Abstract
Organogels composed of 40% hydrolyzed poly(vinyl acetate) (40PVAc) and three arylboronic acids have been formed and characterized in several liquids to gain insights into the role of crosslinker length and structure on the properties of the gels. Data from 1H NMR, steady-state and time-resolved fluorescence, and rheology have been employed to correlate the degrees of crosslinking and the bulk physical properties of the gels. Benzene-1,4-diboronic acid (1,4-BDBA) and 4,4′-biphenyldiboronic acid (bPDBA) formed gels with 40PVAc that were stable for longer periods than those with benzene-1,3-diboronic acid. Surprisingly, the steady-state and time-resolved fluorescence properties of 1,4-BDBA or bPDBA were affected very little in solution upon addition of two model diols (1,3-propanediol and 1,2-ethanediol) or even 40PVAc. Furthermore, 40PVAc appeared to form crosslinks more efficiently, resulting in stiffer gels, with bPDBA than with 1,4-BDBA. We attribute these trends to the greater length, flexibility, and linearity of bPBPA. Overall, the results reported here demonstrate that minor structural changes in the crosslinker can alter significantly many of the bulk properties of two-component organogel networks. They also provide a clear ‘blueprint’ for making gels with specific desired properties for a range of applications that require virtually water-free systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Both a boronic acid (i.e., R-B(OH2) where R is an alkyl or aryl group) and its anion are capable of reacting reversibly with 1,2- and 1,3-diols. [1,2,3] The reaction of arylboronic acids with diol-functionalized polymers, especially those in which pairs of hydroxyl groups are in a 1,3 structural arrangement, can be exploited to produce soft materials. [4, 5] The reaction of boronic acid-functionalized polymers and poly(vinyl alcohol) in aqueous media is known to produce hydrogels [6,7,8] and films [9,10,11,12] that can be modified by addition of glucose or changes in pH. The mixing of boronic acid- and glucose-functionalized polymers has also produced hydrogels that are sensitive to pH. [13] Thus, boronic acid-functionalized polymeric hydrogels have been shown to release diols and monosaccharides for applications in drug delivery. [9, 14,15,16,17] Also, the dynamic equilibrium between boronic acids and boronate esters has been exploited to form some self-healing materials. [18,19,20]
Molecules containing two boronic acid groups, such as benzene-1,4-diboronic acid (1,4-BDBA), can form inter- and intra-molecular crosslinks with polymer chains containing 1,2- or 1,3-diol units. Although 1,4-BDBA is not water-soluble, like boric acid, it is soluble in a wide range of organic liquids. As a result, it has been used to form hydrogels indirectly, by first creating organogels in DMSO and then immersing them in water. [21] It has been employed, as well, to thicken a dilute 0.36 wt% solution of guar, a naturally derived polysaccharide. [22] Also, by placing poly(vinyl alcohol) droplets or fibers into a solution of 1,4-BDBA, it has been possible to form soft materials with a range of morphologies, including microspheres and fibers. [23, 24] In them, the 1,4-BDBA is crosslinked with the poly(vinyl alcohol) to impart temporal and/or thermal stability to the shapes of the materials.
Previously, we reported the formation of a variety of soft, ‘peelable’ organogels, composed of 40% hydrolyzed poly(vinyl acetate) (40PVAc) and benzene-1,4-diboronic acid (1,4-BDBA) as the crosslinker, which are stable under atmospheric conditions. [25] These gels are important from fundamental and applied standpoints because they are virtually water-free (as opposed to other gels based on poly(vinyl alcohol) or partially hydrolyzed poly(vinyl acetate) which have large contents of water as the liquid component), and they exhibit bulk properties that are attractive potentially in art conservation. [25] It should be noted that these organogels do contain small amounts of water: two molecules of water are produced per crosslink formed. Here, we expand the range of these gel structures and properties by employing two additional aryl boronic acid (ABA) crosslinkers of 40PVAc (Fig. 1). Benzene-1,3-diboronic acid (1,3-BDBA) was selected to create nonlinear crosslinks, and biphenyl-4,4′-diboronic acid (bPDBA) was chosen to produce longer and more flexible crosslinks: it is capable of undergoing torsional motions about its two phenyl rings whereas the single aromatic ring of 1,3,-BDBA and 1,4-BDBA can twist only about its boronic acid groups. In this study, only 40PVAc has been employed in order to allow direct comparisons of the effects of changing the ABA structures. In this way, the diversity of the liquids gelated and the resulting physical properties of the gels can be correlated with the nature of the crosslink sites (Fig. 2). In addition, 1H NMR spectra are used to investigate the reaction of these crosslinkers with two monomeric diols, 1,3-propanediol (PD) and 1,2-ethanediol (i.e., ethylene glycol), as well as with 40PVAc. Also, because 1,4-BDBA and bPDPA fluoresce, their photophysical changes when undergoing crosslinking have been probed by steady-state and time-resolved fluorescence experiments. A comparison of the properties of materials formed with the ABAs demonstrates the sensitivity of the crosslinks to the structure of the crosslinkers. As a result, we have gained a deeper understanding of how nanoscale changes to gelator components can affect the macroscale properties of their gels, especially in this case, organogels.
