Abstract
Colloidal noble metal-conducting polymer core–shell nanoparticles show promising applications in a wide diversity of areas, but it remains a great challenge to develop a convenient synthetic method allowing strict control over their size/dimension and facile modulation of their nanostructures. In this work, we report the synthesis of monodisperse gold dendrite@polypyrrole (AuD@PPy) core–shell nanoparticles with an eco-friendly one-step solution reaction by triggering oxidative polymerization of pyrrole with tetrachloroauric acid. As-prepared AuD@PPy nanoparticle is composed of a gold dendrite core coated by a thin PPy shell; the size and the thickness of the PPy shell could be adjusted by simply changing the pyrrole concentration. Benefiting from the protective effect of the PPy shell, the nanoparticles demonstrate enhanced catalytic durability toward the reductive reaction of methylene blue (MB) compared with the citrate-capped gold nanoparticles (AuNPs).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Noble metal colloids such as gold nanoparticles (AuNPs) and silver nanoparticles (AgNPs) demonstrate compelling optical, electronic, and catalytic properties sharply contrasted to their bulky counterparts and have attracted great interest in a wide range of research areas [1, 2]. Well-established methods allow for reliable synthesis of them with the ability to tailor their dimension/shape, and various nanostructures such as spheres, prisms, rods, wires, and cubes have been successfully prepared with encouraging applications for bioimaging, nanomedicine, biosensing, surface-enhanced fluorescence, light scattering, and so forth [3–5]. Meanwhile, conducting polymers such as polypyrrole (PPy) and polyaniline (PANI) with highly π-conjugated polymeric chains exhibit tunable electrical properties, reversible doping/de-doping process, and controllable chemical and electrochemical properties [6]. Modulated by synthetic parameters, they also display a wide diversity of morphologies from zero-dimensional nanospheres to one-dimensional nanowires and two-dimensional nanofilms, which facilitates their applications in various areas including electronics, sensing, and energy storage and conversion [6–9].
Noble metal-conducting polymer colloidal nanocomposites have attracted considerable research interest in recent years. By rationally combining the advantages of individual components while eliminating respective drawbacks, these nanocomposites demonstrate promising applications in a wide variety of practical areas [10–13]. Various noble metal-conducting polymer nanocomposites have been synthesized with different synthetic strategies [11, 12, 14–19]. The first strategy is to grow polymer on the pre-synthesized metal nanostructures. With this method, colloidal gold-conducting polymer nanomaterials including gold nanorod@PANI core–shell nanoparticles and superparticle/PPy core–shell composites have been prepared by oxidative polymerization of corresponding monomer on the surface of colloidal gold particles [12, 14]. Double-layer core–shell structures, triple-layer core–shell, and yolk–shell metal nanoparticle-conducting polymer nanostructures were also prepared by growing conducting polymer shell in surfactant capsules with encapsulated metal nanoparticles using emulsion polymerization [15, 16]. The second strategy is to grow or adsorb metal nanostructure on pre-synthesized conducting polymers. Xiaomiao Feng and coworkers have synthesized PANI hollow spheres with polystyrene nanoparticle as sacrificial templates and further modified the hollow spheres with Au nanoparticles to obtain PANI/Au hollow spheres for electrochemical detection of dopamine [11]. PPy/Au nanocomposites were synthesized by chemical polymerization of pyrrole with FeCl3 as an oxidative reagent, followed by reducing HAuCl4 with NaBH4 [17]. The third strategy involves simultaneous polymerization of monomers and growth of metal nanostructures. Ag@PPy nanosnakes have been prepared by polymerization of pyrrole in the presence of Ag2O under hydrothermal condition [18]; Ag/PPy composite colloids were prepared through the reaction of silver nitrate with pyrrole solution in DMF with the assistance of femtosecond laser pulse or UV lamp [19]. Although impressive progresses have been achieved, these methods are tedious with multiple synthetic steps, and some are unable to strictly control the morphology and/or structure of products, resulting in mixed products without defined nanostructures. In some cases, the surfactant used may damage the potential application of the nanocomposites. Therefore, it remains a great challenge to develop a convenient synthetic method to synthesize the noble metal-conducting polymer colloidal nanocomposites while allowing for control over their size/dimension and facile modulation of their nanostructures.
