Abstract
In the duration of initial flocculation stage, required time for waiting prior to the gravitational collapse of Na montmorillonite suspension in the semi-dilute coagulated regime (with solid volume fraction of 1.097 × 10−4) was analyzed as a function of ionic strength for the two different pH values (pH = 4.0 (acid) and pH = 9.5(alkaline)). The obtained result clearly demonstrated that the duration time correlates the coagulation rate of clay particles. That is, the duration time is shorter (but not to be zero) for the system undergoing rapid coagulation which takes place under sufficiently high ionic strength. In this region, the effect of pH is shielded by the complete compression of electric double layer. However, the duration becomes longer with a decrease of ionic strength in accordance with the decrease of the rate of coagulation. In addition, under the condition approaching to the region near critical coagulation concentration, further longer duration is observed for the case of high pH value as compared to that obtained for the case of lower pH value. The reason for the additional increase of duration was ascribed to the elimination of positive charge along the edge of clay sheet under alkaline condition. In the case of low pH, on the contrary, the effect of the oppositely charged edge(+)-to-face(−) interaction is considered to induce attraction between clay particles which contributes to induce heterocoagulation. This effect will result in the shorter duration time for the case of low pH than that of high pH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Montmorillonite is an important clay mineral for agricultural and environmental processes as well as industrial applications [1]. Most of them are related to an aqueous suspension, which demonstrates very unique and useful characteristics. The remarkable points for considering the characteristics of this suspension are the shape and the charging behavior of unit particle in aqueous suspension. That is, the unit of this clay particle is a sheet of thin layers of alumina silicate with a thickness of approximately 1 nm and length of facial distances of a few hundred nanometers [2]. Face of the sheet is negatively charged due to the isomorphic substitution, and edge of the sheet is charged either positively or negatively due to pH-dependent dissociation or adsorption of chemical groups located at the edge of sheet [3]. Understanding of macroscopic physical property or mechanical behavior of suspension in terms of microscopic interaction between clay particles can be regarded as one of the most fundamental subjects in the domain of clay rheology. It is known that the interaction between clay particles is a function of chemical condition, such as ionic strength and pH values [4]. On the basis of rheological data, Abend and Lagaly [5] summarized the sol-gel transitions of sodium montmorillonite dispersion as functions of volume fraction and ionic strength. They emphasized that the transition from the repulsive gel, sol, attractive gel, and to sediment as the ionic strength is increased. Sedimentation behavior, on the other side, is more or less related to the rheological data and is more directly important in the analysis of the transportation and separation of colloidal particles in the aquatic environment. For example, in the control of the discharge of muddy colloidal dispersion produced by puddling, the prediction of sedimentation behavior of flocculated colloidal soil is critically important. However, relatively little attentions have been paid so far on the sedimentation of the montmorillonite suspension as compared to rheology.
In the previous investigation, we have reported on the rate of sedimentation using coagulated suspension of sodium montmorillonite in the semi-dilute regime as a function of ionic strength for the two different pH values (pH = 4.0 and pH = 9.5) [6]. Semi-dilute regime is defined by the colloid concentration where the coagulated dispersed phase initially demonstrates very slow rate of sedimentation termed flocculation stage, followed by a sudden collapse of coagulated network with maximum sedimentation rate named settling stage and ended by the long lasting consolidation stage [7]. From the analysis of the maximum rate of sedimentation in the second stage as a function of the volume fraction of colloid [8] invoking the method of Richardson and Zaki [9], we have demonstrated that essentially different two types of flocs which are formed in the domain of attractive gel and the sediment will behave in qualitatively different ways. This difference was also verified by the tendency of “ultimate” sediment height brought about by the various chemical conditions. These results were qualitatively consistent with the picture proposed by Abend and Lagaly [5].
In the course of measurements and analysis in our previous study on the sedimentation behavior, we have noted the considerable difference in the duration of initial flocculation stage. The duration of initial stage corresponds to the lag time prior to gel collapse and it can be regarded as one of the most important indexes to characterize gel collapse. So far, gel collapse of many types of aggregation [10,11,12,13,14] has been reported; the prediction of delayed sedimentation behavior has not been fully successful. In addition, it can be pointed out that analyses are rather limited for the system of weakly flocculated dispersions in which colloidal flocculation is induced by the depletion effect or by the effect of secondary minimum attraction. Poon and co-workers have been reported their series of experimental study on the gravitational collapse of colloidal network formed in the colloid and non-adsorbing polymer mixture [15,16,17,18]. Bartlett et al. [14] reported the duration time increases as polymer concentration increases. Buscall et al. [19] tried to interpret the mechanism of gel collapse by the interacting potential which characterizes the gel strength. Developing the concept of gel strength, they reached to the conclusion to introduce the concept of coarsening effect which is added to explain the consequence of heterogeneity at the onset of collapse.