The reactions between 1,3-BDBA and 40PVAc (not shown) are completely analogous to those involving 1,4-BDBA
Materials and methods
Materials
Benzene-1,4-diboronic acid (Sigma-Aldrich, ≥ 95.0%), benzene-1,3-diboronic acid (Alfa Aesar, 97%), 1,3-propanediol (Sigma-Aldrich, 98%), dimethyl sulfoxide (Sigma-Aldrich, > 99.5%), 2-ethoxyethanol (Sigma-Aldrich, 99%), dimethylformamide (EMD, ACS grade), methanol (Sigma-Aldrich, ACS grade, > 99.8), tetrahydrafuran (Sigma-Aldrich, anhydrous, > 99.9%), 2-propanol (Fisher Scientific, HPLC grade), and DMSO-d 6 (Cambridge Isotope Lab, 99.9% D) were used as received. Before its use, 40PVAc (Kuraray Co., Ltd.; 40% hydrolyzed poly(vinyl acetate); ~5 mPa‧s viscosity of a 4% aqueous solution at 20 °C) was rinsed with ice-cold deionized water, filtered, and vacuum dried (~ 125 mmHg) overnight at room temperature. Attempts to determine the average molecular weight of the 40PVAc and its distribution by gel permeation chromatography were unsuccessful because of aggregation in the only solvent we found that dissolves the polymer and is compatible with our columns, tetrahydrafuran. Biphenyl-4,4′-diboronic acid (Alfa Aesar, 95%) (~ 100 mg) was dissolved in ~ 20-mL hot 2-propanol and placed in a fume hood until half of the solvent had evaporated. The white powder that formed was filtered and dried at reduced pressure (~ 125 mmHg) overnight at room temperature. 1H NMR spectra collected before and after crystallization are displayed in Fig. S1, along with an analysis of their purities.
Organogels were prepared by dissolving 40PVAc and an ABA separately in an organic liquid heated to 40 °C. The ABA solution was transferred with a pipette into the 40PVAc solution, and the mixture was stirred vigorously with a spatula for approximately 30 s.
Instrumentation
1H NMR spectra were acquired at 25 °C on a Varian-MR 400 MHz NMR spectrometer. Gels were prepared using a syringe to disperse an ABA solution throughout a 40PVAc solution in an NMR tube, and the material was stirred with the syringe needle. For variable temperature studies, samples were allowed to equilibrate at each temperature (as reported by a thermocouple in the spectrometer) for 5 min before beginning an experiment. UV–Vis spectra of samples in 1-cm quartz cuvettes were recorded on an Agilent 8453 UV–Vis spectrophotometer. Rheological measurements were made on samples 1 day after their preparation on a stress-controlled Anton Paar Physica MCR 302 rheometer with a 25-mm diameter cone (angle of 2.0°) and plate. Strain sweeps were measured at 0.08–300% at a constant angular frequency of 1 rad/s. In the linear viscoelastic regions, frequency sweeps were recorded in the range 0.01–100 rad/s. A Horiba FluoroMax-4 fluorimeter was used to collect excitation and emission spectra of samples in a front-face geometry in a 1-cm triangular quartz cuvette capped with a Teflon plug. Unless noted otherwise, N2 was bubbled through the samples for 15 min before data collection. After steady-state fluorescence measurements, time-correlated single photon decays of the same samples were acquired with an Edinburgh Analytical Instruments single-photon counting system (model FL900) and a H2 lamp operating at 40 kHz. ‘Instrument response functions’ were obtained with Ludox® as the scatterer at the excitation wavelength, 270 nm. Fluorescence intensity was monitored versus time at either 320 or 383 nm. The fluorescence decays had a minimum of 6000 counts in the channel with maximum counts and were measured over 1024 channels with a 0.05 ns time per channel. The monomer and excimer decays were treated with an exponential deconvolution method to minimize χ2 using the FAST software supplied by Edinburgh Analytical Instruments. Fits were deemed acceptable when χ2 ≤ 1.3 and residual plots showed no systematic deviations from zero.
