Abstract
A series of poly(acrylic acid-co-acrylamide) (PAA)/SiO2 hybrid hydrogels were prepared by in situ frontal polymerization. It was found that the increase in the concentration of SiO2 nanoparticles could lead to the increase in front velocity (V f) and the highest front temperature (T max). This may be attributed to the fact that SiO2 nanoparticles could increase the liquid viscosity of reaction mixture. The obtained PAA/SiO2 hybrid hydrogels were characterized by SEM and Fourier transform infrared spectroscopy spectrum and swelling measurements. The pH-sensitive swelling behaviors showed that the prepared PAA/SiO2 hybrid hydrogel had high pH sensitivity in different pH buffer solutions. Mechanical property test indicated that the PAA/SiO2 hybrid hydrogels exhibited a high compressive strength while remaining a high swelling radio (SR). The maximum of compressive strength and SR of the hybrid hydrogel may reach 42.6 kPa and 17.8, respectively, which was much higher than that of pure PAA hydrogel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, environmental-sensitive hydrogels have gained considerable attention because they can exhibit volume or phase transition in response to external environmental stimuli such as pH, temperature, light, electronic and magnetic field, etc. These hydrogels mainly include pH, temperature, light, electronic- and magnetic-sensitive hydrogels, etc. They have great potential application in drug delivery carriers, tissue engineering, artificial muscles, biosensors, adsorbents and bio-separation, etc. [1]. The polyacrylate-based hydrogel is one of the most important pH-sensitive hydrogels, which have many acidic pendent groups in the hydrogel networks. Considering the dissociation of acidic groups and the influx of counter ions, the ionic concentration in the hydrogels is often higher than that in the surrounding solution. Thus, it leads to a difference in osmotic pressure between the hydrogel and solution, which causes the solution molecules to penetrate into the hydrogel and leads to a high degree of swelling of hydrogels. However, this high osmotic pressure often leads to break the hydrogel networks under the effect of internal stress. Therefore, this inherent drawback limits the application of pH-sensitive hydrogels. To overcome this shortcoming, it is necessary to enhance the strength of pH-sensitive hydrogels while remaining their good pH sensitivity [2, 3]. Up till now, some studies have attempted to improve the mechanical strength of pH-sensitive hydrogels by introducing interpenetrating polymer network [4, 5], hydrophobic monomers [6] or inorganic nanoparticles into hydrogels and so on [7–9].
It is well known that frontal polymerization (FP) is a reaction model of converting monomer into polymer via a localized and propagating reaction in a tube, which presents obvious advantage with a fast and efficient way to produce the polymers [10]. Many polymers and polymeric hydrogels, such as polyurethane, poly(N-methylolacrylamide)/polyhedral oligomeric silsesquioxane, monolithic macroporous polymers, poly(N-methylolacrylamide)/polymethylacryamide hybrids, poly(HPA-co-VeoVa10) amphiphilic gels, and poly(N-isopropylacrylamide) hydrogels have been prepared by FP [11–20]. In our previous research, we rapidly prepared poly(acrylic acid-co-acrylamide) (PAA) hydrogels via FP for dye removal [21].
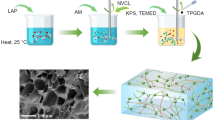
In this study, new pH-sensitive PAA/SiO2 hybrid hydrogels were prepared by in situ FP. SiO2 nanoparticles were rapidly embedded into PAA hydrogel matrix to enhance the mechanical strength of PAA hydrogels via simple FP technique to avoid aggregation of SiO2 nanoparticles during the polymerization of monomers (see Fig. 1). The obtained pH-sensitive PAA/SiO2 hybrid hydrogels were characterized by SEM and Fourier transform infrared spectroscopy (FTIR). The pH sensitivity and compressive strength of PAA/SiO2 hybrid hydrogels were also investigated in details.
