Abstract
The electrokinetic properties of commercial titania and TiO2-SiO2 oxide composite, precipitated from an emulsion system with cyclohexane as the organic phase, are described. To extend the possible range of applications of the TiO2-SiO2 oxide composite, its surface was modified with selected alkoxysilanes: N-2-(aminoethyl)-3-aminopropyltrimethoxysilane, 3-methacryloxypropyltrimethoxysilane and vinyltrimethoxysilane. Modification with selected alkoxysilanes leads to the introduction of new chemical groups on the TiO2-SiO2 surface, which changes its initial properties and also the surface charge, manifested by the values of zeta potential. This study was undertaken to establish the effect of the type and amount of the modifier and type and ionic strength of the electrolyte on the zeta potential of the modified TiO2-SiO2 oxide composite and thus on the stability of the colloidal system. The powders were characterised by FTIR and elemental analysis to confirm the effectiveness of the surface modification. The structure of TiO2-SiO2 oxide composite was resolved by the wide-angle X-ray scattering method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The start of the twenty-first century has seen intense work on the development of new materials or alteration of the physicochemical properties of known ones to extend their range of application. The variables are performed in different ways and lead to materials with specific target properties, e.g. functionalised composites or advanced ceramic materials [1]. Titania is among the most commonly used inorganic pigments. It is used in the cosmetic, food and pharmaceutical industries [2]. Titanium dioxide refined to nanosized particles has unique photocatalytic properties and has often been used as a catalyst in chemical reactions [3]. TiO2 occurs in three polymorphous varieties: anatase, rutile and brookite, differing in their crystalline structure [4]. The tetragonal varieties of anatase and rutile have found the most widespread use and are also synthetically produced. The rutile variety has been proved to have the highest thermal stability [5]. Titanium dioxide, used as a photocatalyst because of its high activity, nontoxicity, low cost and high stability in photochemical reactions, unfortunately also shows poor mechanical performance, low surface area and low thermal stability [6, 7]. In view of this, a search has been undertaken for new modifications which would eliminate the above drawbacks. The catalytic and photocatalytic properties of titania modifications depend on the content of TiO2 [6]. A combination of TiO2 of crystalline structure and SiO2 of amorphous structure permits the elimination of the majority of the above-mentioned drawbacks. The presence of SiO2 brings a reduction in the particle size and an increase in the surface area or in the mechanical strength [8–10]. The composite is chemically neutral and UV transparent and has a large surface area [6]. The presence of SiO2 is also responsible for the increased thermal stability of the composite, which prevents transformation of anatase TiO2 into its rutile variety [8]; this is of profound importance as the anatase variety shows the highest photocatalytic activity out of all three [9]. TiO2-SiO2 oxide composites are used as effective catalysts and photocatalysts of, for example, polycondensation of ethylene polyterephthalate, hydration of carbon oxide or selective oxidation by peroxides of organic compounds in the liquid phase. TiO2-SiO2 oxide composite also acts as a photocatalyst in the neutralisation of waste water after production of nitriles, nylon, plastics, synthetic rubber, benzene, nitrobenzene, herbicides and gold [9].
The proper dispersion and stabilisation of inorganic compounds such as TiO2 or TiO2-SiO2 oxide composite in water solutions poses a great problem in many technological processes and has a significant effect on the properties of the final product [11]. One of the most important controllable properties of each suspension is its stability. According to the Derjaguin, Landau, Verwey and Overbeek (DLVO) theory, the stability of a colloidal system is determined by the London–van der Waals and electrostatic interactions. The DLVO theory gives a physical description of the mechanism of stabilisation of colloidal particles, according to which the repulsive electric interaction of the double layers of the two surfaces endowed with the same charge and the attracting van der Waals interactions reach a compromise at an equilibrium state at which the colloidal particles are stable [12].
