Abstract
In this study, a series of shear thickening fluids based on the suspension of silica nanoparticles in polyethylene glycol (PEG) were prepared, and the influences of particle size, concentration, and shear on the shear thickening effect were investigated by rheological measurements. It was observed that with the increase of silica concentration, the viscosity and critical shear rate of the fluid become higher, and the shear thickening phenomenon becomes more obvious. Under the same particle concentration, higher shear thickening effect and lower critical shear thickening concentration appear for smaller nanoparticles but for larger micro particles. As the temperature increases, the enhanced Brownian motion leads to the rising of critical shear rate and the strengthening of shear thickening effect. The rheological properties of these fluids are very sensitive to the shear rate with fast viscosity change for response, and smaller nanoparticles can recover their network structures more easily after shear breakage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shear thickening fluid (STF) consists of extremely small particles dispersed in the organic solvent or aqueous phase or ionomers in the nonpolar solvents. STF is slightly viscous in the normal state, and its apparent viscosity can increase sharply when subjected to shock, showing an impact resistance effect of solid. When the impact force disappears, the fluid returns to the original flexible state, and this is a reversible process [1, 2]. Due to the excellent rheological response of impact stress and energy absorptive capacity, STF has been widely used in applications such as soft armor, dampers, liquid couplings, shock absorption, and ballistic protection [2,3,4,5].
In recent years, more and more studies on STF have been reported. Qin et al. [6] investigated the dispersions of 50 nm hydrophilic silica nanoparticles in 1-butyl-methylimidazolium tetrafluoroborate under the steady shear and oscillatory shear, respectively. They found that this fluid presented shear thinning, notable shear thickening, and shear thinning behaviors successively with increasing shear rate, and the shear thickening behavior was strongly controlled by the nanoparticle content and temperature. STF also exhibited reversible property and transient response ability. Feng et al. [7] reported that the aramid fabrics treated with STF (fumed or submicron silica particles dispersed in PEG 200) exhibited a significant enhancement in quasi-static stab resistance, and the quasi-static stab-resistant properties of the treated fabrics containing submicron silica particles were better than those of the treated fabrics containing fumed silica particles. Shan et al. [8] observed that the increase in the pH value of the dispersion of 14 nm silica particles in ethylene glycol (EG) shifted the critical shear stress at the onset of shear thickening to higher values, as well as increased the low-shear-rate viscosity and led to stronger shear thinning behavior after shear thickening. Tian et al. [9] prepared shear thickening fluid with 14 nm fumed silica particles and EG at three typical temperatures, and this fluid showed larger critical shear rate and lower shear thickening ratio at higher temperature. He et al. [10] dispersed porous and non-porous silica nanoparticles into EG and noted that the porous nature in dispersed nanoparticles could remarkably influence the shear thickening effect.
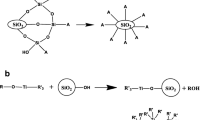
Until now, the mechanism of shear thickening is mainly described by “particle cluster” theory [11,12,13,14,15]. Since the surface of hydrophilic silica aggregates has a large amount of silanol groups (Si–OH), when silica is dispersed in an organic medium, its Si–OH groups react with the –OH groups of organic media to form hydrogen bonds, which in turn helps to form a stable network structure. At lower shear rate, the Brownian motion and intermolecular force make the damaged spatial structure recover very quickly due to smaller applied force, so the fluid viscosity does not change significantly. With the increase of shear rate, the shear force destroys the network structure and results in decreased viscosity, and when the viscosity reaches the lowest value, the fluid force just balances the Brownian force and intermolecular force. As the shear rate increases to a critical value, the fluid force becomes the main force and promotes the system of silica aggregates to form “cluster”. This cluster is separated by thin medium, which plays a significant role in hindering the flow of liquid, so the fluid viscosity increases. The smaller the viscosity of the dispersion medium is, the more intense the Brownian motion is, and the greater fluid force is required to balance.
