Abstract
We report the thermal conductivity, κ, and the specific heat, C p, of dispersion of 0.95-nm-thick, 25-nm-diameter disks of Laponite in water and in ice, as well as the thermal effects during gelation of several compositions, and the temperature dependence of the gelation time. The κ values of its 5.0 wt% sol and gel states at T > 273 K are ∼3 % larger than those of pure water. In the frozen state of water, κ is lower than that of hexagonal ice and the difference increases on cooling. κ of the sol and gel calculated from Maxwell’s mixture model agrees with the measured κ. During the course of homogenization and formation of the gel state, κ and C p do not change significantly. The time for gel formation, t gel, decreases rapidly when the sol is aged at high temperatures. The change occurs almost according to the relation, log10(t gel) ∝1/T. The accelerated formation of the Laponite gel at high T is distinguished from that of organic, mostly protein gels which form more rapidly at low T. The gels are not thermo-reversible. We consider the consequences of our findings for the current understanding of the phonon propagation and electrostatic interactions between H2O molecules and Laponite disks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colloidal dispersions are known to be usually translucent or opaque. But, when nanometer-dimensions particles are used as a dispersed phase, colloidal dispersions are optically clear, except when the particles are composed of elements in which there are free electrons (5-nm-size gold particles for example do not produce an optically clear dispersion). Nevertheless, all colloidal dispersions show shear thinning, i.e., flow easily when gently shaken or slightly stirred. In recent years, preparation and use of optically clear colloidal solutions have been of unusually high interest. Solid Laponite, whose colloidal solutions led to the unusual interest, is a synthetic, layered structure of Na-Mg-silicate. It consists of ∼0.95-nm-thick, 25-nm-diameter disk-shaped crystals of large surface charge density with negative charge on the surface and positive charge on the rim and an anisotropic charge distribution. In transparent, thixotropic dispersion in water, the crystals can have attractive or repulsive interactions [1–4], depending upon the Laponite concentration. At high enough Laponite concentration, its dispersion in the sol state transforms to a gel state with time, when kept isothermally [1–4]. (To maintain distinction between a true solution and a colloidal solution, we use the term sol for the dispersed state of Laponite.) Conversion of sol to a gel state of aqueous Laponite dispersions is detected by observing the loss of (non-Newtonian) viscous flow. In the studies of sol-gel formation, Laponite dispersions in water have been studied by a variety of techniques [1–15], in some cases with the purpose of investigating the unusually high shear thinning of different concentrations of the dispersions used by the industry to produce a transparent material.
Several groups have studied the properties of Laponite dispersions, by measuring in most cases (i) the complex shear modulus, which is related to complex (frequency-dependent) viscosity [10, 12–17]; (ii) the static and dynamic light scattering [1–3, 12, 13, 18]; and (iii) the small-angle X-ray scattering [2–4]. Cummins [1] and others [2–4] have published comprehensive reviews of the studies on Laponite dispersions, and they also studied the change in the properties on aging of the gels at ambient temperature. New experiments, specifically on aging effects, were performed by Shahin and Joshi [6]. Joshi and coworkers have studied the changes in the shear modulus [6], thermal conductivity [5], and refractive index [9], during the conversion of Laponite sol to gel. Because of their thixotropy (shear-dependent flow), Laponite dispersions were regarded as a “non-Newtonian” nano-fluid [8, 19] and were suggested as a load-bearing thermal conductor for use in miniature electronic devices [5, 8, 19]. These studies focused on inter-particle interactions of Laponite crystals in water.
Colloidal suspensions are usually made with water as a dispersing medium. It is also widely known that properties of water are in general affected by interaction with ions or by other charged entities dissolved in it. In contrast, properties of bulk ice are affected by accumulation of similarly charged entities at its crystal lattice site and at intercrystal, or grain boundaries and at grain junctions when the bulk ice is polycrystalline. In Laponite dispersions in water, the highly electrically charged surface of a Laponite particle is expected to have a significant role in determining the thermodynamic and heat transport properties of water. Briefly, the specific heat is affected by any change in the vibrational and/or configurational parts of the properties of water, and thermal conductivity is affected not only by the intrinsically higher thermal conductivity of Laponite [20], but also, according to the Debye theory, by the change in the specific heat and mean free path for phonons.
Dispersed particles also affect the dipolar alignment of the dispersing (liquid) medium. When this medium is water, alignment of H2O dipoles by electrostatic interaction with ions, or ion-hydration, also alters the configurational, vibrational, and dynamic properties of water. While both the ions and the electrically charged particles affect the intermolecular hydrogen bond formation in water, the experimentally observed effects of interactions of H2O dipoles with ions differ from the observed effects of interaction of H2O dipoles with charged surfaces. This is mainly because the nature of interactions in ionic solutions and their consequences differ from the nature of electrostatic interactions in colloidal dispersions and their consequences.
Inter-particle interaction, the H2O-particle interaction, and intermolecular hydrogen bond interaction, all contribute to the macroscopic properties of a water-based colloidal dispersion. While remaining in dispersion as a result of inter-particle electrostatic interactions, the dispersed particles in dilute colloidal dispersions have their own Brownian diffusion dynamics, which is different from the dynamics of H2O molecules. At low concentrations, the dispersed particles diffuse independently of the H2O molecules. But as the particle concentration is increased, or when a colloidal dispersion is aged, inter-particle interaction is said to lead, in some cases, to more complex diffusion process and to structural transformation to fractal structures, compact clusters, cluster gels, repulsive or attractive glasses, and liquid-crystal phases [1–4]. Zaccarelli [2], Cummins [1], Ruzicka and Zaccarelli [3], and Angelini et al. [4] critically reviewed this subject and provided citations and further information on these aspects.