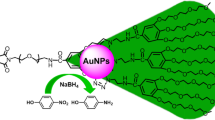
In this work, we report a facile one-step method to synthesize monodisperse gold dendrite@polypyrrole (AuD@PPy) core–shell colloidal nanoparticles. The method involves simply mixing pyrrole monomer with tetrachloroauric acid (HAuCl4) in an aqueous solution, where the HAuCl4 is spontaneously reduced by pyrrole to form gold dendrite (AuD) while the pyrrole is simultaneously oxidized to grow PPy thin film and deposit on AuDs. The whole reaction progresses at room temperature without the need of heating, irradiation, or surfactant. The growth mechanism was proposed, and the catalytic activity and durability of AuD@PPy nanoparticles toward the reduction of methylene blue (MB) dye were investigated.
Experimental section
Synthesis of AuD@PPy core–shell colloidal nanoparticles
At room temperature, 1.0 mL of 0.3-M pyrrole solution was rapidly added into 10 mL 0.01 % (wt) HAuCl4 solution with vigorous stirring. The solution was further stirred for 2 h, and the product was collected by centrifugation and re-dispersed in DI water.
Characterization of AuD@PPy
Transmission electron microscopy (TEM) images were obtained on a JEM-1400 system (JEOL, Japan) operated at 100 kV. Scanning electron microscopy (SEM) images were obtained on JEM-6700F FEG system (JEOL, Japan). X-ray diffraction (XRD) patterns were collected using a Shimadzu XRD-7000 system. Atomic force microscopy (AFM) measurements were conducted with tapping mode by Nanoman AFM (Veeco Metrology Group, USA) at ambient temperature. UV–vis spectra were collected with a Shimadzu UV-2550 spectrometer. Raman characterizations were carried out using a confocal Raman microscope system CRM 200 from WITec, Germany. Zeta-potential measurements were carried out with a malvern ZEN3690 system.
AuD@PPy catalyzed reduction of MB
The catalytic activity and durability of AuD@PPy nanoparticles toward the reduction of MB dye were evaluated and compared with that of citrate-capped AuNPs. In a typical procedure, a certain amount of the AuD@PPy nanoparticles was homogeneously dispersed into the aqueous solution of MB dye, followed by rapid injection of ice-cold aqueous solution of NaBH4 under stirring. The UV–vis spectra of the solution were collected at different times.
Results and discussion
Nanostructure of AuD@PPy nanoparticles and pyrrole’s effect on their nanostructure
When pyrrole was added into the HAuCl4 solution under stirring, the color of the solution was changed to dark red within 2 h, suggesting the formation of AuD@PPy core–shell nanoparticles. The resulted suspension possesses excellent disperse stability in water, possibly because the PPy shell brings positive charges to prevent the aggregation. It can be easily separated and re-dispersed in water by centrifugation without the risk of aggregation.
The morphology and structure of the AuD@PPy core–shell nanoparticles are unveiled by SEM and TEM. As shown in Fig. 1a, b, the products demonstrate irregular sphere appearance and the size distribution is very uniform with diameter around 30–40 nm according to the SEM images. The TEM images in Fig. 1c, d reveal core–shell structured nanoparticles composed of a dendrite core covered with a thin layer shell, which are believed to be gold dendrite and PPy film, respectively, because of their different brightness on TEM. Each gold dendrite is formed by several bent nanorod-like gold branches covered with an integral PPy thin film. The thickness of the polymer shell varies in several nanometer range.
The AFM images shown in Fig. 2 exhibit an average diameter of around 30 nm of the AuD@PPy core–shell nanoparticle, which is consistent with the observation of SEM and TEM in Fig. 1. Meanwhile, the nanoparticles scatter on the mica substrate in a separated form, indicating excellent monodispersity of the nanoparticles in the suspension.