Although a lot of work was reported on silica shear thickening fluids, the effects of particle size, especially the difference between nanoparticles and microparticles on the shear thickening efficiency of the silica fluids, have not been widely and deeply investigated, and the shear stability and reversibility were also rarely mentioned. In this paper, the silica particles with a wide particle size range from 15 nm to 10 μm were used to disperse in polyethylene glycol, and a series of colloids with different particle concentrations were prepared. The effects of particle size, concentration, and temperature on the shear thickening behaviors of these silica fluids were studied systematically by rheological measurements. Moreover, multiple shear rate scans and alternating shear rate scans between 0.1 and 100 s−1 were also operated to observe the shear thickening reversibility and viscosity recovery of these silica fluids.
Materials and methods
Materials and preparation
Five kinds of silica particles with different particle size (15 nm, 30 nm, 2 μm, 5 μm, and 10 μm) were purchased from Aladdin Reagent Co. (China). Polyethylene glycol with average molecular weight of 400 g mol−1 (PEG 400) was purchased from Sinopharm Chemical Reagent Co. (China) and used as continuous phase without further treatment.
Silica particles were dried under vacuum at 100 °C over 48 h. The silica particles were gradually added into PEG and agitated using a magnetic stirrer to obtain well-dispersed and stable solutions with different silica concentrations in weight. The resultant transparent suspensions were then left undisturbed at room temperature for 48 h to remove air bubbles.
Rheological measurements
The rheological measurements were performed at 25 °C by using a Physica MCR-302 rheometer (Anton Paar, Germany) with a cone-and-plate geometry (50 mm in diameter and 0.985° in cone angle). To eliminate any previous shear history and allow the samples establish their equilibrium structure, a steady pre-shear was applied for 300 s at a shear rate of 10 s−1 followed by a 60-s rest period before each measurement. Steady shear measurements were carried out as a function of shear rate from 0.01 to 1000 s−1, and rheological parameters of shear stress and apparent viscosity were obtained for each shear rate. The initial viscosity (η0) means the viscosity before shear rate scans, the peak viscosity means (ηmax) the maximum viscosity after shear thickening, the critical shear rate (\( \dot{\gamma} \)c) means the shear rate at which the shear thickening begins, and the peak shear rate (\( \dot{\gamma} \)P) means the shear rate at which the maximum viscosity appears after shear thickening. Multiple shear rate scanning tests with 5 cycles and alternating shear rate scanning between 0.1 and 100 s−1 were also operated. Shear thickening ratio (R) was used to characterize the shear thickening effect of the silica fluids:
Results and discussion
Effects of particle size and concentration on the shear-thickening behaviors
In this paper, we systematically studied the effects of particle size, including both the nanoparticles and microparticles, on the shear thickening behaviors of the silica fluids. Figure 1 illustrates the plots of viscosity versus shear rate for the silica fluids with different particle size and concentration, and the values of critical shear rate (\( \dot{\gamma} \)c), peak shear rate (\( \dot{\gamma} \)p), initial viscosity (η0), peak viscosity (ηmax), and shear thickening ratio (R) obtained from Fig. 1 are listed in Table 1. It can be seen from Fig. 1 that although the particle size and concentration are different, the trend of each curve with shear thickening effect is basically the same. With the increase of shear rate, the viscosity of the fluid decreases. As soon as the shear rate increases to the critical point (critical shear rate), the viscosity begins to increase rapidly, much higher than the initial viscosity. However, when the shear rate continues to increase, the viscosity begins to decline after experiencing the maximum. The molecular chains of the dispersion medium PEG contain O atoms and –OH groups, the surface of silica agglomerates has a large amount of Si–OH groups, and when silica powder is dispersed into the PEG medium, Si–OH groups on the surface of silica aggregates can react with the –OH groups in PEG to form hydrogen bonds. After the network structure is formed between these two phases, a stable dispersion system is obtained. With the increase of shear rate, the network structure in the dispersion system is destroyed with the formation of some relatively isolated silica aggregates, and then the viscosity begins to decrease. When the shear rate is further increased, the fluid force has become the main force and promotes the isolated silica aggregates to form cluster, and these particle clusters are separated by the thin medium. Although this combination is not stable, the flow resistance is greatly enhanced, thus making the viscosity increase and resulting in the occurrence of shear thickening phenomenon. However, when the shear rate continues to increase, the particle clusters are broken under strong shear stress, which leads to a shear thinning behavior again.