Most studies of Laponite dispersions have focused on the gel-like, liquid-like, and glass-like states [1–17] because interrelations between these states are relevant to their thixotropic behavior. In this study of Laponite dispersed in pure water, we consider a different aspect, namely, the Laponite nanoparticles acting as highly (electrostatically) charged entities that modify the structure of water on a different size scale than ions of a salt do in aqueous solutions. Here, we report a study of the heat effects, the specific heat and thermal conductivity during the course of formation of Laponite gel with time, the effect of temperature on their ability to form a gel, and the change in the apparent viscosity with time during the sol to gel conversion. For comparison against the behavior of organic gels, in which ionic interactions are largely absent and which form thermo-reversible gels (convert to sol on heating and revert to gel on cooling), we investigated whether or not the Laponite sol to gel transformation is thermo-reversible. We also consider the effect of dispersed Laponite on the properties of water, the dispersant media before and after its freezing to ice.
There is a general interest in studies of colloidal dispersions and their gel formation for almost a century. It stems not only from the fact that their thixotropic characteristics are essential to their use as a variety of consumer materials, viz., food products, pharmaceuticals, cosmetics, paints and inks, etc., but also because of the need to understand their macroscopic behavior at a fundamental level. Since Laponite gels are already used in such products and in some technologies, the study reported here would also be of general interest.
Materials and methods
Laponite RD (chemical formula, Na+ 0.7[(Si8 Mg5.5 Li0.3)O20(OH)4]− 0.7) is a synthetic layered silicate of density 2.53 g cm−3, which is insoluble in water but is highly hygroscopic. Its powder easily hydrates in the presence of moisture and swells to give clear and colorless colloidal dispersions of viscosity close to that of water when the Laponite concentration is below 2 %. The viscosity increases gradually with time, until the colloidal dispersion becomes a transparent gel. At concentrations of 2 % or greater in water, it forms thixotropic gels in a relatively short time. We chose Laponite RD specially for this study, because it has been used previously by others in their investigations of the optical and other properties of its dispersions in water [11], aging effects of its gelled state [12], and the properties of its composites with polymers [21]. We purchased Laponite RD powder (58935) from Kremer Pigmente GmbH, Germany. The stated specifications of their product are the following: surface area as measured by the BET method is 370 m2 g−1, and the packed density of bulk powder grades is 0.95–1 g cm−3.
The as-received Laponite RD powder was first dried in a glove box for 4 h in an atmosphere of argon at 350 °C. The moisture content of the powder was measured by weighing the as-received and the dried powder. Its as-received powder was found to contain ∼9.7 wt% water as moisture. (for comparison, the sample used by Cummins [1] had a producer-stated, moisture content of 6.8 %, but he also stated that in his study, [1] “The moisture content was measured during preparation of the samples with a Sartorius MA100C moisture analyzer and was found to be 9.8 %.” We prepared the Laponite-water dispersions by magnetic stirring. Briefly, the dried powder was dispersed in MilliQ deionized water, stirred vigorously for 30 min at 1000 rpm and filtered subsequently by a 0.45-μm syringe filter, and the dispersion transferred to a custom made Teflon cell of dimensions 38-mm internal diameter and 20-mm height. Three compositions of the Laponite dispersion were made, 2.8, 3.2, and 5 wt% Laponite in water before filtration. The 5 wt% dispersion formed a gel within 30 min after mixing; therefore, this dispersion could not be filtered. The 2.8, 3.2, and 5 wt% dispersions were first measured as a function of time until the dispersion transformed to a gel-like state. Thereafter, the thermal conductivity κ and the heat capacity per unit volume ρC p (ρ being the density) of their gel-like state were measured on cooling at ∼0.5 K/min to 100 K and thereafter heating at 0.2 K/min rate to 300 K. In order to investigate if heating to higher temperatures would show new effects, in one case, we also studied the gel-like state by heating at 0.2 K/min rate to 350 K.
The hot-wire technique was used to determine the κ and ρC p of the dispersions at different temperatures. The procedure has been described previously in detail [22]. Briefly, we used a 0.1-mm-diameter, ∼40-mm-long Ni wire as hot-wire inside the sample containing Teflon cell. We used the same hot-wire in the cell for all measurements and changed samples as needed for the study. The cell was fitted inside an aluminum cylinder equipped with heater. The assembly was kept immersed in a fine-sand filled, thermally isolated container, which in turn was cooled by using liquid nitrogen. The hot-wire, surrounded by the sample, was heated by a 1.4-s (duration) pulse of nominally constant power, and its electrical resistance was measured as a function of time. In this technique, the wire acts as both heater and sensor for measuring the temperature rise. This rise was calculated by using the relation between its electrical resistance and temperature. The analytical solution for the temperature rise with time was fitted to the data points for the hot-wire temperature rise, thereby yielding κ and ρC p with estimated inaccuracies of ±2 and ±5 %, respectively. By a calibration procedure in which we used the thermal conductivity of pure water as standard, we increased the accuracy of the data. In the calibration procedure, we used the length of the probe wire as a variable and adjusted the initial value obtained from a measurement in a workshop microscope until the measured thermal conductivity agreed with that of pure water. We estimate that this procedure reduced the inaccuracy in κ to about 0.5 % at room temperature.
The values for measured ρC p are less accurate than those for κ, and ρC p is also more sensitive to non-ideal conditions such as poor thermal contact and heterogeneities, e.g., small air bubbles or particles that affects the initial temperature rise of the hot-wire. During the measurements, we found that when the Laponite particles were not thoroughly mixed or otherwise dispersed in water, ρC p anomalously decreased by up to 8 % for a 5 wt% Laponite sample. A large systematic deviation of the fitted analytical solution from the measured temperature rise appeared when an exothermic or endothermic transformation of the sample occurred. In this case, the real-time fitting routine occasionally failed by the run time error when the sample froze to ice or when the ice melted, and the data collection automatically terminated. In such cases, we manually initiated the data accumulation in the temperature range near these transitions.