Experimental
Materials
Acrylic acid (AAc) was purchased from Tianjin Chemical Reagent Co. (Tianjin, China) and was distilled before use. Acrylamide (AM), potassium persulfate (KPS), N,N′-methylenebisacrylamide (MBA), and N,N′-dimethylformamide (DMF) was purchased from Shanghai Reagent Co. (Shanghai, China). SiO2 nanoparticles were purchased from Haitai Nano-materials Co. (Nanjing, China). The Brunauer–Emmett–Teller surface area is 220 m2 g−1. The average diameter of nanoparticles is about 500 nm. All the reagents were of analytical grade and used as received without any further purification.
Preparation of hybrid hydrogels by in situ FP
First, the appropriate amount of SiO2 nanoparticles was sonicated in DMF and mixed with AAc, AM, MBA, and KPS. Then the mixture was placed for 5 min and was poured into a glass test tube (20 mL; 12.8 mm diameter). To monitor the temperature change, a K-type thermocouple connected to a digital thermometer was immersed into the mixture at about 3 cm from the bottom of the tube. The filled tube was clamped into a holder 1 cm from the top of the tube and kept at ambient temperature. The upper side of the mixture composed of monomers and SiO2 nanoparticles was heated by a soldering iron until the hot propagating front commenced. After the completion of the reaction, the sample was removed from the tube, cut into small pieces, and immersed in deionized water over 3 days to dissolve soluble materials. The obtained hybrid hydrogel products were dried in vacuum at 50 °C to a constant weight and stored for further use. The feed composition of samples during in situ FP was listed and designed as FPn in Table 1.
Velocity and temperature measurements
The front velocities were confirmed by measuring the front position as a function of time. Temperature profiles were determined by measuring the temperature at a fixed point as a function of time, using a K-type thermocouple and converted to spatial ones using front velocities. After the completion of the reaction, the samples were cooled to room temperature and removed from the tube for further investigation.
Characterization and mechanical properties of hybrid hydrogels
Morphology of dry hydrogels was observed by SEM (JSM-5610LV) with an acceleration voltage of 10 kV. The equilibrium-swollen gels were frozen by liquid nitrogen for 6 h, freeze dried, and fractured. The fractured specimens were covered with gold vapor, and then the morphology of the fractured surface of the dry hydrogel was observed by SEM. FTIR spectroscopy was used to confirm the structure of composite hydrogels. The composite hydrogel samples were analyzed on a Bruker EQUNINOX FTIR spectrophotometer in the region of 400–4,000 cm−1. Before the measurements, the dried composite hydrogel samples were crushed down (KBr, pellet). Compressive strength of the swollen hydrogels was tested using a self-prepared equipment described earlier [6]. Compressive strength (Ss) was calculated as follows:
Where, F is force applied and S is the surface area of the swollen hydrogel.
Swelling studies of hybrid hydrogels
The swelling properties of the as-prepared hybrid hydrogels were performed by gravimetric analysis. Briefly, the dry hybrid hydrogel was immersed in the swelling medium at 25 °C. At regular time intervals, the gels were removed from the medium and the weight of the swollen hybrid hydrogel was determined after the removal of the surface water through blotting with filter paper. The measurements were continued until the weight of hydrogels reached a constant value. The swelling ratio (SR) was calculated by the following equation:
Where, Wt is the mass of the swollen hydrogels at t, and W0 is the initial mass of dry hydrogel. All of the experiments were triplicated.
Results and discussion
FP and influence of SiO2 nanoparticles
The PAA/SiO2 hybrid hydrogels were prepared by in situ FP. To obtain PAA/SiO2 hybrid hydrogels with homogeneous dispersion of SiO2 nanoparticles, SiO2 nanoparticles were first sonicated in DMF and then mixed with AAc, AM, and KPS to carry out in situ FP experiments. Figure 2 presents the typical images of propagating fronts of the PAA/SiO2 hybrid hydrogel. An interface between the hybrid and mixture composed of unreacted monomers and SiO2 nanoparticles can be obviously seen. The upper homogeneous white layer was PAA/SiO2 hybrid, and the lower transparent layer was the mixture consisted of unreacted monomers and SiO2 nanoparticles. Furthermore, the front moved downward steadily without bubbles with the increase in time. No “finger phenomenon” was observed [10].