As shown in Fig. 1a with a particle size of 15 nm, a remarkable shear thickening behavior occurs when the silica concentration is no less than 10%, and the viscosity increases greatly from 1.18 to 19.52 Pa s−1 for the 15% fluid after shear thickening. As for Fig. 1c with a particle size of 2 μm, significant shear thickening behavior appears at much higher particle concentration of 70%, and the viscosity increases from 1.13 to 18.98 Pa s−1. In both cases, whether for silica nanoparticles or micro-particles, the viscosity has increased by an order of magnitude after shear thickening of fluid, resulting in an extremely high shear thickening effect above 1500%. However, silica micro-particles help to obtain greater particle concentration fluids and exhibit higher critical shear thickening concentration and worse stability with particle sedimentation after long period storage when compared with silica nanoparticles. It can also be observed from Fig. 1 and Table 1 that under the same particle size, the higher the silica concentration is, the greater the initial viscosity and peak viscosity of the fluid are, the higher the critical shear rate and peak shear rate are, and the more remarkable the shear thickening effect is. When the concentration is low, the viscosity of the fluid does not change significantly. At low concentration, the reduction of solid particles leads to the weakening of the interactions between silica and PEG, and the viscosity of the fluid decreases correspondingly. After the shear rate increases, few particle clusters form, and the flow resistance is low so that the critical shear rate is low and the thickening effect is not obvious. Although both nanometer and micro scale silica particles show similar increasing viscosity of the fluid with particle size increasing, they exhibit different influences of particle size on the shear thickening of the fluid, with higher shear thickening effect and lower critical shear thickening concentration for smaller nanoparticles but for larger micro particles under the same particle concentration. Thus it can be seen that the fluid with smaller nanoparticles is more sensitive to the external shear field and exhibits greater shear thickening effect even at lower particle concentration, which should be attributed to its stronger particle interactions and faster smart response.
Feng et al. [7] also explored the effect of particle size on the shear thickening behavior of silica fluid. They dispersed 100 nm fumed silica particles and 500 nm submicron silica particles into PEG with a molecular weight of 200 g mol−1. It was found that fumed silica-STFs exhibit a shear thickening phenomenon at lower solid content comparing to submicron silica-STFs. This phenomenon is explained by the existing form of silica particles in STFs. Submicron silica particles are monodisperse and fumed silica particles are aggregates in STFs. The open nature of fractal aggregates allows fumed silica particles to occlude considerable amounts of liquid, providing a much larger effective solid volume fraction which contributes to higher viscosity. Higher viscosity leads to weaker Brownian motion, and the shear force can easily drive particles together to form clusters at lower shear rate, because Brownian motion is no longer capable of restoring the equilibrium microstructure. Therefore, fumed silica-STFs with nanometer size particles exhibit a shear thickening phenomenon at a lower silica concentration compared to submicron silica-STFs. This is consistent with our experimental conclusion.
Even for the fluids of silica nanoparticles in the same PEG solvent, the particle size still exhibits great influence on the shear thickening behaviors. The 15 wt% fluid of 12 nm silica in PEG 400 shows a critical shear rate of 60 s−1 and a shear thickening ratio of about 900% [10], and the 20 wt% fluid of 20 nm silica in PEG 400 gives a critical shear rate of 1 s−1 and a shear thickening ratio of about 300% [16], while the 23 wt% fluid of 50 nm silica in PEG 400 presents a much higher critical shear rate of 100 s−1 and a much lower shear thickening ratio of 11% [6]. It seems that both the critical shear rate and shear thickening ratio decrease with the increase of particle size. In the fluid containing larger particles, the hydrogen bonds between particles and dispersion medium become less, and the resultant network structure will be more easily destroyed by the shear force, thus leading to a decrease in the critical shear rate. Meanwhile, at the same particle concentration, larger particle system means the reduction of the amount of particles and the corresponding particle clusters, thus causing the remarkable decrease in the resistance to flow and the shear thickening ratio. In our study, the 15 wt% fluid of 15 nm silica, 30 wt% fluid of 30 nm silica, and 20 wt% fluid of 30 nm silica show much higher shear thickening ratio values of 1554.2, 944.9, and 327.5% than those of previous work reported, which may be due to the better particle dispersion and more uniform particle size in our silica fluids.