In another set of experiments, Laponite dispersions were made in the composition range 0.5 to 3.2 wt% Laponite in water and aged by keeping the sol inside a glass vial immersed in a temperature-controlled water bath or an oil bath, or by keeping the glass vial in a refrigerator. Since the 0.5 wt% sample did not form a gel within the maximum aging time of ∼135 days at 347 K and ∼120 days at room temperature, it did not provide data for the gelation time.
The dynamic viscosity of the sols was measured at 347, 333, and 303 K by using a falling ball viscometer, known as Thermo Scientific HAAKE Falling Ball Viscometer type B. It comes with its own temperature-controlled water bath (Grant Instruments Ltd). The change in the apparent viscosity with time was measured while the sample was kept in the viscometer at 347, 333, and 303 K during the 4-, 50-, and 80-h aging time experiments. At each temperature, the apparent viscosity was typically measured by 3–4 falling ball measurements without allowing any waiting time between the measurements. The sample was then kept for a fixed time at the relevant temperature, e.g., for ∼30 min at 347 K, before the next measurement. The measurements were terminated when the ball was unable to fall through the dispersion.
Results
In the first set of this study, we measured κ and ρC p during the homogenization and gelation of several compositions of Laponite-water dispersions kept at ambient temperature of 297 K. Figure 1 shows the plots of κ and ρC p against time of 2.8 wt% (a, b, c) and 3.2 wt% (d, e, f) Laponite dispersion. The plots of the temperature against time of the same samples are also provided for the purpose of showing thermal effects during the homogenization and gel formation. These show slow temperature variations by ±1 K with time, with a period of about 24 h. This was attributed to slow variations in the ambient temperature over 1 day. Therefore, no change in the temperature due to gelation was observed (Corresponding measurements at about 350 K gave similar results, as shown in Figure S2.) This indicates that if there is a thermal effect on gel formation, it is too small or occurs too slowly, to be observed. The plots for the same composition in Fig. 1 also show that κ increases with the rise of temperature during its fluctuations, and this variation is consistent with the dκ/dT being positive.
The κ, ρC p, and the temperature of 2.8 wt% (a, b, c) and 3.2 wt% (d, e, f) Laponite dispersion in water measured at 1 bar pressure are plotted against time during the course of their homogenization to thixotropic states. Arrows indicate the temperature rise and the vertical lines indicate the consequent change in κ and ρC p
Figure 2 shows the plots of κ measured on cooling the gelled states together with the previously reported results for ice [22] and hydrogels [23]. After slight supercooling to ∼269 K, all the Laponite samples abruptly crystallized and the κ value for all Laponite compositions increased to about 2 W m−1 K−1, a value slightly less than that of hexagonal ice [22]. On further cooling, κ of the different compositions remains almost the same and increases, which is typical of crystalline materials. But the rate of increase on cooling is much less than that for hexagonal ice.
a Thermal conductivity of 2.8 wt% (red, empty triangle), 3.2 wt% (blue, empty square), and 5 wt% (black, empty circle) Laponite/water dispersion plotted against the temperature. The values for hexagonal ice [22] and hydrogel [23] are included for comparison. b The quantity x (=−d ln κ/d ln T) of the relation κ ∼ T −x of frozen Laponite gels and pure ice is plotted against temperature
Figure 3 shows the plots of κ and ρC p against the concentration of the gelled state of Laponite-water dispersions at 295 K. Figure 3a shows that κ increases with increase in the Laponite content, whereas ρC p decreases. The increase in κ i ∼2.6 % for a 5 wt% Laponite gel, which is outside the experimental inaccuracy.
a Thermal conductivity plotted against the wt% of Laponite dispersion in the gelled state at 295 K. b The corresponding plots of the heat capacity per unit volume. The dashed line in panel (a) is the thermal conductivity of Laponite-water dispersion, as calculated by using the Maxwell’s model. The dashed line in panel (b) is the heat capacity per unit volume estimated by mixture rule. The error bar is ±0.5 % for thermal conductivity and ±5 % for heat capacity per unit volume. A linear fit of the plot of ρC p against the Laponite content indicates that there is a slight decrease in ρC p with increase in Laponite concentration: (−30 ± 20) kJ m−3 K−1 wt%−1, with the inaccuracy given as one standard error
In order to investigate the effect of temperature on gel formation, we made visual observation, as done previously by others [4], for the flow of the Laponite dispersion after keeping it for certain duration at a fixed temperature. For this purpose, ∼ 2 ml of freshly prepared 2.8 and 3.2 wt% Laponite-water dispersion were transferred to a screw-top sealed 13.0-mm-diameter glass bottle. The container was weighed in order to determine if any water entered or was lost during the aging experiment. The sealed bottles were either immersed in thermostated water baths at temperatures of 295, 310, 336, and 347 ± 1.5 K, or kept in a refrigerator at 283 ± 1.5 K. After definite time intervals, the bottles were taken out of the bath or the refrigerator, laid horizontally, and the samples were observed visually, as done by others previously [2, 4], to see if they showed any flow (see Figure S1 in Electronic supplementary material). We found that after a certain time period, the sample did not flow when observed for 30 s, and that time period was taken as the gelation time, t gel, at that temperature. The data in Table 1 provide a list of the composition, the temperature, and t gel. The 3.2 wt% Laponite-water dispersion formed a gel in 96 h at 295 K, but only in 1.7 h at 347 K. It is evident that increase in the temperature decreases t gel. This demonstrates that hydration of Laponite is accelerated by higher thermal energy and the accelerated hydration leads to more rapid formation of the gel clusters. A plot of log10(t gel) against 1/T is shown in Fig. 4. It should be stressed that the gelation time measured by different techniques often depends upon the technique used, and a variety of techniques are used for the purpose, viz., measurement of shear modulus, of surface tension, of light scattering, or of simple penetration of the liquid or the rate of its flow through a narrow pore. The technique used here is a simple and a common one. Since we used these techniques consistently for all the samples, the relative differences in t gel are of significance here. Other techniques may give different absolute values of t gel, but the relative differences in t gel obtained from those techniques are expected to remain the same.