As we all know, it is an important features of FP without simultaneous spontaneous polymerization (SP) that the front velocity remains a constant [13, 14]. Figure 3a shows a typical curve for the front position as a function of time. As it can be seen, the experimental data was well fitted by a straight line, meaning that the front velocity was a constant, 0.95 cm/min. This indicated that the pure FP had occurred. Moreover, a typical temperature profile was obtained during the FP experiments as shown in Fig. 3b. The temperature remained constant until the front reached its junction, indicating SP was not occurring simultaneously [15].
The influence of concentration of SiO2 nanoparticles on V f and T max is also shown in Fig. 4. It was found that V f increased from 0.08 to 1.09 cm min−1 as the concentration of SiO2 nanoparticles increased from 0 to 0.025 g mL−1. The increase in the concentration of SiO2 nanoparticles also caused the increase in T max from 85 to 144 °C, which was similar to the front velocity trend. This may be attributed to the fact that more SiO2 nanoparticles could increase the liquid viscosity of reaction mixture and would result in a higher front temperature. Therefore, the higher the polymerization temperature was, the quicker the V f was. Similar results are reported in a previous research [22].
Characterization of PAA/SiO2 hybrid hydrogels
Figure 5 is the SEM micrographs of the prepared FP2 hydrogel. It could be seen that the white circular paeticles in the SEM micrographs were the SiO2 inorganic nanoparticles. The black background was the hydrogel matrix. We could not see the porous structures in the present SEM micrographs because the hydrogel network was in a collapsed (impact) status. In the preparation procedure of the hydrogel, the dry method has an important effect on the morphology of the hydrogel. In general, the hydrogel network is in a collapsed (impact) status when it is dried by a thermal air-drying method. Therefore, smooth (impact), nonporous structure is often observed in this case. However, the hydrogel network is in a swelling (loose) status when it is dried by a frozen dry method. The porous structure of the hydrogel remained because of water sublimate in the form of ice crystal. Thus, the porous structure is often observed [23]. In our experiment, although the equilibrium-swollen gels were frozen by liquid nitrogen for 6 h, the frozen dried results of liquid nitrogen is not good. Thus, the equilibrium-swollen gels were reduced in a collapsed (impact) status because the water in the hydrogel disappeared and dried in air. Therefore, smooth (impact), nonporous structure was observed. Similar results were seen in our previous reports [24]. In addition, it can be seen that nanosized SiO2 particles with an average diameter of about 500 nm were dispersed in the hydrogel well. The distribution of SiO2 nanoparticles in the PAA hydrogel was highly homogeneous without aggregation. This may be attributed to the fact that monomers polymerized rapidly and SiO2 nanoparticles fast locked into the hydrogel networks were formed, which was also one of the obvious advantages of in situ FP. Figure 6 shows the FTIR spectra of PAA and PAA/SiO2 hybrid hydrogels. It can be observed that the band with a weak shoulder at 2,926 and a medium band at 1,459 cm−1 belonged to the stretching of aliphatic –CH2– and –CHR– groups, respectively. The characteristic absorption peak around 3,400 cm−1 assigned to the –NH2 and –OH groups could be observed in both PAA and PAA/SiO2 hybrid hydrogels. In comparing the FTIR spectra of the PAA/SiO2 hybrid hydrogel and PAA hydrogel, it was found that the intensity of the characteristic absorption peak around 3,400 and 1,600 cm−1 dramatically decreased in PAA/ SiO2 hybrid hydrogel because of the existence of SiO2 nanoparticles.