We can say, however, flocculation/coagulation which is ubiquitous in nature and engineered system are not only limited to weakly flocculated system and are mostly controlled irreversibly by the rate process; the criteria on the basis of only interacting potential are not sufficient. Using kaolinite suspension dipped in 1 M NaCl, Nakaishi et al. carried out careful observation of the sedimentation behavior of clay suspension, reporting significant effect of container size [20]. Clay particles are expected to have more or less anisotropic nature with respect to shape and charging behavior; these factors can be considered to be affected in the sedimentation. Very recently, Ali and Bandyopadhyay [21] tracked the collapsing interface of sodium montmorillonite gel prepared for different ionic strengths by applying optical method. They confirmed that duration of initial stage will decrease by increasing ionic strength and also pointed out the importance of edge(+)-to-face(−) attractive interactions. Tombacz et al. [22] reported systematic analysis of the stability of montmorillonite suspension based on the surface characterization by classical titration. That is, the edge(+)-to-face(−) heterocoagulation can reduce the value of CCC (critical coagulation concentration) in the acidic condition where the pH value is lower than that of PZC (point of zero charge) of the sheet edge, while more probable situation of face(−)-to-face(−) homocoagulation at alkaline condition can enhance the colloidal stability of the montmorillonite suspension. These two reports [21, 22] are critically important to understand the sedimentation behavior of montmorillonite gel. In order to confirm their validity, one needs to design the experiment to measure the settling velocity of coagulated montmorillonite suspension as a function of ionic strength and pH with rather large-scale setup taking into account the result of [20] and size of individual flocs.
In the present study, we restrict our attention to the early stage of sedimentation for different pH and ionic strength to clarify if the microscopic heterogeneous interaction between clay particles will influence the macroscopic behavior of sedimentation. For this purpose, we focus on the duration of initial stage sedimentation of sodium montmorillonite flocs as function of ionic strength and pH.
Experimental section
Determination of the flocculation time
When studying the mechanics of coastal cohesive sediment, Imai [7] classified three patterns of sedimentation behavior based on the concentration of solid in the dispersion. They are free settling, zone settling, and consolidation. The three regimes can be, in other words, called as dilute, semi-dilute, and concentrated, respectively. Here, we focus on the second regime. In this regime, the sedimentation can be recorded simply by the location of boundary between the region occupied by coagulated floc and supernatant. As schematically illustrated in the previous study [6], the sedimentation process can be separated into three stages. The first stage is characterized by very slow or apparently zero-velocity of the boundary. Duration of this stage is called as the flocculation time. As demonstrated in Fig. 1, this time was determined by the corresponding time to reach the cross point of the two tangent lines of the first stage and the second sedimentation stage where the maximum sedimentation rate is observed [16].
The schematic drawing for determination of temporal scale for the flocculation stage (the flocculation time, t f , min) of the suspensions as intersection point of tangents at linear parts between the first stage as flocculation process and the second stage with maximum sedimentation rate in the plots of the boundary (between supernatant and sediment) location (H, mm) vs. the elapsed time (t, min)
Materials
High-purity montmorillonite powder (Kunipia-F) was purchased from Kunimine Co. Ltd., Tokyo. The powder was dispersed in the deionized water, and the suspension was left undisturbed to remove the coarse components by sedimentation. Sufficiently, high concentration of NaCl (1.0 M) was used to replace the exchangeable cations with Na+. The dispersion was dialyzed against distilled water until the electrical conductivity of the percolate decreased to 2 μs/cm. After dialysis, the dispersion was stored in a refrigerator as the stock dispersion. Prior to use, the stock dispersion was stirred for 30 min and then ultrasonicated for another 30 min.
