, Bassett DR (1998) A structural model of hydrophobically modified urethane-ethoxylate (HEUR) associative polymers in shear flows. Macromolecules 31:4149–4159
Tanaka F, Edwards SF (1992) Viscoelastic properties of reversibly crosslinked polymer networks—Transient Network Theory. Macromolecules 25(5):1516–1523
Tanaka F, Koga T (2006) Nonaffine transient network theory of associating polymer solutions. Macromolecules 39:5913–5920
Yoshida K, Nakamura A, Nakajima Y, Fukuhara T, Inoue H, Kaneda I (2007) Use of associating polymers as multifunctional thickeners: Studies of their structure in aqueous solutions via NMR, QELS, fluorescence, and rheology measurements. IFSCC magazine 10:1–6
Acknowledgements
This work is partly supported by a Grant-in-Aid for Scientific Research on Priority Areas “Soft Matter Physics” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We wish to acknowledge their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaneda, I., Koga, T. & Tanaka, F. Rheological properties of physical gel formed by hydrophobically modified urethane ethoxylate (HEUR) associative polymers in methanol–water mixtures. Rheol Acta 51, 89–96 (2012). https://doi.org/10.1007/s00397-011-0571-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00397-011-0571-0