PH-sensitive swelling behaviors of PAA/SiO2 hybrid hydrogels
To investigate the pH sensitivity of PAA/SiO2 hybrid hydrogels, the swelling kinetics of the hydrogels in various pH values ranging from 2.2 to 8.0 were studied, respectively. As shown in Fig. 7, for every hybrid hydrogel, the SR of hydrogel increased with the increase in pH values. At the same pH values, the SR of hybrid hydrogels first increased with the increase in content of SiO2 nanoparticles (see FP1 and FP2 hydrogels). This is perhaps because the SiO2 nanoparticles dispersed in FP2 was much better than that in FP1 and was more proper for the water absorbency. However, when the content of SiO2 nanoparticles continued to increase, a decrease in SR can be observed (see FP3 and FP4 hydrogels). It is probable because the hydrogel networks became impact and thicker in these cases, which is not suitable for water absorbency [25, 26].
The influence of pH values on the equilibrium SR of hybrid hydrogels was determined in different pH buffer solutions (i.e., 2.2, 4, 6, 7.4, and 8.0) at 25 °C. Figure 8 shows the change of the equilibrium SR of hybrid hydrogels with pH. Obviously, for all hybrid hydrogels, the equilibrium SR showed a sharp increase when the pH value changed from 2.2 to 6.0. This is mainly attributed to the ionization of carboxyl groups [6]. Furthermore, the ability of sharp increase in SR is parallel to the trend of swelling kinetics stated above.
Figure 9 shows the reversible pH-dependent swelling behaviors of the hybrid hydrogels in the pH 2.2 and 7.4 buffer solutions. When the hybrid hydrogels were first placed in pH 7.4 buffer solution, they swelled to a great degree because of the electrostatic repulsion among the ionized carboxyl groups of hydrogels. When the hybrid hydrogels were transferred into pH 2.2 buffer solution, the ionized carboxyl groups of hydrogels patted to proton. The hybrid hydrogels shrunk gradually due to the hydrogen bonding interaction between the non-ionized carboxyl groups [6]. For all hybrid hydrogels, the sudden and sharp swelling–deswelling behavior in different pH buffer solutions implied that they have high pH sensitivity and can be used for tailoring pulsatile (on–off swelling behavior) drug delivery. Here, inspection of FP2, especially, shows a continuous increase in variation (dynamic range) of SR with time. The main reason may be that the equilibrium SR of FP2 hydrogel was highest in all hybrid hydrogels because of its unique structure. The macromolecular chain of FP2 hydrogel was easier to relax than other hybrid hydrogels when the ionized carboxyl groups of hydrogels patted to proton [6]. In addition, after a cycle of reversible swelling–deswelling, all hybrid hydrogels were stable enough, which can be attributed that the introduction of SiO2 nanoparticles can improve the mechanical strength of the PAA hydrogel.
Mechanical properties of PAA/SiO2 hybrid hydrogels
The influence of different content of SiO2 nanoparticles on the mechanical properties of the hybrid hydrogels were further investigated (Table 2). As shown in Table 2, as a result, the compressive strength of hyrogel increased with the increase in content of SiO2 nanoparticles. The maximum of compressive strength of hyrogel may reach 42.6 kPa (FP3 hybrid hydrogel). In comparison, the compressive strength of a pure PAA hydrogel only reached 12.1 kPa. It appeared that the SiO2 nanoparticles played an important role in enhancing the compressive strength of PAA hydrogels. For FP2 hybrid hydrogel, the compressive strength was the second highest among all hydrogels and remained a high-equilibrium SR. Figure 10 shows the photographs of FP2 hybrid hydrogel before compression test, during test, and after test, respectively. Interestingly, during the tests, FP2 hybrid hydrogel did not break, even under extreme high straining, and quickly recovered its original shape after the release of its load. This phenomenon also indicated that good hybrid hydrogels with high compressive strength in swollen status could be obtained via simple in situ FP technique. However, although the compressive strength of the hybrid hydrogels was high, too high content of SiO2 nanoparticles also led to a decrease in the equilibrium SR of the hydrogel (FP3 and FP4 hybrid hydrogels).