Effects of temperature on the shear-thickening behaviors
The effect of temperature on the viscoelasticity of the silica fluids is given in Fig. 2. The 15% silica fluid with 15-nm particles presents obvious shear thickening at each temperature, even at 85 °C. As the temperature increases from 5 to 85 °C, although the viscosity of the fluid decreases by two orders of magnitude or so, the basic trend of viscosity curve remains unchanged, which implies that the temperature does not affect the shear thickening performance of silica fluid, and these STFs are very stable and could bear a range of temperatures in their further practical applications. The shifting of the viscosity curves downwards should be attributed to the strengthening of the interaction force between the particles and the intensifying of Brownian exercise with temperature increasing.
The plots of critical shear rate and shear thickening ratio versus temperature for the 15% silica fluid with 15-nm particles are illustrated in Fig. 3. The critical shear rates of the silica fluid are 3.56, 121, and 91 s−1 for the temperatures of 5, 65, and 85 °C, respectively, increasing with the temperature exponentially until 65 °C and then decreasing at 85 °C. Obviously, the critical shear rate is very sensitive to the temperature field and changes greatly with the rising of temperature. The shear thickening ratio of the silica fluid changes greatly with temperature too, especially at high temperature, increasing by six times from 5 to 85 °C. Due to the increase of Brownian motion at higher temperature, silica particles move more quickly and then are far away from each other. As the shear rate increases, the force balance ruptures, and the hydrodynamic force exceeds the interactions between the particles, and then the shear thickening behavior occurs for the silica fluids. At higher temperature, hydrodynamic force makes the particles approaching and clusters formation more difficult. In this case, stronger force is required, so the critical shear rate increases accordingly. The rheological behaviors of the STF with 680 nm silica particles in ethylene glycol (EG) at 42.5 wt% showed similar results when the temperature varies from 5 to 40 °C [10]. However, the fluid of 7 nm silica in three-armed glycerol poly(propylene oxide) with a molecular weight of 700 g mol−1 (PPG 700) merely showed obvious shear thickening effect at low temperature, and the shear thickening behavior disappeared above 40 °C [16]. Thus, it can be seen that different particles and different solvents may lead to different effects and sensitivity of temperature as for the shear thickening behaviors.
Shear-thickening reversibility
The effect of multiple shear rate scans on the shear-thickening behaviors of the silica fluids is shown in Fig. 4 with the viscosity as a function of shear rate for the 15% fluid with 15-nm particles. It can be noted from Fig. 4 that the 15 nm silica fluid can still exhibit remarkable shear thickening behavior even after five continuous shear scans. The peak viscosity from the first scan is much higher than those from the other following scans, although the initial viscosity does not change too much for these five scans. The significant viscosity decrease indicates that the first shear run has destroyed some of the network structures inside the fluid, but the fluid recovers quickly (even not fully recovered) and becomes stable after the second shear scan with little change of the viscosity and shear thickening effect. Thus, it can be seen that it is much easier for smaller nanoparticles to reorganize and reconstruct network structures after shear fracture.