The gelation time plotted logarithmically against the inverse temperature for 2.8 wt% (filled squares) and 3.2 wt% (open circles) Laponite-water. The aging time when the effect of thixotropy was observed in falling ball measurements of 3.2 wt% Laponite-water dispersions, t thixo, is shown for comparison (filled triangles). The bars indicate the estimated inaccuracies in the time and temperature measurements. The dashed and dotted lines represent linear fits to the gelation time data for the 3.2 wt% and 2.8 wt% samples, respectively
We did a number of experiments to investigate whether or not the sol-gel transformation of Laponite dispersions is thermo-reversible, i.e., whether or not on heating the Laponite gel converts to a sol, which on cooling reverts to the gel state. These experiments could help to further distinguish the behavior of Laponite gel from the behavior of (thermo-reversible) protein gels. Here, we describe three such experiments in which the Laponite gel was aged by keeping at a fixed temperature for a certain period. In one experiment, we prepared a 3.2 wt% Laponite gel by keeping the sol inside a screw-top sealed, 5-ml capacity, glass bottle at 295 K. The bottle was then immersed vertically up to the level of the gel in a water bath kept at 336 K, and its empty neck in air was at T < 336 K. After keeping for 24 h at 336 K, we found that a thin fluid-like layer appeared on top of the gel. When the sample was thereafter kept at 295 K, the fluid layer slowly vanished and an (apparently) homogeneous gel was again obtained after 24 h. In the second experiment, we first prepared an apparently completely gelled state of 3.2 wt% Laponite dispersion (the same concentration as before) by keeping the sol similarly immersed in water for 4 h at 336 K. It was then kept for a further period of 24 h, at 336 K with the bottle’s empty neck in air at T < 336 K. As in the first experiment, we again found that after 24 h at 336 K, a thin fluid-like layer appeared on top of also this gel, and when the sample was thereafter kept at 295 K, the fluid layer slowly vanished and an (apparently) homogeneous gel formed after 24 h. In the third experiment, we made a 3.2 wt% Laponite gel by keeping in the sealed bottle at 336 K for 24 h, examined the gel visually at 295 K, and then submerged the entire bottle with the empty neck in a water bath kept at 336 K. When the bottle was then taken out and kept at 295 K for a few minutes, it showed a small amount of fluid attached to the inside wall of the bottle’s neck, but there was no fluid-like layer on top of the gel. We interpret these findings in terms of the dilution of the top part of the gel inside the container, as follows: during aging of the gel at 336 K, water from the gel at 336 K evaporated and condensed on the wall of the empty part of the bottle. This water then flowed down and accumulated on top of the gel, thus diluting the top layer of the gel and causing the large clusters to break down into small clusters that deformed and flowed.
A second set of Laponite dispersions were made to study the gelation time at lower concentrations, from 2.0 wt% down to 0.5 wt%. For these studies, we used an oil bath instead of a water bath to study gelation over a longer period without the need to refill the water bath. The 0.5 wt% sample did not form a gel within the maximum aging time of ∼135 days at 347 K, and the 1.5 wt% sample did not form a gel within the maximum aging time of ∼240 days at room temperature. The results obtained are shown in Fig. 5.
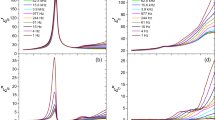
To study the manner in which the viscosity of the sol increased with time as it converted to gel, we measured the (apparent) viscosity as a function of time for a 3.2 wt% Laponite dispersion by using a falling ball viscometer in which the sample of Laponite dispersion was hermetically sealed. The results obtained at different temperatures are shown in Fig. 6, where we have indicated the “measured viscosity” as apparent viscosity because of the non-Newtonian behavior of the Laponite dispersions. When repeat measurements were made on the same sample in the viscometer, the falling ball time was found to decrease in the second measurement and then to remain almost constant in the subsequent measurements that started within an interval of less than a few minutes after the ball had dropped to the bottom of the viscometer. The decrease was interpreted as the effect of thixotropy (shear thinning), and the results are shown in Fig. 6. For long aging times, the viscosity calculated from the first measured falling ball time (open symbols in Fig. 6) is larger than that calculated from the average of the 2–3 consecutive falling ball times (filled symbols in Fig. 6). This effect was observed at all temperatures, but the time after which the effect of thixotropy was observed, t thixo, decreased with increase in T, as seen by comparing the plots for different T in Fig. 6. For comparison with the change of t gel with the temperature, the values for t thixo are included in Fig. 4.