Results and discussion
Clear, homogeneous organogels were produced by crosslinking 40PVAc with the three different ABAs in DMSO, DMF, and 2-ethoxyethanol (Table 1). Mixing a 40PVAc solution with an ABA solution resulted in a clearly perceptible increase in viscosity in less than 1 min. Although an inhomogeneous mixture formed upon combining 40PVAc and 1,4-BDBA in methanol, the mixture became homogeneous after being left undisturbed at room temperature for 24 h. Only sols resulted upon mixing 40PVAc and either 1,3-BDBA or bPDBA in methanol.
The stability of the organogels was monitored visually at room temperature in closed vials: some of the organogels exhibited syneresis (visually noted) after long time periods, ranging from 2 to 7 days, while others returned to a sol in as little as 20 min (Table 1). Also, those organogels that did form with 1,3-BDBA were significantly less stable than those formed with 1,4-BDBA or bPDBA, in terms of the amount of time before the sample either returned to a sol or underwent syneresis (Table 1). We hypothesize that the geometry of the 1,3-BDBA crosslinks may decrease the ratio of inter-chain to intra-chain crosslinks. Also, the meta orientation of the boronic acid groups of 1,3-BDBA may result in less stable ester linkages for steric reasons than those involving the para oriented groups of 1,4-BDBA and bPDBA.
The bulk physical properties of materials formed from 40PVAc and either 1,4-BDBA or bPDBA were compared by oscillatory rheology 1 day after preparation. Only samples stable for long periods of time were studied in order to avoid collecting data on samples that had undergone syneresis. Materials prepared with 1,3-BDBA as a crosslinker were not stable for a sufficiently long period to be studied in this way. 2-Ethoxyethanol is a high boiling solvent with a low UV-cutoff wavelength, making it an appropriate choice for both rheological and photophysical studies. Both the strain and frequency sweeps of 40PVAc/1,4-BDBA and 40PVAc/bPDBA mixtures showed that gels are formed with both crosslinkers: G′ is larger than G″ at all strain and angular frequency values examined (Fig. 3). Although the molar concentrations of crosslinker were kept constant, the organogel formed from 6 wt% 40PVAc and 0.29 wt% bPDBA was slightly stiffer than that formed from 6 wt% 40PVAc and 0.2 wt% 1,4-BDBA. These data suggest that the longer, more flexible crosslinker, bPDBA, forms crosslinks more efficiently with 40PVAc than the shorter, less flexible crosslinker, 1,4-BDBA, or the non-linear crosslinker, 1,3-BDBA.
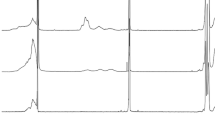
The reactions between ABAs and 40PVAc were monitored by 1H NMR spectroscopy as well. The spectra of 40PVAc with each of the ABAs indicate that the acids reacted completely with the polymer, as inferred from the lack of apparent signals from the acids (Fig. 4a, c, and e) after addition of 40PVAc (Fig. 4b, d, and f). Although the 40PVAc/ABA materials did not flow for at least 1 min after being inverted, indicating gelation due to the presence of crosslinks, the 1H NMR spectra showed more signals than would be expected if only di-esterified species were present. Therefore, the addition of a molar excess of 40PVAc likely results in a material with some mono-esterified species as well.