The amount of pyrrole for the preparation shows significant effect on the size and nanostructure of the AuD@PPy nanoparticles. When the added pyrrole solution decreases to 0.2 and 0.05 mL, respectively, the average size of the nanoparticles increases to around 60 and 90–100 nm according to the SEM and TEM images shown in Figs. 3 and 4. The gold dendrite core is bigger and more compact compared with that prepared with 1.0-mL pyrrole solution (shown in Fig. 1). The polymer shell is not integral with varying thickness and cannot fully cover the dendrite core, and some nanoparticles in close proximity adhere to one another connected by the polymer, as displayed by the TEM images. The AFM images in Figs. 5 and 6 further confirm that the average size of the nanoparticles increase to 60 and 90–100 nm, respectively, when 0.2- and 0.05-mL pyrrole solution were used to prepare the AuD@PPy nanoparticles.
Spectroscopic and XRD characterizations of AuD@PPy nanoparticles
UV–vis spectra of resulted AuD@PPy nanoparticle suspensions are shown in Fig. 7. All spectra demonstrate evident adsorption peaks around 529–549 nm, which are located in the characteristic adsorption wavelength range of gold nanostructure, implying the growth of gold nanostructure in the suspension. With the decrease of pyrrole solution volume from 1.0 to 0.05 mL, the peak wavelength shifts from 529 to 549 nm, suggesting increased dimension of the resulted gold nanostructure [20], which is consistent with the SEM, TEM, and AFM observations shown above. It is further confirmed by the observation that the stability of the suspension increases with the increase of added pyrrole. At the same time, the peak widths of the UV–vis spectra decrease with the increase of added pyrrole amount, suggesting narrow size distribution of as-prepared products.
XRD analysis was employed to investigate the crystal structure of the AuD@PPy nanoparticles. As shown in Fig. 8, all three patterns exhibit a weak broad peak centered at the 2θ value of 25°, which could be assigned to the amorphous PPy film [21]. The peaks located at 38.3°, 44.4°, 65.1°, and 77.6°could be well indexed to the diffractions of 111, 200, 220, and 311 planes of face-centered cubic (fcc) gold structure (JCPDS No. 04–0784) [22]. It is notable that the full widths at half maximum of characteristic diffraction peaks from fcc gold increases with the added pyrrole solution amount, suggesting decreased crystallite size of gold core according to Scherrer equation [23, 24], which is consistent with the results presented above. The XRD patterns unambiguously confirm the presence of polymer and gold in the nanoparticles.
The Raman spectra further confirm the chemical composition of the PPy polymer in the nanoparticles, as shown in Fig. 9. The peaks located around 1,586 and 1,328 cm−1 corresponds to the C=C backbone stretching and ring stretching mode of the PPy backbone, respectively [25, 26]. The peak at 1,040 cm−1 can be assigned to the C–H in-plane deformation of PPy [25, 26]. The small peak around 985 cm−1 could be assigned to the quinoidpolaronic and/or bipolaronic structure of PPy [27].
Growth mechanism of AuD@PPy nanoparticles
Based on the experimental observations, the mechanism for the one-step formation of the AuD@PPy nanoparticles is proposed. It is well known that pyrrole could be oxidized chemically or electrochemically to generate various oligomers, which further aggregate to form PPy polymer [7, 28, 29]. When mixed with HAuCl4, a strong oxidative reagent, the pyrrole was spontaneously oxidized and the HAuCl4 was reduced to form gold nucleus [30]. Due to the presence of nitrogen atom on the pyrrole ring and its flexible nature, the PPy oligomer possesses high affinity toward the gold surface and would adsorb to form an incomplete covering layer on the gold core, which directs the gold core to further grow from the uncovered surface to a dendrite nanostructure rather than a spherical gold nanoparticle until the AuD@PPy nanoparticle was formed when all the precursors (pyrrole and HAuCl4) were consumed. Meanwhile, due to the presence of positively charged PPy shell (the zeta potential of the AuD@PPy nanoparticle measured to be +17.2 ± 1.4 mV, data not shown), the nanoparticles keep away from one another to prevent aggregation; thus, the final product exhibit excellent monodispersity [29]. Therefore, the role of pyrrole is threefold in the preparation. First, it reduces HAuCl4 to elemental gold as a reducing reagent; at the same time, its oxidative product, i.e., PPy, acts as a ligand to cap and guide the growth of gold nanostructure and prevents the formation of large gold core; also, the PPy shell confers the monodispersity of the AuD@PPy nanoparticle by inter-particle electrostatic repulsion. When the pyrrole amount decreases, less surface of the gold core is covered by the PPy oligomer, and excess HAuCl4 would further oxidize the PPy oligomer/polymer and deposit on the gold core to produce bigger and more compact gold cores and inhomogeneous PPy shells. It also results in possible aggregation of the nanoparticles during the growth as the repulsive force between the nanoparticles decreases due to the low PPy coverage on gold core.