The apparent viscosity of 3.2 wt% of Laponite in water dispersion at 347, 333, and 303 K during gelation process. Open symbols show the viscosity calculated from the falling ball time in the first experiment, and the filled symbols show the viscosity calculated by using an average falling ball times in the two to three consecutive experiments on the same sample. The effect of thixotropy makes these distinguishable and the time of this observation, t thixo, is plotted in Fig. 4
Discussion
General features
In our studies of κ and ρC p, the time period for which the sol was kept at 295 K (room temperature) is at most 3 to 7 days. The 2.8, 3.2, and 5 wt% Laponite sol formed a gel at 295 K within 200 h or less, and we found that a 7 wt% Laponite dispersion at 295 K formed a gel immediately during mixing, which prohibited filling of the sample cell; therefore, its thermal properties could not be studied. It has been suggested that a high-density gel forms [1] quickly when the Laponite sol contains ions in a concentration high enough that Coulombic, attractive interactions dominate. In such cases, it has been suggested that the Laponite disks dispersed in the gel may arrange in a “house of cards” configuration, in which positively charged edges of the disks face the negatively charged surfaces of the disks [1]. In contrast, Lemaire et al. [24] and Kroon et al. [25], who studied the structure of the gels by using small-angle X-ray scattering (SAXS), found evidence for nematic order of the liquid crystal type, i.e., the Laponite disks are directionally oriented in loose parallel lines. This is in contradiction to the “house of cards” conjecture. On the basis of their observation of the change in the SAXS data with time, Kroon et al. [25] suggested that gelation of Laponite dispersions is a two-step process, which involves first an orientational ordering of the disks (equivalent to growth of nematic order). Thereafter, a thermally activated process restores the random orientation of the Laponite disks, thereby producing the gel state. Other mechanisms have also been proposed: In their computer simulation, Aray et al. [26] concluded that adsorption of water on electrically charged Laponite surface occurs as a result of electrostatic repulsion between the negative lone pairs on the water molecules and the surface oxygen atoms of Laponite structure. Thus, H2O molecules bind to the surface in a perpendicular and tilted manner, an occurrence that minimizes the repulsive interactions.
Here, we first discuss our findings for the thermal properties of the Laponite sol, gel, and frozen states, and then, we discuss the implications of our gelation time results on the models for the gelation process.
The magnitude of κ, its variation with the state and temperature
The plots in Fig. 2 show that κ of pure water and Laponite gels are the same within 2.6 %. On thermodynamic freezing, κ increases, as is normally observed on freezing to a crystalline solid. A comparison of these plots against those for pure water and hydrogel, also provided in Fig. 2, shows that the increase in κ on freezing of Laponite gels is ∼6.3 % less than that observed on freezing of pure water, and it is 14.2 % more than that observed for freezing of the hydrogel. Since Coulombic interactions among Laponite particles would affect structural disorder and produce steric effects, such interactions can cause a change in phonon scattering and hence have a considerable effect on κ of the thermodynamically frozen state of the gels. We argue that ∼25-nm-diameter disks of Laponite would not occupy the lattice sites of hexagonal ice crystals and, like any other dissolved material in water, freezing of water to ice would reject the material from the ice crystal lattice, thereby causing it to occupy the grain boundaries and grain junctions of a polycrystalline sample, a process generally known as freeze concentration. Thus, one expects that the dispersed Laponite crystals of nanometer size would affect the crystallinity of ice at a microstructural level by producing smaller grain size, and these grain sizes would vary with the composition of the frozen gel. It is known that when crystal grains are of small size, the volume fraction of the liquid at grain boundaries and grain junctions is large, and even impurity-free polycrystalline ice at T < 273 K contains a significant volume fraction of water [27, 28]. When polycrystalline ice is formed by freezing impure water, freeze concentration of the impurities occurs at the solid’s grain boundaries and grain junctions. Therefore, polycrystalline ice formed on freezing Laponite dispersions is expected to contain concentrated Laponite dispersion at the grain boundaries of polycrystalline hexagonal ice. Accordingly, part of the lowering of κ in the frozen Laponite gel at T < 273 K would be due to presence of water in the sample. Therefore, one expects that the amount of this water would increase with increase in the amount of Laponite; therefore, κ would decrease as the amount of Laponite is increased. One may alternatively consider that the Laponite disks present in the grain junctions increase the grain boundary scattering; this too would decrease κ with increase in the Laponite concentration. The plots in Fig. 2 show indeed that κ of the frozen Laponite dispersion is less than that of pure ice and that the difference increases on cooling, but also that these effects depend only weakly on the Laponite concentration.
To analyze our findings further, we recall that, according to the Debye theory for a continuous medium [29],
where ρ is the mass density, C V is the specific heat capacity at constant volume, v is the phonon velocity, and l is the phonon mean free path. Equation 1 is commonly used to discuss the temperature dependence of κ of crystals at relatively high temperatures, i.e., near or above the Debye temperature. According to Eq. 1, the change of κ with change in temperature can be expressed as follows:
Since acoustic phonons are main carriers of heat, the heat capacity will be roughly constant at high temperatures. The density and the phonon velocity typically decrease slightly with decrease in T, but these vary much less strongly than l. Unless crystals have very small size or contain a large population of point defects, dislocations, and impurities, it is expected that the mean free path is limited mainly by three phonon-phonon Umklapp scattering [30]. Since the number of phonons that can participate in Umklapp scattering processes increases in direct proportion to T at high temperatures, l will vary inversely with T. Thus, κ varies as T −1, as has been found for most crystals. However, in some cases, the variation of κ is slightly stronger than T −1, which indicates that other phonon-phonon scattering processes, such as those involving optic phonons, become effective in limiting l and/or that the thermal expansion causes a significant decrease in κ. Slightly weaker temperature dependence, as observed here for the frozen-to-ice Laponite gels, indicates that structural scattering, such as scattering on grain boundaries, impurities, and point defects, is also significant.