1H NMR spectra of ABAs, before and after adding a molar excess of OH40PVAc, in DMSO-d 6 at 25 °C. a 1,3-BDBA, b 6 wt% 40PVAc and 0.2 wt% 1,3-BDBA, c 1,4-BDBA, d 6 wt% 40PVAc and 0.2 wt% 1,4-BDBA, e bPDBA, and f 6 wt% 40PVAc and 0.29 wt% bPDBA. The OH40PVAc/ABA ratio is always 29:1. All spectra were acquired 24 h after sample preparation, and the signals with the largest intensities in the displayed ppm range were normalized with respect to each other for comparison
Two of the ABAs, 1,4-BDBA and bPDBA, were reacted with 1,3-propanediol (PD) to form boronate esters with six-membered rings, similar to those that were formed upon reaction with 40PVAc. However, the rotational and translational diffusion of the boronate esters formed with 1,3-propanediol are not impeded severely, as they are when the diol groups are a part of 40PVAc. Thus, signal resolution is not lost. The addition of small amounts of 1,3-propanediol to 1,4-BDBA led to the formation of mostly the mono-esterified boronate ester (with a unique boronic acid proton signal at 8.03 ppm); at PD:1,4-BDBA ratios of 3.1:1 and higher, the di-esterified boronate (Fig. 5a) was formed predominantly. Similar experiments tracked the reaction of 1,4-BDBA with 1,2-ethanediol (Fig. S2), which reacts with 1,4-BDBA to form mono- and di-esterified species with five-membered rings. The signals were sufficiently well-resolved to be identified and integrated (Table S1), allowing the portion of each species present to be estimated (Table S2). Upon addition of a molar excess of 1,2-ethanediol, only 23% of the 1,4-BDBA exists in the di-esterified form (with a five-membered ring). However, upon addition of a molar excess of 1,3-propanediol, the di-esterified boronate ester species (with a six-membered ring) is formed more efficiently (Tables S3 and S4): for a 0.2 wt% 1,4-BDBA solution, > 80% of the 1,4-BDBA is in its di-esterified form when PD:1,4-BDBA is > 3.1:1 (i.e., > 0.3 wt%).
1H NMR spectra of 1,3-propanediol and ABAs in DMSO-d 6 . a 0.2 wt% 1,4-BDBA, and upon addition of 0 (i), 0.05 wt% (ii), 0.1 wt% (iii), 0.2 wt% (iv), 0.3 wt% (v), and 0.4 wt% (vi) 1,3-propanediol. PD/1,4-BDBA: (i) 0:1, (ii) 0.5:1, (iii) 1.0:1, (iv) 2.2:1, (v) 3.1:1, and (vi) 5.4:1. b 0.3 wt% bPDBA, and upon addition of 0 (i), 0.05 wt% (ii), 0.1 wt% (iii), 0.2 wt% (iv), 0.3 wt% (v), and 0.4 wt% (vi) 1,3-propanediol. PD/bPDBA: 0:1 (i), 0.6:1 (ii), 1.1:1 (iii), 2.3:1 (iv), 3.4:1 (v), and 5.7:1 (vi)
1H NMR spectra from the addition of small amounts of 1,3-propanediol to bPDBA (Fig. 5b) showed a similar trend. Addition of 1,3-propanediol to a 0.3 wt% bPDBA solution led to the initial formation of the mono-esterified boronate ester (with a unique boronic acid proton signal at 8.09 ppm), and a preponderance of the di-esterified species when PD/bPDBA was > 3.4:1 (i.e., 0.3 wt%)). For these reasons, additional 1H NMR and fluorescence spectra were recorded on samples with a large excess of 1,3-propanediol (to increase the fraction of di-esterified species).
Figure 6a, b shows the spectra acquired from reacting 1,4-BDBA or bPDBA with either 1,3-propanediol or 40PVAc. With both ABAs, a large excess of 1,3-propanediol, as determined by the results reported in Fig. 5, was added in order to ensure the presence of predominantly the di-esterified boronate species. The spectra indicate—but do not demonstrate conclusively—that the aromatic signals from the mono-esterified boronate resonate farther downfield than those from di-esterified boronate; our analyses assume this to be the case. In Fig. 6aiii, the signal resonating near 7.75 ppm appears to be larger than the one near 7.65 ppm (i.e., there is a greater amount of mono-esterified than di-esterified boronate ester present). In Fig. 6biii, the intensities of the signals near 7.65 and 7.88 ppm are similar, suggesting that the mono- and di-esterified boronate esters are present in comparable quantities. Insofar as these assignments are correct, bPDBA forms di-esterified boronate ester crosslinks more efficiently than 1,4-BDBA. We attribute this to the increased length and flexibility of the bPDBA: the increased length of the biphenyl ring allows a greater distance between polymer chains at the crosslinking points, and the biphenyl rings can undergo torsional motions in a manner not possible structurally with 1,4-BDBA.