It is worthy of a note that although the Au/PPy nanocomposite has been previously prepared by reacting HAuCl4 with pyrrole, no well-defined core–shell nanoparticle was obtained [31]. It is possibly due to the presence of high-concentration surfactant and hydrochloric acid (HCl) in the reaction solution in that work. The HCl changes the pH value of the solution and thus changes the growth kinetics of both elemental gold and PPy polymer; the high ionic strength in the reaction solution would screen the electrostatic repulsion between PPy polymer, resulting big aggregates. At the same time, the surfactant may also prevent the fast adsorption of PPy on gold surface.
Catalytic activity and durability of AuD@PPy nanoparticles toward MB reduction
Nanostructured gold and its composites have demonstrated high catalytic activity in a wide variety of reactions such as the reduction of various dyes [14, 32]. As-prepared AuD@PPy nanoparticles may show unique catalytic properties due to their well-defined core–shell nanostructure and rational combination of gold dendrite and conducting polymer. As a proof-of-concept, we investigated its catalytic activity and durability toward the reduction of MB dye by NaBH4, which is usually used to evaluate the catalytic activity of gold nanostructures or gold composites [14]. As shown as the UV–vis spectra in Fig. 10, MB exhibits characteristic peaks at 664 and 615 nm in aqueous solution (curve a), and when abundant reducing reagent, NaBH4, was added into the solution for 15-min reaction, the absorbance at 664 nm slightly decrease, suggesting that very small portion of blue MB was reduced to its colorless reduced form. In sharp contrast, in the presence of pre-dispersed AuD@PPy nanoparticles, the color of MB solution quickly disappears in 5 min, and the absorbance intensity on the UV–vis spectrum also vanishes, indicating that all MB is reduced by NaBH4 due to the presence of the AuD@PPy nanoparticles, which possess high catalytic activity toward the reduction of MB. It is not surprising even though the active gold surface was coated by the PPy shell, because the PPy thin film allow for free access of small molecules to the underlying gold surface due to its porous nature [33].
The reusability of catalyst is also essentially important for gold-based heterogeneous catalysis. The catalytic durability of AuD@PPy nanoparticles was further investigated by successive catalytic reaction of MB reduction. In each cycle, after the MB solution turned colorless, the AuD@PPy nanoparticles were collected from the reaction solution by simple centrifugation and re-dispersed in MB solution for the next cycle of catalysis. Our experiments reveal that even after eight cycles of reactions, the MB solution changes to colorless solution in several minutes in the presence of AuD@PPy nanoparticles, suggesting that the AuD@PPy nanoparticles retain their high catalytic activity toward MB reduction reaction. Our control experiments further unveil that the citrate-capped AuNPs completely lose their catalytic activity after only two successive MB reduction reaction, which is consistent with previous observation, possibly due to the low efficiency of catalyst separation and aggregation-induced deterioration of catalytic activity [32]. This comparison highlights the excellent catalytic durability of AuD@PPy nanoparticles, very likely contributed from the protection of the PPy shell to prevent nanoparticle aggregation.