According to the general relation, κ ∼ T −x, the quantity −d ln κ/d ln T, would be equal to x. For the frozen Laponite gels at low temperatures, this quantity x (=−d ln κ/d ln T) is considerably less than that of hexagonal ice. The magnitude of the difference is seen in the plots of x in the 100–265 K range shown in Fig. 2b, where the quantity d ln κ/d ln T is the sum of the changes in κ, ρC v, and in l with change in T. The plots of x against T in Fig. 2b show that this sum for Laponite dispersions differs from that for ice most at low T. The difference gradually vanishes as T is increased. Since the Laponite particles would not occupy sites in the ice lattice, the properties of the ice crystals would be unaltered, i.e., the density, heat capacity, and sound velocity of ice with Laponite would not be significantly different from that of pure ice. This suggests that the Laponite nanoparticles induce structural scattering, which is independent or is only weakly dependent on temperature and that the effect of this scattering increases at low temperatures. This does not necessarily mean that structural scattering increases, but rather that its relative importance increases due to diminishing Umklapp scattering. Laponite particles can cause structural scattering both directly and indirectly, i.e., phonons can be scattered directly by the Laponite particles at the grain boundaries and indirectly by the defects in ice microcrystals caused by freezing of the gels that contains the Laponite particles. Therefore, we conclude that structural scattering caused by Laponite particles present at the grain boundaries and grain junctions of hexagonal ice cause the deviation of κ(T) of the frozen Laponite gels from that of hexagonal ice. It also seems that the supercooling of Laponite gels below 273 K indicates that Laponite disks do not act as heterogeneous nucleation sites.
Composition dependence of κ and ρC p
The κ of Laponite/water dispersions can be estimated by the Maxwell’s mixture model [31],
where κ La/wa, κ water, and κ Laponite are the κ of Laponite/water dispersion, water, and Laponite, respectively. The volume fraction of Laponite, ϕ, is given by,
where W water and W Laponite are the respective weight fractions of water and Laponite and ρ water and ρ Laponite are their respective densities. For κ water, we use 0.606 W m−1 K−1at 298 K [32]. In the absence of κ Laponite values for the nanometer-size particles of Laponite, we have to use the estimated value of 1.37 W m−1 K−1 at 300 K given by Lee et al. [20], which is the value previously measured by Cahill for amorphous silica [33]. We use ρ Laponite = 2.53 g cm−3 and ρ water = 1.0 g cm−3 and plot the calculated values in Fig. 3. The plots show that the Maxwell’s model predicts a weak increase of κ with increasing amount of Laponite. The experimentally measured κ of the dispersions is slightly higher than model-predicted value. However, the κ values we found are much less than the κ values reported by Bhandari et al. [5]. Briefly, they [5] measured the thermal diffusivity by using laser interferometry in an experiment performed under non-steady state conditions of heat diffusion and, from analysis of the data, they deduced that κ was as high as 0.8 W m−1 K−1 for a 1.2 vol% (3 wt%) Laponite-water dispersion. Their result of 0.64 W m−1 K−1 for 1 vol% Laponite agrees with our value of 0.615 W m−1 K−1 to within their inaccuracy of about 0.025 W m−1 K−1. It is possible that their measurements under non-steady state condition were more influenced by mass convection (and the resulting increase in thermal diffusion) than our measurements during the short (1.4 s) heat pulse of the hot-wire. It is also possible that our respective samples and the preparation methods are different from theirs [5]. A resolution of the abovementioned different κ values can be reached if κ of the same sample is determined by both methods, or κ of a sample of known thermal conductivity is determined by their technique [5], or else κ of the (3 wt%) Laponite-water dispersion is determined by yet another technique. The ρC p of Laponite/water dispersions was estimated by using the mixture rule:
where C p, water and C p, Laponite are the heat capacity of water and Laponite, respectively. By using C p, water = 4.18 J g−1 K−1 and C p, Laponite = 1.03 J g−1 K−1 from ref. 5. in Eq. 3, we calculated ρC p of the Laponite dispersion. Its value is 4.15 MJ m−3 K−1 for 5 wt% composition, which is less than 1 % from that of pure water. As shown by the plot in Fig. 3b, this change in ρC p is marginally small, which is consistent with the experimental value plotted in Fig. 3b, showing that adding small amount of Laponite does not change ρC p significantly.
Effect of temperature on the sol-gel transformation
Studies of the effect of temperature on the sol-gel transformation have been rarely reported. Nevertheless, it has been stated in product information [34] that the dispersion time of Laponite in water can be reduced by increasing the temperature of the mixture up to 40–50 °C after the Laponite-R powder is fully wetted out or by use of a high shear mixer. But our samples in their containers were already mixed before they were kept in a water bath or oil bath at high temperatures, and we used the same method for mixing as Cummins [1] and Ruzicka and coworkers [3, 18] had used. After extensive search in the literature, we found that in his Ph.D. thesis, Kroon reported: [35] “Furthermore, at elevated temperatures the gelation times are up to an order of magnitude shorter than at room temperature. Apparently, the gelation is a thermally driven process, where a high temperature means a higher kinetic energy by which the process of gelation runs faster.” But no data were given in ref. 35.
In Fig. 4, t gel for a 3.2 wt% Laponite dispersion decreases from 96 h at 295 K to 1.7 h at 347 K, indicating that the process of gelation is thermally activated. By fitting a straight line to the plot of log10 (tgel) against 1/T in Fig. 4, we obtain an Arrhenius activation energy of ∼63 kJ/mol for the gelation process. As shown in Fig. 4, t thixo also changed with temperature in a similar manner as the gelation time. Therefore, the results obtained for t gel measurements are consistent with the temperature-induced changes in the time for the observed thixotropic behavior in our falling ball viscometer measurements, i.e., the activation energies calculated from these independent results are of the same magnitude.
According to the model of Kroon et al. [25], reorientation of the Laponite disks rather than their aggregation is the mechanism for gel formation. If so, the consequences of this “mechanism” should be unambiguous, namely either t gel would increase with increase in the extent of the reorientational motions or decrease. Since all reorientational motions are thermally activated, an increase in T would normally cause the reorientation to occur faster and the extent of reorientation to increase, as the intermolecular distance increase with increase in volume. We argue that according to the Kroon et al.’s model, t gel should change with T monotonically, i.e., either t gel would decrease with increase in T, or increase, and the former is consistent with our results.