1H NMR spectra of the ABAs at different concentrations of 1,3-propanediol or 40PVAc in DMSO-d 6 . a 0.2 wt% 1,4-BDBA (i), 0.2 wt% 1,4-BDBA and 3 wt% 1,3-propanediol (ii), and 0.2 wt% 1,4-BDBA and 6 wt% 40PVAc (iii). b 0.29 wt% bPDBA (i), 0.29 wt% bPDBA and 3 wt% 1,3-propanediol (ii), and 0.29 wt% bPDBA and 6 wt% 40PVAc (iii). All spectra were acquired 24 h after sample preparation, and the signals with the largest intensities in the displayed ppm range were normalized to each other for comparison. The OH40PVAc/ABA molar ratio is 29:1, and the OHPD/ABA is 62:1, where ‘OHPD’ refers to the amounts of hydroxyl groups on 1,3-propanediol
Fluorescence excitation and emission spectra were recorded to probe the changes in local environments that accompany reactions between 1,4-BDBA or bPDBA and either 1,3-propanediol or 40PVAc. We anticipated that comparison between boronate esters formed with 1,3-propanediol and 40PVAc would provide such insights because esterification of the polymer might force the ABAs into more constrained environments.
However, reaction between 1,4-BDBA and either 1,3-propanediol or 40PVAc resulted in only small changes in the absorbance (Fig. S3a), excitation, and emission (Fig. 7a) spectra. The emission spectra of 1,4-BDBA broadened only very slightly more upon reaction with 1,3-propanediol than with 40PVAc. In fact, the differences caused by esterification with 1,3-propanediol and 40PVAc are too small to conclude that there is a significant environmental difference. The reaction of bPDBA with either 1,3-propanediol or 40PVAc led to similarly small differences in absorbance (Fig. S3b), excitation, and emission spectra (Fig. 7b). Although the emission spectra show different ratios of intensity for the excimer and monomer, they may arise from traces of dioxygen that remain after the purging with nitrogen (Fig. S5). Thus, there are no dramatic effects in the emission spectra of the ABAs upon their esterification with the monomeric or polymeric diol source.
a Excitation (dashed lines; λem 330 nm) and emission (solid lines; λex 270 nm) spectra of 0.2 wt% (1.1 × 10−2 M) 1,4-BDBA in 2-ethoxyethanol in the absence and presence of 3 wt% 1,3-propanediol or 6 wt% 40PVAc. b Excitation (dashed; λem 330 nm) and emission (solid; λex 270 nm) spectra of 0.29 wt% (1.1 × 10−2 M) bPDBA in 2-ethoxyethanol in the absence and presence of 3 wt% 1,3-propanediol or 6 wt% 40PVAc. The OH40PVAc/ABA ratio is 29:1, and the OHPD/ABA ratio is 62:1. All intensity values are normalized with respect to intensities at λmax. Non-normalized spectra are displayed in Fig. S6
1,2-Ethanediol was also combined with bPDBA in order to assess potential changes to the emission and excitation spectra upon the formation of five-membered boronate ester rings. Because the previously mentioned 1H NMR studies indicate that bPDBA is in equilibrium with the five-membered cyclic boronate ester ring species, the excitation and emission spectra represent both species in solution. Similar to the observations when 1,3-propanediol was employed, no discernible spectral differences were noted between the emission spectra for bPDBA in the absence and presence of 1,2-ethanediol (Fig. S7). A slight increase in the ratio of monomer to excimer may be the result of small concentration differences between the acid and ester forms or to the effect of slightly different amounts of dioxygen present in the nitrogen purged samples. Thus, there are no dramatic effects in the emission spectra of bPDBA between esterification with the 1,2- or 1,3-diol source.
In an effort to extract more information from the emission of the ABAs, time-resolved fluorescent decays were collected. The decay times of 1,4-BDBA both with and without 1,3-propanediol or 40PVAc were virtually indistinguishable at the two emission wavelengths probed, 320 and 383 nm (Fig. 8). The decay curves were fitted to a sum of exponentials (Table 2) in which there was a minor, short-lived component of ~ 2 ns and a dominant decay of ~ 8–9 ns.