Values of critical shear rate, initial viscosity, peak viscosity, and shear thickening ratio obtained from multiple shear rate scans for the 15% silica fluid with 15-nm particles are given in Table 2. As seen from Table 2, during multiple shear scans, the critical and peak shear rates are the smallest, but the peak viscosity is the largest for the first shear scan. Due to the first shear breakage, not only the initial and peak viscosity decrease obviously, but also the shear thickening ratio becomes much lower after the first shear scan. Yeh et al. [17] conducted the same rheological tests on the mixed liquid of 12 nm hydrophobic fumed silica nanoparticles into PPG with a molecular weight of 3000 g mol−1 to investigate the recoverability of the mixed liquid after being disturbed. They found that the larger the number of tests was, the lower the viscosity of the fluid would be, and the experimental result began to stabilize from the second test due to the dispersed aggregation. The viscosity of the silica/PPG system dropped by about 4% after the first shear, less than 87% of the result in Fig. 4, which may be attributed to the relatively high molecular weight of the dispersed solvent. The multiple shear experimental results verify that the silica fluids have a very stable shear thickening effect after the first shear fracture and can be reused for several times, which will be very important for practical applications.
The shear thickening stability was also investigated by alternating shear rate scans between 0.1 and 100 s−1, and the results of the 15% silica fluid with 15-nm particles and 20% silica fluid with 30-nm particles are illustrated in Fig. 5 with the viscosity as a function of time. The silica fluids suffer the great change of shear rate from 0.1 to 100 s−1 by turns, lasting 120 s for each scan every time. In Fig. 5a, the viscosity of the 15 nm silica fluid keeps about 1.5 Pa s−1 for 0.1 s−1 all along, without any shear thickening behavior. However, when the shear rate is increased to 100 s−1, the viscosity of the fluid increases to 11 Pa s−1 instantaneously. Obviously, the silica fluid is experiencing the shear thickening process. After the shear rate is decreased to 0.1 s−1, the viscosity of the fluid decreases to about 1.5 Pa s−1 immediately again. As seen from Fig. 5b, the 30 nm silica fluid also experiences shear thickening when the shear rate is increased from 0.1 to 100 s−1, but its viscosity and shear thickening effect are lower than those of the 15 nm silica fluid. Moreover, after cycling alternative shear rate scans, the viscosity and shear thickening effect keep decreasing for the 30 nm silica fluid while keeping gradual increasing until stable for the 15 nm silica fluid. Similar to the results of multiple shear rate scans, it is much easier for the silica fluid containing smaller nanoparticles to recover their original network structures after shear breakage, and the fluid can still exhibit higher viscosity and remarkable shear thickening behavior even after cycling shear scans for several times. Nevertheless, different from multiple shear scans, alternating shear scan does not influence the viscosity of the 15 nm silica fluid at high shear rate (100 s−1), and the viscosity even becomes a little higher after particle structural recovery. The silica fluids present transient response ability. The remarkable change of viscosity suggests that the silica fluid is very sensitive to shear rate, which can be explained by the instability of the nanoparticle clusters formed in the shear thickening region. The reversible property and the transient response ability imply that the silica nanoparticle clusters can transform dynamically with the change of shear rate [6].
Shear-thickening mechanism
As shown in Fig. 6, a theoretical model is developed to describe the strain-thickening mechanism of the silica fluids. Silica nanoparticles are dispersed randomly in PEG at the initial phase (Fig. 6 (a)). At the onset of shear, the silica nanoparticles are arranged into layers, which makes the viscosity reduced (Fig. 6 (b)). At the critical shear rate, hydrodynamic force causes instability, and the broken layered flow forces the silica nanoparticles to aggregate with the formation of some clusters, thus resulting in the increase of viscosity in low solid content fluids (Fig. 6 (c)). When more clusters are formed and become jam in high solid content fluids (Fig. 6 (d)), the viscosity of the fluid increases notably. Moreover, nanoparticle aggregation is a metastable structure, and a partial section will be continuously broken and rebuilt under shearing.
Conclusions
In order to better understand the shear thickening properties and design the components needed for practical applications, the rheological behaviors of silica fluid were investigated at different particle sizes, concentrations, and temperatures, and multiple shear rate scans and alternating shear rate scans were also performed besides the normal steady shear test. The silica fluid behaves as a non-Newtonian fluid with shear thickening behavior. With the increase of shear rate, the system presents shear thinning, shear thickening, and then shear thinning phenomenon in turn. As the silica concentration increases, the critical and peak shear rate of the fluid increases, and the initial viscosity, peak viscosity, and shear thickening effect become larger. Silica microparticles exhibit higher critical shear thickening concentration and worse stability when compared with silica nanoparticles. Brownian motion becomes stronger at higher temperature, which leads to the reduction of viscosity but the increase of critical shear rate and the enhancement of shear thickening effect. During multiple and alternating shear rate scans, the silica fluid with smaller nanoparticles shows better structural recovery, higher stability, and stronger shear thickening effect.