It is well known that protein gels form in a few hours only when the sol is cooled and kept at subambient temperature and that the process is thermally reversible in that when the gel is heated it becomes a sol [36]. Djabourov et al. have reported that “Low temperatures give more rapid, but less reproducible gelation (tgel, 30 min)” [37]. It was also found that a supercooled sol of a protein gel, gelatin, forms a gel on continuous heating at a constant rate, as identified by exothermic effect in studies using differential scanning calorimetry [38]. Such exothermic effect is very small if any for the Laponite’s sol-gel conversion. Djabourov et al.’s finding indicate that for protein gels, whose sol-gel transformation is thermally reversible, t gel is less at lower T than at higher T, i.e., t gel increases with increase in T. Therefore, our finding that t gel of the Laponite sol decreases with increase in T is the opposite of the behavior of protein gels. Although protein sol/gels differ from the Laponite sol/gels in as much as the latter have no entangled hydrogen-bonded structure of an extended protein molecules with water, the finding here does indicates that increase in the thermal energy of both the H2O molecules and of the nanometer-size Laponite disks in the sol state affects the gelation time. In this sense, gel formation of Laponite sol is distinguished from that of protein gels. Thus, we conclude that gelation of protein sols by hydrogen bond interaction with H2O molecules differs from the gelation by Coulombic forces in Laponite sols. In this context, Kroon wrote [35], “Unlike gelatin the clay/water system does not form thermotropic gels, i.e., clay gels are not reversible under temperature variation. For the clay/water suspensions shear takes the role of temperature.” It still remains unclear whether the accelerated gelation of Laponite sol at a higher T is caused by increase in the reorientation of Laponite disks, increase in the diffusion coefficient of H2O, weakening of electrostatic interactions relative to thermal energy (or vice versa), or by some other effect.
The results also raise the issue as to whether there is a temperature at which thermal energy would be high enough to destructure the Laponite gel. Our experiments showed that after the 2.8 and 3.2 wt% Laponite gels were heated to 336 K, and kept at 336 K for 24 h; a fluid layer appeared on top of the gel. This could indicate destructuring of the gel with time. But we found that the liquid layer slowly vanished when the sealed glass vial was completely immersed in the water bath. Therefore, we conclude that the liquid layer was formed by vaporization of water from the gel that condensed on the (colder) neck of the sealed vial and then drained back on to the surface of the gel, and this process continued until an equilibrium between the evaporation and condensation rates was attained. Accumulation of liquid on top of the gel should not be taken as evidence of destructuring or thermoreversibility of a Laponite gel.
It is also worth pointing out that a Laponite gel network is distinguished from the covalently bonded gel network which, in some addition polymerization processes, forms first and further polymerization turns the gel to a glass [39]. Cooling of such a covalently bonded polymer gel structure vitrifies it to a glassy state.
Conclusions
Coulombic interactions of 0.95-nm-thick, ∼25-nm-diameter disks, interactions of the H2O dipoles with the highly negatively charged particles, and the effects on intermolecular hydrogen bonds serve to increase the thermal conductivity of the sol and gel states of the Laponite dispersions at T > 273 K. But when the gelled sample freezes on cooling below 273 K, the Laponite particles would be rejected into the intergranular regions (grain boundaries and grain junctions) of hexagonal ice, because there is not enough space in the unit cell of the ice lattice to accommodate 25-nm-diameter disks. The phenomenon is reminiscent of the freeze concentration (rejection of impurities to the liquid-containing grain boundaries of crystals on freezing of aqueous and other solutions), and it is used for purifying materials by the melt-freezing method. Since the grain boundaries in a microcrystalline sample are spatially random, the segregation of Laponite particles would be spatially irregular, and this random distribution of the segregated Laponite particles at the microcrystals grain boundaries would scatter phonons. It is also possible that the Laponite particles prevent freezing of a certain amount of water, thereby decreasing the amount of crystalline ice. For these reasons, we attribute the lowering of the thermal conductivity of the Laponite-ice mixture relative to pure microcrystalline hexagonal ice, as observed here, to these two effects.
Studies performed over the 100–273 K temperature range show that the difference between the frozen gels and pure hexagonal ice increases when the temperature is decreased. This is due to the increased relative importance of phonon scattering caused directly or indirectly by the presence of the Laponite particles. In comparison with the poly(vinyl alcohol) hydrogel [23], the changes in κ on freezing at 273 K of the Laponite gel are larger. The increased effect may be partly due to higher intrinsic thermal conductivity of Laponite, its clustered state, and the nature of crystals formed on freezing. For both the sol and gel states of the Laponite dispersions, the κ value calculated from the Maxwell’s model agrees well with the κ measured at low concentration, but is slightly low at the highest concentration. During the time period in which the sol converted to a homogeneous gel, κ and ρC p do not change significantly.
Finally, we note that a study of the temperature dependence of the reorientation motion of Laponite disks in colloidal dispersions could be rewarding in an effort to elucidate the gelation process. Such data could indicate whether or not the reorientational motions of the Laponite disks govern the gelation process.