Time-resolved fluorescence decays (λex 270 nm) of 0.2 wt% (1.1 × 10−2 M) 1,4-BDBA in 2-ethoxyethanol in the absence and presence of either 3 wt% 1,3-propanediol or 6 wt% 40PVAc at λem = 320 nm (a) and 383 nm (b). The OH40PVAc/ABA and OHPD/ABA molar ratios are 29:1 and 62:1, respectively. Instrument response functions (dots showing shortest decay) are from scattering by Ludox®
Although the decays of bPDBA in 2-ethoxyethanol, with and without the addition of 1,3-propanediol or 40PVAc, showed no differences at an emission wavelength of 320 nm, the presence of a longer-lived species at an emission wavelength of 383 nm in the decay of the bPDBA/40PVAc solution was apparent; it was not present in the bPDBA or bPDBA/1,3-propanediol solutions (Fig. 9). The decay curves obtained from excitation at 270 nm and emission at 320 nm for bPDBA with and without the addition of 1,3-propanediol or 40PVAc could be fitted well to a major, shorter-lived component of ~ 2 ns and a minor, longer component of ~ 5–8 ns (Table 3). However, when collecting decays at 383 nm (i.e., primarily from excimer), the longest-lived component of ~ 12–17 ns increased in importance from ~ 1 to ~ 18% (i.e., ~ 1 to ~ 16%, as reported in Table 3 when components are included that are too short to be measured reliably and may be from light scatter) upon addition of 40PVAc. An attractive hypothesis to explain this observation is that there is an increase in the proportion of polymer-bound crosslinker which should be less capable of undergoing the internal motions necessary to facilitate internal conversion than the unesterified bPDBA. However, this hypothesis is not consistent with another observation: a smaller (but clear) increase, from ~ 1 to ~ 5% (~ 1 to ~ 4% as reported in Table 3 when components are included that are too short to be measured reliably and may be from light scatter) of the longest-lived component of ~ 12–17 ns is also observed when the monomeric diol, 1,3-propanediol, is added to the bPDBA solution. Thus, the source of the longest-lived decay component is unclear at this time. Overall, because the observed spectral differences among bPDBA and 1,4-BDBA esterification/crosslinking reactions are subtler and smaller than anticipated, we conclude that the local environments experienced by the ABAs in their free and crosslinked forms are very similar. This conclusion was unexpected for another reason: we anticipated that the acid and ester forms of the excited singlet states of the ABAs would exhibit significantly different rates of internal conversion. Our results indicate that they do not, at least in a solvent of high polarity like 2-ethoxyethanol.
Decays from an experiment conducted on a bPDBA/1,3-propanediol solution in 2-ethoxyethanol at an excitation wavelength of 260 nm were virtually indistinguishable from the corresponding one employing 270 nm excitation (Fig. S8). At least within this wavelength range, the decays are independent of excitation wavelength (i.e., the same species are being excited).
Conclusions
The stability and bulk physical properties of organogels derived from 40PVAc and three aryldiboronic acids are affected acutely by the length and structure of the crosslinkers. Thus, the organogels formed from 40PVAc and either 1,4-BDBA or bPDBA in a range of organic liquids were stable under normal atmospheric conditions for long periods, while those formed with 1,3-BDBA reverted to sols after shorter periods. We hypothesize that the geometry of the crosslinks afforded by 1,3-BDBA may decrease somewhat the ratio of inter-chain to intra-chain crosslinks and, more importantly, the meta orientation of the boronic acid groups of 1,3-BDBA makes their ester linkages intrinsically less stable for steric reasons than those involving the para oriented groups of 1,4-BDBA and bPDBA.
Regardless, 1H NMR spectroscopic investigations indicate that the three ABAs react fully with 40PVAc when a large excess of OH groups is present. Although the poor resolution of the gel spectra, a consequence of the lowered mobility that attends gelation, were not amenable to definitive signal identifications, a comparison with spectra collected from the ABAs and 1,3-propanediol did allow for tentative assignments and comparisons: the bPDBA forms boronate di-ester crosslinks more efficiently than does 1,4-BDBA. This conclusion is consistent with the data from oscillatory rheology that gels made with bPDBA were slightly stiffer than those employing 1,4-BDBA as the crosslinker.