References
Lee YS, Wagner NJ (2003) Dynamic properties of shear thickening colloidal suspensions. Rheol Acta 42:199–208
Lee YS, Wetzel ED, Wagner NJ (2003) The ballistic impact characteristics of Kevlar® woven fabrics impregnated with a colloidal shear thickening fluid. J Mater Sci 38:2825–2833
Gong XL, Xu YL, Zhu W, Xuan SH, Jiang WF, Jiang WQ (2014) Study of the knife stab and puncture-resistant performance for shear thickening fluid enhanced fabric. J Compos Mater 48:641–657
Zhang XZ, Li WH, Gong XL (2008) The rheology of shear thickening fluid (STF) and the dynamic performance of an STF-filled damper. Smart Mater Struct 17:035027
Decker MJ, Halbach CJ, Nam CH, Wagner NJ, Wetzel ED (2007) Stab resistance of shear thickening fluid (STF)-treated fabrics. Compos Sci Technol 67:565–578
Qin J, Zhang G, Shi X, Tao M (2015) Study of a shear thickening fluid: the dispersions of silica nanoparticles in 1-butyl-3-methylimidazolium tetrafluoroborate. J Nanopart Res 17(8):333
Feng XY, Li SK, Wang Y, Wang YC, Liu JX (2014) Effects of different silica particles on quasi-static stab resistant properties of fabrics impregnated with shear thickening fluids. Mater Des 64:456–461
Shan L, Tian Y, Jiang JL, Zhang XJ, Meng YG (2015) Effects of pH on shear thinning and thickening behaviors of fumed silica suspensions. Colloids Surf A Physicochem Eng Asp 464:1–7
Tian TF, Peng GR, Li WH, Ding J, Nakano M (2015) Experimental and modelling study of the effect of temperature on shear thickening fluids. Korea-Aust Rheol J 27:17–24
He QY, Gong XL, Xuan SH, Jiang WQ, Chen Q (2015) Shear thickening of suspensions of porous silica nanoparticles. J Mater Sci 50:6041–6049
Boersma WH, Laven J, Stein HN (1995) Computer simulations of shear thickening of concentrated dispersions. J Rheol 39:841–860
Bossis G, Brady JF (1989) The rheology of Brownian suspensions. J Chem Phys 91:1866–1874
Maranzano BJ, Wagner NJ (2001) The effects of particle size on reversible shear thickening of concentrated colloidal dispersions. J Chem Phys 114:10514–10527
Maranzano BJ, Wagner NJ (2002) Flow-small angle neutron scattering measurements of colloidal dispersion microstructure evolution through the shear thickening transition. J Chem Phys 117:10291–10302
Wu QM, Ruan JM, Zhou ZC, Huang BY (2006) Study on the rheological properties of silica/polyethylene glycol non-Newtonian flow. Acta Phys -Chim Sin 22:48–52
Warren J, Offenberger S, Toghiani H, Pittman Jr CU, Lacy TE, Kundu S (2015) Effect of temperature on the shear-thickening behavior of fumed silica suspensions. ACS Appl Mater Interfaces 7:18650–18661
Yeh FY, Chang KC, Chen TW, Yu CH (2014) The dynamic performance of a shear thickening fluid viscous damper. J Chin Inst Eng 37:983–994
Funding
This research was supported by the Natural Science Foundation of China (Grant No. 11572189).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yu, M., Qiao, X., Dong, X. et al. Shear thickening effect of the suspensions of silica nanoparticles in PEG with different particle size, concentration, and shear. Colloid Polym Sci 296, 1119–1126 (2018). https://doi.org/10.1007/s00396-018-4325-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-018-4325-8