Conclusions
In summary, we reported a facile one-step method for synthesis of monodisperse AuD@PPy core–shell colloidal nanoparticles by simply mixing pyrrole with HAuCl4 solution at room temperature without the need of heating, irradiation, or surfactant. In this reaction, the pyrrole reduces HAuCl4 to elemental gold and its oxidative product, i.e., PPy, acts as a ligand to cap and guide the growth of gold nanostructure and prevents the formation of large gold core; also, the positively charged PPy shell confers the monodispersity of the AuD@PPy nanoparticles by inter-particle electrostatic repulsion. Contributed from their well-defined core–shell nanostructure, as-prepared AuD@PPy nanoparticles exhibit high catalytic activity and long-term stability toward the reduction of MB dye.
References
Hu W, Chen H, Shi Z, Yu L (2014) Dual signal amplification of surface plasmon resonance imaging for sensitive immunoassay of tumor marker. Anal Biochem 453:16–21
Hu W, Chen H, Zhang H, He G, Li X, Zhang X, Liu Y, Li CM (2014) Sensitive detection of multiple mycotoxins by SPRi with gold nanoparticles as signal amplification tags. J Colloid Interface Sci 431:71–76
Xia X, Wang Y, Ruditskiy A, Xia Y (2013) 25th anniversary article: galvanic replacement: a simple and versatile route to hollow nanostructures with tunable and well-controlled properties. Adv Mater 25:6313–6333
Lohse SE, Murphy CJ (2013) The quest for shape control: a history of gold nanorod synthesis. Chem Mater 25:1250–1261
Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, Baxter SC (2008) Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc Chem Res 41:1721–1730
Au KM, Lu Z, Matcher SJ, Armes SP (2011) Polypyrrole nanoparticles: a potential optical coherence tomography contrast agent for cancer imaging. Adv Mater 23:5792–5795
Zang J, Li CM, Bao S-J, Cui X, Bao Q, Sun CQ (2008) Template-free electrochemical synthesis of superhydrophilic polypyrrole nanofiber network. Macromolecules 41:7053–7057
Patil DS, Pawar SA, Devan RS, Gang MG, Ma Y-R, Kim JH, Patil PS (2013) Electrochemical supercapacitor electrode material based on polyacrylic acid/polypyrrole/silver composite. Electrochim Acta 105:569–577
Chen W, Li C-M, Yu L, Lu Z, Zhou Q (2008) In situ AFM study of electrochemical synthesis of polypyrrole/Au nanocomposite. Electrochem Commun 10:1340–1343
He YH, Yuan JY, Shi GQ (2005) Fabrication of gold nanocrystal-coated polypyrrole nanotubules. J Mater Chem 15:859–862
Feng XM, Mao CJ, Yang G, Hou WH, Zhu JJ (2006) Polyaniline/Au composite hollow spheres: synthesis, characterization, and application to the detection of dopamine. Langmuir 22:4384–4389
Jiang N, Shao L, Wang J (2014) (Gold nanorod core)/(polyaniline shell) plasmonic switches with large plasmon shifts and modulation depths. Adv Mater 26:3282–3289
Ye S, Lu Y (2008) Optical properties of Ag@polypyrrole nanoparticles calculated by Mie theory. J Phys Chem C 112:8767–8772
Wu J, Zhang X, Yao T, Li J, Zhang H, Yang B (2010) Improvement of the stability of colloidal gold superparticles by polypyrrole modification. Langmuir 26:8751–8755
Xing, S., Tan, L.H., Chen, T., Yang, Y., Chen, H. (2009) Facile fabrication of triple-layer (Au@Ag)@polypyrrole core-shell and (Au@H2O)@polypyrrole yolk-shell nanostructures. Chem Commun 1653–1654
Xing S, Tan LH, Yang M, Pan M, Lv Y, Tang Q, Yang Y, Chen H (2009) Highly controlled core/shell structures: tunable conductive polymer shells on gold nanoparticles and nanochains. J Mater Chem 19:3286–3291
Zhang J, Liu X, Wu S, Xu H, Cao B (2013) One-pot fabrication of uniform polypyrrole/Au nanocomposites and investigation for gas sensing. Sensors Actuators B Chem 186:695–700