Surprisingly, the steady-state spectra and time-resolved fluorescence decays from solutions of 1,4-BDBA and bPDBA were affected very little by the addition of 40PVAc or 1,3-propanediol, despite the fact that the acids and esters should undergo radiationless decays from their excited singlet states in very different ways. These results suggest that the local environments of the ABAs in their free and crosslinked forms are very similar, that the formation of crosslinks is efficient, and that the acid or ester groups of the ABAs are not strongly linked electronically to the aromatic parts. Interestingly, the ABAs are prone to ground-state aggregation even in media where they are solubilized reasonably well.
The stability of the organogels characterized here suggests several potential uses for them, including the softening and removal of varnishes on painted surfaces, [25] and others that require application of gels that contain virtually no water under atmospheric conditions. Future studies will be directed to exploit them as well as to design related gels using the information obtained here.
References
Hall DG (2011) Boronic acids. Wiley-VCH Verlag GmbH & Co KGaA, Weinheim
Yan J, Springsteen G, Deeter S, Wang B (2004) The relationship among pK a, pH, and binding constants in the interactions between boronic acids and diols - it is not as simple as it appears. Tetrahedron 60:11205–11209. https://doi.org/10.1016/j.tet.2004.08.051
Adamczyk-Woźniak A, Jakubczyk M, Jankowski P, Sporzyński A, Urbański PM (2013) Influence of the diol structure on the Lewis acidity of phenylboronates. J Phys Org Chem 26:415–419. https://doi.org/10.1002/poc.3102
Guan Y, Zhang Y (2013) Boronic acid-containing hydrogels: synthesis and their applications. Chem Soc Rev 42:8106–8121. https://doi.org/10.1039/c3cs60152h
Brooks WLA, Sumerlin BS (2016) Synthesis and applications of Boronic acid-containing polymers: from materials to medicine. Chem Rev 116:1375–1397. https://doi.org/10.1021/acs.chemrev.5b00300
Kikuchi A, Suzuki K, Okabayashi O, Hoshino H, Kataoka K, Sakurai Y, Okano T (1996) Glucose-sensing electrode coated with polymer complex gel containing Phenylboronic acid. Anal Chem 68:823–828. https://doi.org/10.1021/ac950748d
Piest M, Zhang X, Trinidad J, Engbersen JFJ (2011) pH-responsive, dynamically restructuring hydrogels formed by reversible crosslinking of PVA with phenylboronic acid functionalised PPO–PEO–PPO spacers (Jeffamines®). Soft Matter 7:11111–11118. https://doi.org/10.1039/c1sm06230a
Meng H, Zheng J, Wen XF, Cai Z, Zhang J, Chen T (2015) PH- and sugar-induced shape memory hydrogel based on reversible phenylboronic acid-diol ester bonds. Macromol Rapid Commun 36:533–537. https://doi.org/10.1002/marc.201400648
Ding Z, Guan Y, Zhang Y, Zhu XX (2009) Layer-by-layer multilayer films linked with reversible boronate ester bonds with glucose-sensitivity under physiological conditions. Soft Matter 5:2302–2309. https://doi.org/10.1039/b901910c
Zhang X, Guan Y, Zhang Y (2012) Ultrathin hydrogel films for rapid optical biosensing. Biomacromolecules 13:92–97. https://doi.org/10.1021/bm2012696
Zhao YN, Yuan Q, Li C, Guan Y, Zhang Y (2015) Dynamic layer-by-layer films: a platform for zero-order release. Biomacromolecules 16:2032–2039. https://doi.org/10.1021/acs.biomac.5b00438
Zhang D, Yu G, Long Z, Yang G, Wang B (2016) Controllable layer-by-layer assembly of PVA and phenylboronic acid-derivatized chitosan. Carbohydr Polym 140:228–232. https://doi.org/10.1016/j.carbpol.2015.12.032
Kotsuchibashi Y, Ebara M, Sato T, Wang Y, Rajender R, Hall DG, Narain R, Aoyagi T (2015) Spatiotemporal control of synergistic gel disintegration consisting of boroxole- and glyco-based polymers via photoinduced proton transfer. J Phys Chem B 119:2323–2329. https://doi.org/10.1021/jp506478p