References
Cummins HZ (2007) Liquid, glass, gel: the phases of colloidal laponite. J Non-Cryst Solids 353:3891–3905
Zaccarelli E (2007) Colloidal gels: equilibrium and non-equilibrium routes. J Phys Condens Matter 19:323101
Ruzicka B, Zaccarelli E (2011) A fresh look at the Laponite phase diagram. Soft Matter 7:1268–1286
Angelini R, Ruzicka B, Zaccarelli E, Zulian L, Sztucki M, Moussaid A, Narayanan T, Sciortino F (2013) Observation of empty liquids and equilibrium gels in a colloidal clay. AIP Conf Proc 1518:384–390
Bhandari SS, Muralidhar K, Joshi YM (2013) Enhanced thermal transport through a soft glassy nanodisk paste. Phys Rev E 87:022301
Shahin A, Joshi YM (2012) Physicochemical effects in aging aqueous Laponite suspensions. Langmuir 28:15674–15686
Liu AJ, Wyart M, van Saarloos W, Nagel SR (2011) Dynamical heterogeneities in glasses. Oxford University Press, Oxford, Colloids and Granular Materials
Wang X-Q, Mujumdar AS (2008) A review on nanofluids—part II: experiments and applications. Braz J Chem Eng 25:631–648
Ravi Kumar NVN, Muralidhar K, Joshi YM (2008) On the refractive index of ageing dispersions of Laponite. Appl Clay Sci 42:326–330
Bonn D, Coussot P, Huynh HT, Bertrand F, Debrégeas G (2002) Rheology of soft glassy materials. Europhys Lett 59:786
Dinsmore AD, Weeks ER, Prasad V, Levitt AC, Weitz DA (2001) Three-dimensional confocal microscopy of colloids. Appl Opt 40:4152–4159
Bonn D, Tanaka H, Wegdam G, Kellay H, Meunier J (1999) Aging of a colloidal “Wigner” glass. Europhys Lett 45:52
Bonn D, Kellay H, Tanaka H, Wegdam G, Meunier J (1999) Laponite: what is the difference between a gel and a glass? Langmuir 15:7534–7536
Mourchid A, Lécolier E, Van Damme H, Levitz P (1998) On viscoelastic, birefringent, and swelling properties of laponite clay suspensions: revisited phase diagram. Langmuir 14:4718–4723
Mourchid A, Delville A, Lambard J, LeColier E, Levitz P (1995) Phase diagram of colloidal dispersions of anisotropic charged particles: equilibrium properties, structure, and rheology of laponite suspensions. Langmuir 11:1942–1950
Bellon L, Ciliberto S (2002) Experimental study of the fluctuation dissipation relation during an aging process. Phys D 168–169:325–335
Pignon F, Magnin A, Piau J-M (1998) Thixotropic behavior of clay dispersions: combinations of scattering and rheometric techniques. J Rheol 42:1349–1373
Tudisca V, Ricci MA, Angelini R, Ruzicka B (2012) Isotopic effect on the aging dynamics of a charged colloidal system. RSC Adv 2:11111–11116
Khandekar S, Joshi YM, Mehta B (2008) Thermal performance of closed two-phase thermosyphon using nanofluids. Int J Therm Sci 47:659–667
Lee SH, Kim JE, Song HH, Kim SW (2004) Thermal properties of maleated polyethylene/layered silicate nanocomposites. Int J Thermophys 25:1585–1595
Morariu S, Bercea M (2012) Effect of temperature and aging time on the rheological behavior of aqueous poly(ethylene glycol)/laponite rd dispersions. J Phys Chem B 116:48–54
Andersson O, Inaba A (2005) Thermal conductivity of crystalline and amorphous ices and its implications on amorphization and glassy water. Phys Chem Chem Phys 7:1441–1449
Andersson O, Johari GP (2011) Effect of pressure on thermal conductivity and pressure collapse of ice in a polymer-hydrogel and kinetic unfreezing at 1 GPa. J Chem Phys 134:124903
Lemaire BJ, Panine P, Gabriel JCP, Davidson P (2002) The measurement by SAXS of the nematic order parameter of laponite gels. Europhys Lett 59:55
Kroon M, Vos WL, Wegdam GH (1998) Structure and formation of a gel of colloidal disks. Phys Rev E 57:1962–1970
Aray Y, Marquez M, Rodríguez J, Coll S, Simón-Manso Y, Gonzalez C, Weitz DA (2003) Electrostatics for exploring the nature of water adsorption on the laponite sheets’ surface. J Phys Chem B 107:8946–8952
Salvetti G, Tombari E, Johari GP (1995) Calorimetric effects of intergranular water in ice. J Chem Phys 102:4987–4990
Johari GP, Pascheto W, Jones SJ (1994) Intergranular liquid in solids and premelting of ice. J Chem Phys 100:4548–4553
Berman R (1949) Thermal conductivity of glasses at low temperatures. Phys Rev 76:315–316
Ashcroft NW, Mermin ND (1976) Solid state physics. Saunders College Publishing, Philadelphia
Maxwell JC (1873) Electricity and magnetism. Clarendon, Oxford
Toulokian YS, Liley E, Saxena SC (1970) Thermal conductivity, nonmetallic liquids and gases, vol 3. Thermophysical properties of matter, Plenum, New York
Cahill DG (1990) Thermal conductivity measurement from 30 to 750 K: the 3 omega method. Rev Sci Instrum 61:802–808
Laponite Technical Bulletin: Laponite: structure, chemistry and relationship to natural clays (1990), vol L104-90-A
Kroon M (1998) Structure and formation of a gel of colloidal disks. Academische Pers B.V, The Netherlands
Cole G (2000) Gelatin in encyclopedia of food science and technology, vol 4. Encyclopedia of food science and technology, 2nd edn. Wiley, New York
Djabourov M, Leblond J, Papon P (1988) Gelation of aqueous gelatin solutions. II. Rheology of the sol-gel transition. J Phys France 49:333–343
Guigo N, Sbirrazzuoli N, Vyazovkin S (2012) Gelation on heating of supercooled gelatin solutions. Macromol Rapid Commun 33:698–702
Johari GP (1994) Dynamics of irreversibly forming macromolecules in disorder effects in relaxational processes. Disorder effects in relaxational processes. Springer-Verlag
Acknowledgments
This work was supported by The Swedish Research Council and the Faculty of Science and Technology, Umeå University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 370 kb)
Rights and permissions
About this article
Cite this article
Yu, J., Andersson, O. & Johari, G.P. Effects of nanometer-size Laponite disks on thermal conductivity and specific heat of water and ice, and the gelation time. Colloid Polym Sci 293, 901–911 (2015). https://doi.org/10.1007/s00396-014-3481-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-014-3481-8