Conclusions
In this study, high pH-sensitive PAA/SiO2 hybrid hydrogels with high strength were rapidly prepared by in situ FP. The data of front velocity and front temperature implied that the pure FP had occurred. The rapid conversion reaction of monomer into the PAA hydrogel via the in situ FP could effectively avoid the precipitation of SiO2 nanoparticles during FP. The increase in the concentration of SiO2 nanoparticles led to an increase of V f and T max. For all hybrid hydrogels, the equilibrium SR showed a sharp increase when the pH value changed from 2.2 to 6.0, which was attributed to the ionization of carboxyl groups. After a cycle of reversible swelling–deswelling, all hybrid hydrogels were stable enough. The compressive experiments results indicated that the incorporation of SiO2 nanoparticles into the PAA hydrogel may enhance the strength of the hydrogels.
References
Roy D, Cambre J, Sumerlin B (2010) Prog Polym Sci 35:278
Horkay F, Han M, Han I, Bang I, Magda J (2006) Polymer 47:7335
Jin X, Hsieh Y (2005) Polymer 14:5149
Tang Q, Sun X, Li Q, Wu J, Lin J (2009) J Colloid Interface Sci 339:45
Tang Q, Sun X, Li Q, Wu J, Lin J (2009) J Colloid Interface Sci 346:91
Li S, Li H, Yang Y, Yang X, Xu H (2007) J Appl Polym Sci 106:3792
Wu Q, Zhou C (2011) Colloids Surf A: Biointerface 84:155
Wang N, Han Y, Liu Y, Bai T, Gao H, Zhang P, Wang W, Liu W (2012) J Hazard Mater 213–214:258
Qin X, Zhao F, Liu Y, Feng S (2011) Eur Polym J 47:1903
Pojman JA (1991) J Am Chem Soc 113:6284
Fiori S, Mariani A, Ricco L, Russo S (2003) Macromolecules 36:2674
Fang Y, Chen L, Chen S (2009) J Polym Sci Part A: Polym Chem 47:1136
Yan Q, Lu G, Zhang W, Ma X, Ge CC (2007) Adv Funct Mater 17:3355
Hu T, Fang Y, Yu H, Chen L, Chen S (2007) Colloid Polym Sci 285:891
Cui Y, Yang J, Zhan Y, Zeng Z, Chen Y (2008) Colloid Polym Sci 286:97
Alzari V, Monticelli O, Nuvoli D, Kenny J, Mariani A (2009) Biomacromolecules 10:2672
Yu C, Zhou J, Wang C, Chen L, Chen S (2010) J Polym Sci Part A: Polym Chem 48:2000
Liu N, Shao H, Wang C, Chen Q, Chen S (2013) Colloid Polym Sci. 291:1871
Feng Q, Li F, Yan Q, Zhu Y, Ge C (2010) Colloid Polym Sci 288:915
Feng Q, Yan Q, Ge C (2013) Colloid Polym Sci 291:1163
Li S, Zhang H, Feng J, Xu R, Liu X (2011) Desalination 280:95
Sanna R, Sanna D, Alzari V, Nuvoli D, Scognamillo S, Piccinini M, Lazzari M, Gioffredi E, Malucelli, Mariani A (2012) J Polym Sci Part A: Polym Chem 50:4110
Kang H, Tabata Y, Ikada Y (1999) Biomaterials 20:355
Li S, Huang H, Tao M, Liu X, Cheng T (2013) J Appl Polym Sci 129:3737
Garnweitner G, Smarsly B, Assink R (2003) J Am Chem Soc 125:5626
Liu H, Huang C, Teng X, Wang H (2008) Mater Sci Eng, A 487:258
Acknowledgments
The authors are grateful to the science research project of Hubei Provincial Department of Education (grant no. D20123001) and Hubei Key Laboratory of Mine Environmental Pollution Control and Remediation (grant no. 2012101), and Innovative Team (grant no. T201323) of Hubei Polytechnic University for the financial support on this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, S., Wang, H., Huang, W. et al. Facile preparation of pH-sensitive poly(acrylic acid-co-acrylamide)/SiO2 hybrid hydrogels with high strength by in situ frontal polymerization. Colloid Polym Sci 292, 107–113 (2014). https://doi.org/10.1007/s00396-013-3050-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-013-3050-6