Kataoka K, Miyazaki H, Bunya M, Okano T, Sakurai Y (1998) Totally synthetic polymer gels responding to external glucose concentration: their preparation and application to on-off regulation of insulin release. J Am Chem Soc 120:12694–12695. https://doi.org/10.1021/ja982975d
Shiino D, Murata Y, Kataoka K, Koyama Y, Yokoyama M, Okano T, Sakurai Y (1994) Preparation and characterization of a glucose-responsive insulin-releasing polymer device. Biomaterials 15:121–128. https://doi.org/10.1016/0142-9612(94)90261-5
Ge H, Ding Y, Ma C, Zhang G (2006) Temperature-controlled release of diols from N-isopropylacrylamide-co-acrylamidophenylboronic acid microgels. J Phys Chem B 110:20635–20639. https://doi.org/10.1021/jp060914t
Du X, Jiang G, Li L et al (2015) Photo-induced synthesis glucose-responsive carriers for controlled release of insulin in vitro. Colloid Polym Sci 293:2129–2135. https://doi.org/10.1007/s00396-015-3625-5
Cash JJ, Kubo T, Bapat AP, Sumerlin BS (2015) Room-temperature self-healing polymers based on dynamic-covalent boronic esters. Macromolecules 48:2098–2106. https://doi.org/10.1021/acs.macromol.5b00210
Cromwell OR, Chung J, Guan Z (2015) Malleable and self-healing covalent polymer networks through tunable dynamic Boronic Ester bonds. J Am Chem Soc 137:6492–6495
Deng CC, Brooks WLA, Abboud KA, Sumerlin BS (2015) Boronic acid-based hydrogels undergo self-healing at neutral and acidic pH. ACS Macro Lett 4:220–224. https://doi.org/10.1021/acsmacrolett.5b00018
Nishiyabu R, Kobayashi H, Kubo Y (2012) Dansyl-containing boronate hydrogel film as fluorescent chemosensor of copper ions in water. RSC Adv 2:6555–6561. https://doi.org/10.1039/c2ra20516e
Parris MD, MacKay BA, Rathke JW et al (2008) Influence of Pressure on Boron Cross-Linked Polymer Gels. Macromol (Washington, DC, United States) 41:8181–8186. https://doi.org/10.1021/ma801187q
Nunes MAP, Martins S, Rosa ME, Gois PMP, Fernandes PCB, Ribeiro MHL (2015) Improved thermostable polyvinyl alcohol electrospun nanofibers with entangled naringinase used in a novel mini-packed bed reactor. Bioresour Technol 213:208–215. https://doi.org/10.1016/j.biortech.2016.03.058
Nunes MAP, Gois PMP, Rosa ME, Martins S, Fernandes PCB, Ribeiro MHL (2016) Boronic acids as efficient cross linkers for PVA: synthesis and application of tunable hollow microspheres in biocatalysis. Tetrahedron 72:7293–7305. https://doi.org/10.1016/j.tet.2016.02.017
Duncan T, Berrie B, Weiss R (2017) Soft, Peelable Organogels from partially hydrolyzed poly(vinyl acetate) and Benzene-1,4-diboronic acid: applications to clean works of art. ACS Appl Mater Interfaces 9:28069–28078
Acknowledgements
We thank the US National Science Foundation (Grant CHE-1502856) for supporting part of this research. TTD thanks the Achievement Rewards for College Scientists/Metro Washington Chapter for a scholar award. The Kuraray Co., Ltd. is thanked for donating the xPVAc samples employed in this research. We are grateful to Ting-An Chen for collecting the time-resolved fluorescence decays.
Dedication
We dedicate this article to Dr. Eduardo Lissi Gervaso who became ‘Profesor Emérito’ of the Universidad de Santiago de Chile on 29 November 2017. He has been a wonderful inspiration to many in his roles as a superb scientist and a principled human being. Congratulations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 1015 kb)
Rights and permissions
About this article
Cite this article
Duncan, T.T., Weiss, R.G. Influence of length and structure of aryl boronic acid crosslinkers on organogels with partially hydrolyzed poly(vinyl acetate). Colloid Polym Sci 296, 1047–1056 (2018). https://doi.org/10.1007/s00396-018-4326-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-018-4326-7