Sample preparation
The solid content in suspension (1.097 × 10−4, v/v) was determined on the basis of preliminarily experiments beginning with zone settling at the highest ionic strength (1.5 M NaCl) in this study. The pH values of the samples were adjusted at 9.5 ± 0.3 and 4.0 ± 0.3 by the addition of NaOH solution, guaranteeing that the edges were charged negatively and positively, respectively [22]. The NaCl concentration was lowered down to 50 mM (just above the CCC in case of suspension under acidic condition [3, 22]. The initial height of the suspensions was 15.8 cm (the inner height of the cylinder was 20.0 cm), as in the previous study [23]. The cylinder diameter was 5.0 cm to take into account adequate elimination of the “wall effect” and hydrodynamic wake capture [24]. The mixtures containing chemicals and solids were rotated end-over-end for five times at a frequency 1/2 [Hz]; then, the suspension was immersed in a water tank for 1 min and subjected to ultrasound at 100 kHz. The sonication time was included in data acquisition. The room temperature was maintained at 20 ± 1 °C. The settling curve at each pH value and ionic strength was obtained from the plot of boundary position between sediment and supernatant against elapsed time.
Results
Locations of the boundary between flocculated sediment and supernatant are plotted as a function of elapsed time for different ionic strength in Fig. 2. Figure 2a represents the result at pH 4.0 and Fig. 2b at pH 9.5, respectively. The demonstrated result for the high pH value agreed with those reported by Miyahara et al. [23, 25]. As demonstrated in these figures, not only the rate of sedimentation [6], but also the duration of initial flocculation stage is strongly influenced by the ionic strength. As the main experimental manifestation of this study, the duration of initial flocculation stage for the serial variation of pH and ionic strength is summarized in Fig. 3. As demonstrated in this figure, the time declines abruptly in the range of NaCl 0.5–0.75 M with an increase of ionic strength. In contrast to the condition of lower ionic strength, the effect of pH is diminished under the condition of higher ionic strength.
Flocculation time as a function of ionic strength for the different pH values (pH = 4.5 and pH = 9.0). Due to the un-coagulated suspension under the condition of pH 9.0 and ionic strength 0.05 M NaCl, the relative movement of boundary between supernatant and coagulated suspension could not be recorded in Fig. 2. Therefore, the duration time at the condition (pH 9.0 and NaCl 0.05 M) cannot be calculated
There are two points which should be focused on in the result of the duration time for the lower ionic strength. One is that the duration time is much longer than the case of high ionic strength. The other is, in the region of low ionic strength, the remarkable difference of the duration of time was confirmed by the difference of pH. That is, longer duration was detected in the case of high pH. In addition, for the case of high pH, clear boundary between sediment and supernatant was not observed for the case of lowest ionic strength c.a. 0.1 M.
Discussion
Understanding of the macroscopic behavior in terms of microscopic interaction between colloidal particles can be regarded as one of the most important subjects of the rheology of colloidal dispersion. Our focus of the macroscopic behavior in the present study is the duration of initial flocculation stage in the sedimentation of flocculated suspension of montmorillonite. This issue is related to so-called gravitational collapse of gel network which has attracted many attentions mainly under the condition of weakly flocculated system [10,11,12,13,14,15,16,17,18,19,20,21]. Based on the observation throughout the experiments in the present study, the scenario of gel collapse for weakly flocculated suspension can be drawn as follows.
In Fig. 4, the schematic representation on the progress of sedimentation in the initial flocculation stage is outlined. In the beginning, when the mixture of dispersion was left to stand, small flocs are suspended independently as illustrated in Fig. 4a. The rate of sedimentation at this stage is very slow reflecting the small size of flocs composing the system. However, each floc will touch and stick each other upon collision induced by sedimentation or weak fluid flow or Brownian motion to form a weak network as depicted in Fig. 4b. When the concentration is sufficiently high, the formed network will soon fill the space of the container. With this situation, a certain part of floc structure will act as a stress-carrying strand which will essentially resist against the compression due to self-weight of sediment. Consequently, the rate of sedimentation becomes apparently small. However, the sticking between flocs is not completely rigid allowing the relative motion of the two touching clusters to result in the growth of flocs as illustrated in Fig. 4c. An increase of floc size will result in the generation of big pore and the concentration of stress in the strand which is depicted in dotted black particles in the right figure of Fig. 4c. Under such condition, channeling and densification of flocs due to the rearrangement between clusters will be also enhanced. If the concentrated stress overcomes the yield strength of gel structure at this stage, a big deformation that was induced by the breakup of a developed floc to induce the gel collapse will be induced. Probably, this sequence starting from the first breakup to big deformation is a kind of feed-forward system. Coarsening effect due to heterogeneity suggested by Buscall et al. is presumably related to this scenario.