Munoz-Rojas D, Oro-Sole J, Ayyad O, Gomez-Romero P (2011) Shaping hybrid nanostructures with polymer matrices: the formation mechanism of silver-polypyrrole core/shell nanostructures. J Mater Chem 21:2078–2086
Zhao C, Zhao Q, Zhao Q, Qiu J, Zhu C, Guo S (2007) Preparation and optical properties of Ag/PPy composite colloids. J Photochem Photobiol A Chem 187:146–151
Ghosh SK, Pal T (2007) Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: from theory to applications. Chem Rev 107:4797–4862
Zhang WX, Wen XG, Yang SH (2003) Synthesis and characterization of uniform arrays of copper sulfide nanorods coated with nanolayers of polypyrrole. Langmuir 19:4420–4426
Liu YC (2002) Evidence of chemical effect on surface-enhanced Raman scattering of polypyrrole films electrodeposited on roughened gold substrates. Langmuir 18:174–181
Viswanathan S, Narayanan B, Yaakob Z, Periyat P, Padikkaparambil S (2014) Selective formation of aniline over nanogold incorporated cobalt loaded SBA 15 catalysts. J Porous Mater 21:251–262
Dilshad N, Ansari MS, Beamson G, Schiffrin DJ (2012) Amines as dual function ligands in the two-phase synthesis of stable AuxCu(1-x) binary nanoalloys. J Mater Chem 22:10514–10524
Wang Z-L, He X-J, Ye S-H, Tong Y-X, Li G-R (2014) Design of polypyrrole/polyaniline double-walled nanotube arrays for electrochemical energy storage. ACS Appl Mater Interfaces 6:642–647
Chen W, Lu Z, Li CM (2008) Sensitive human interleukin 5 impedimetric sensor based on polypyrrole-pyrrolepropylic acid-gold nanocomposite. Anal Chem 80:8485–8492
Biswas S, Drzal LT (2010) Multilayered nanoarchitecture of graphene nanosheets and polypyrrole nanowires for high performance supercapacitor electrodes. Chem Mater 22:5667–5671
Hu W, Li CM, Dong H (2008) Poly(pyrrole-co-pyrrole propylic acid) film and its application in label-free surface plasmon resonance immunosensors. Anal Chim Acta 630:67–74
Hu W, Li CM, Cui X, Dong H, Zhou Q (2007) In situ studies of protein adsorptions on poly(pyrrole-co-pyrrole propylic acid) film by electrochemical surface plasmon resonance. Langmuir 23:2761–2767
Selvan ST, Nogami M (1998) Novel gold-polypyrrole anisotropic colloids: a TEM investigation. J Mater Sci Lett 17:1385–1388
Henry MC, Hsueh C-C, Timko BP, Freund MS (2001) Reaction of pyrrole and chlorauric acid a New route to composite colloids. J Electrochem Soc 148:D155–D162
Lee J, Park JC, Song H (2008) A nanoreactor framework of a Au@SiO2 yolk/shell structure for catalytic reduction of p-nitrophenol. Adv Mater 20:1523–1528
Hao L, Zhu C, Chen C, Kang P, Hu Y, Fan W, Chen Z (2003) Fabrication of silica core–conductive polymer polypyrrole shell composite particles and polypyrrole capsule on monodispersed silica templates. Synth Met 139:391–396
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (nos. 21205098 and 21273173), Natural Science Foundation Project of CQ CSTC (cstc2012jjA10099), Chongqing Key Laboratory for Advanced Materials and Technologies of Clean Energies, Chongqing International Collaboration Base for Science and Technology (Southwest University), Start-up grant under SWU111071 from Southwest University, Chongqing Science and Technology Commission under cstc2012gjhz90002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, W., Chen, H. & Li, C.M. One-step synthesis of monodisperse gold dendrite@polypyrrole core-shell nanoparticles and their enhanced catalytic durability. Colloid Polym Sci 293, 505–512 (2015). https://doi.org/10.1007/s00396-014-3440-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-014-3440-4