Schematic representation for the temporal variation of initial flocculation stage prior to gel collapse. a Just after mixing, small flocs composed of the lamellae of montmorillonite are formed and suspended independently. In this stage, the rate of sedimentation is essentially small due to the small size flocs. b Secondly, the formed flocs connect each other to form the network structure spanning-fill throughout the container. The stress-carrying strand marked as dotted black circles will resist against the compression by self-weight of network. Due to this resistance, apparent rates of sedimentation are essentially low. Particles or clusters colored in gray will contribute to the self-weight. White particles or clusters do contribute either self-weight or strength of network. c In the process of flocculation stage, the size of flocs indicated with a dotted circle will grow due to the collision with suspended clusters. The size of flocs in the gel network will also increase by the rearrangement process between neighboring flocs in the sediment. By the progress of initial flocculation stage, stress concentration will be taken places. This situation is illustrated as dotted circles in the right figure of (c)
This scenario is rather speculative; however, it is consistent when we regard that the duration time is an index of time when the system reaches the certain degree of coagulation. That is, the faster the rate of coagulation, the shorter the duration becomes. The difference of the observed duration time between that of higher salt concentration (higher than 0.75 M) and that of lower salt concentration is consistent with this picture. In addition, in the comparison of the slow coagulation regime, the rate of coagulation under low pH is faster than that under high pH as being analogous to the analysis of colloidal stability in montmorillonite suspension [22]. That is, the faster rate of coagulation is because of higher probability of edge-to-face collisions due to positively charged sheet edge at low pH in this work. Our results of the duration time obtained for lower ionic strength (lower than 0.5 M) are qualitatively consistent with this picture. In the analysis of colloidal stability, the approach of two particles to overcome the electrostatic repulsion is discussed. In this sense, the situation which the two particles touching each other making a contact after the consequence of successful approach is slightly different. Our experimental result can be regarded as an evidence of the validity of the stability analysis for the extensive use in the flocculated region.
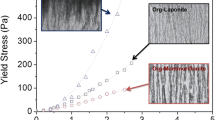
In addition, there seems a qualitatively remarkable difference of floc strength against compaction. That is, flocs formed in the region of salt concentration higher than 0.75 M have stronger bonds which result in the large or coarse floc as compared with that those formed in the region of lower salt concentration as demonstrated in Fig. 5. It can be considered that this difference can be corresponded to the boundary between sediment and attractive gel noted by Abend and Lagaly [5]. Further investigations on the direct observation of morphology of sediment under gravitational collapse with the resolution level of individual flocs [26] are necessary.
Conclusions
Gel collapse or gravitational collapse in sedimentation process of flocculated colloidal suspension is conveniently defined as experimental phenomenon of abrupt increase in settling velocity of boundary between sediment and supernatant. In the present study, coagulated suspension of sodium montmorillonite in semi-dilute regime was chosen for the analysis. In this regime, the initial stage is essentially regarded as a dominative stage of flocculation. In this stage, flocs grow keeping suspended state until a certain degree of coagulation is withstanding against large deformation by self-weight. When the rate of flocculation of suspending particles is faster, the required time to reach the certain degree of flocculation becomes shorter. Alternatively, the duration of this stage can be regarded as an index of the rate of flocculation. In this context, the sedimentation behavior was observed as a function of NaCl concentration ranging from CCC to 1.5 M for two different pH values (pH = 4.0 and 9.5). From the result of experiment, it was confirmed that (1) increase of ionic strength reduces the duration time and (2) effect of pH is remarkable in the range of low ionic strength with the longer time at pH 9.5 than at pH 4.0. In the latter, positively charged edges are considered to attract to negatively charged face of montmorillonite sheet. However, the difference becomes negligibly small for the system of sufficiently high ionic strength.
References
van Olphen (1977) An introduction to clay colloid chemistry, 2nd edn. Wiley, London,
Adachi Y, Nakaishi K, Tamaki M (1998) Viscosity of dilute suspension of sodium montmorillonite in an electrostatically stable condision. J Colloid Interface Sci 198:100–105
Lagaly G, Dekany I (2013) Colloid clay science. In: Bergaya F, Lagaly G (eds) Handbook of clay science, Part A: fundamentals, 2nd edn. Elsevier, Amsterdam, pp. 243–345
Luckham PF, Rossi S (1999) The colloidal and rheological properties of bentonite suspensions. Adv Colloid Interf Sci 82:43–92
Abend S, Lagaly G (2000) Sol–gel transitions of sodium montmorillonite dispersions. Appl Clay Sci 16:201–227
MY W, Adachi Y (2016) Effects of electrolyte concentration and pH on the sedimentation rate of coagulated suspension of sodium montmorillonite. Colloids Surf A Physicochem Eng Asp 506:686–693
Imai G (1980) Settling behavior of clay suspension. Soils Found 20:61–77
Michaels AS, Bolger JC (1962) Settling rates and sediment volumes of flocculated kaolin suspensions. Ind Eng Chem Fundam 1:24–33
Richardson JF, Zaki WN (1954) Sedimentation and fluidization: Part I. Trans Inst Chem Eng 32:35–53
Allain C, Cloitre M, Wafra M (1995) Aggregation and sedimentation in colloidal suspensions. Phys Rev Lett 741:478–1481
Cipelletti L, Manley S, Ball RC, Weitz DA (2000) Universal aging features in the restructuring of fractal colloidal gels. Phys Rev Lett 84:2275–2278
Manley S, Skotheim JM, Mahadevan L, Weitz DA (2005) Gravitational collapse of colloidal gels. Phys Rev Lett 94:218–302
Poon WCK, Selfe JS, Robertson MB, Ilett SM, Pirie AD, Pusey PN (1993) An experimental study of a model colloid-polymer mixture. J Phys II France 3:1075–1086
Bartlett P, Teece LJ, Faers MA (2012) Sudden collapse of a colloidal gel. Phys Rev E 85:021404
Poon WCK, Starrs L, Meeker SP, Moussaïd A, Evans RML, Pusey PN, Robins MM (1999) Delayed sedimentation of transient gels in colloid–polymer mixtures: dark-field observation, rheology and dynamic light scattering studies. Faraday Discuss 112:143–154
Starrs L, Poon WCK, Hibberd DJ, Robins MM (2002) Collapse of transient gels in colloid-polymer mixtures. J Phys Condens Matter 14:2485–2505
Poon WCK (2002) The physics of a model colloid–polymer mixture. J Phys Condens Matter 14:R859–R880
Harich R, Blythe TW, Hermes M, Zaccarelli E, Sederman AJ, Gladden LF, Poon WCK (2016) Gravitational collapse of depletion-induced colloidal gels. Soft Matter 12:4300–4308
Buscall R, Chounhury TH, Faers MA, Goodwin JW, Luckham PA, Partridge SJ (2009) Towards rationalising collapse times for the delayed sedimentation of weakly-aggregated colloidal gels. Soft Matter 5:1345–1349
Nakaishi K, Ooi S, Kobayashi M (2012) Effects of container diameter and volume fraction on the sedimentation process of flocculated clay suspensions. Nihon Reoroji Gakkaishi (J Soc Rheol, Jpn) 40:205–208
Ali S, Bandyopadhyay R (2016) Aggregation and stability of anisotropic charged clay colloids in aqueous medium in the presence of salt. Faraday Discuss 186:455–471
Tombácz E, Szekeres M (2004) Colloidal behavior of aqueous montmorillonite suspensions: the specific role of pH in the presence of indifferent electrolytes. Appl Clay Sci 27:75–94
Miyahara K, Ohtsubo M, Nakaishi K, Adachi Y (2001) Sedimentation rate of sodium montmorillonite suspension under high ionic strength. Nendo Kagaku (J Clay Sci Soc, Jpn) 40:179–184 (in Japanese with English abstract)
Lovell CJ, Rose CW (1991) The effects of sediment concentration and tube-diameter on particle settling velocity measured beyond stokes’ range: experiment and theory. J Sediment Petrol 61:583–589
Miyahara K, Adachi Y, Nakaishi K (1998) The viscosity of a dilute suspension of sodium montmorillonite in an alkaline state. Colloids Surf A Physicochem Eng Asp 131:69–75
Auzerais FM, Jackson R, Russel WB, Murphy WF (1990) Transient settling of stable and flocculated suspension. J Fluid Mech 221:613–639
Acknowledgements
We thank the Research Facility Center for Science and Technology of the University of Tsukuba for manufacturing the settling tubes.
Funding
This research was partly supported by JSPS Kakenhi (16H06382) and Kenkyu Kiban Shien Project Type B from the University of Tsukuba.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wu, MY., Adachi, Y. Duration of initial flocculation stage in the sedimentation of sodium montmorillonite suspension in the semi-dilute regime. Colloid Polym Sci 296, 71–76 (2018). https://doi.org/10.1007/s00396-017-4222-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4222-6