The parameter providing information about the stability of colloidal systems is the electrokinetic (zeta) potential. This is a potential that appears on the shear plane between the mobile and immobilised part of the broadened electric double layer. High positive (>30 mV) or high negative (<−30 mV) values of zeta potential mean that the colloidal system studied is stable [12]. The potential value depends on, among other things, the type of solid suspension, surface of the powder, pH of the suspension, ionic strength of the solution and type of electrolyte used. Even a small amount of a compound adsorbed on the surface of a given suspended substance can significantly change the surface charge and hence also the zeta potential and stability of the dispersion [17]. As the values of zeta potential provide important information on the surface chemistry and stability of materials, its knowledge permits their selective and effective use. Measurements of zeta potential have been applied in the pharmaceutical, paper and ceramic industries, at water treatment plants and in biomedicine. Many authors have been interested in the zeta potential of TiO2. Kosmulski et al. [13] determined the effect of multivalent cations on the isoelectric point (IEP) of titanium dioxide. According to these authors, for the anatase variety of TiO2, in the presence of a high concentration of multivalent cations, no IEP is reached and the zeta potential values are positive over the entire pH range studied. The same authors also reported the electrokinetic properties of anatase and rutile varieties of TiO2 as a function of the type and ionic strength of the electrolyte used [14–18]. Similar studies have been performed by Gustafsson et al. [19].
The aim of this study is to determine the zeta potential of commercial titania of the rutile variety, obtained by the sulphate method, and the synthesised TiO2-SiO2 oxide composite. The influence of surface modification, type of electrolyte and its ionic strength on the zeta potential is also studied. Knowledge of the zeta potential values permitted evaluation of the stability of the colloidal systems studied.
Materials and methods
Materials
The commercial titanium white was Tytanpol® RS, produced by the sulphate method at the POLICE SA chemical plant. This pigment is based on the rutile variety of titanium dioxide, contains 1 % of Al2O3 used for surface treatment and is modified with hydrophobic organic compounds.
TiO2-SiO2 oxide composites were obtained by precipitation from emulsion with the use of cyclohexane (POCh SA) as the organic phase. TiO2-SiO2 oxide composites were precipitated from solutions of titanium sulphate (POLICE SA) and sodium silicate (Vitrosilicon SA) which were used as precursors of Ti and Si, respectively. The emulsifiers were nonylphenylpolyoxyethyleneglycol ethers with a mean degree of oxyethylenation of 3 (NP3) and 6 (NP6). Additionally, rutile nuclei of crystallisation were introduced to the reaction system.
The morphology of the TiO2-SiO2 oxide composite and its hydrophobic/hydrophilic properties could be changed thanks to the introduction of silane pro-adhesive compounds in quantities of 1, 3, 5 and 10 parts by mass (Table 1) on the surface of the composites. The effect of the quantity of silane on the zeta potential values was determined. The modification was performed according to procedures described earlier [20, 21].
Physicochemical analysis
The adsorption properties of the samples was evaluated on the basis of surface area, total pore volume and size, measured using an ASAP 2020 instrument made by Micromeritics Instrument Co. Prior to the measurements, the samples were degassed at 120 °C for 4 h.
Dispersive properties were characterised on the basis of particle size distributions measured by the noninvasive back scattering method using a Zetasizer Nano ZS apparatus and by calculation of the polydispersity index.
Information on grain morphology, particle shape, character of agglomerations and dispersion was obtained from scanning electron microscope images (Zeiss EVO40) and TEM microphotographs (Jeol 1200 EX II).
Zeta potential was measured by the electrophoretic light scattering method using a Zetasizer Nano ZS instrument equipped with an autotitrator made by Malvern Instruments Ltd., which enables measurement of the electrophoretic mobility of particles with diameters ranging from 5 nm to 100 μm. The zeta potential was determined in the pH range 2–11. Three types of electrolytes were used: KCl, NaCl and NaNO3 of ionic strength 0.001, 0.01 and 0.1 M, respectively. The measurements of conductivity and pH of the suspensions were performed simultaneously on the same instrument. The instrument was calibrated by measuring the zeta potential of a standard latex suspension and the pH of standard buffer solutions for pH 4 and 9. Before the measurement was performed, the analysed dispersions were stabilised for 15 min in an ultrasonic bath. To avoid possible measurement errors, every sample was measured three times, and the mean and standard deviation were taken for the three measurements. The standard deviation of the zeta potential at a given pH was ±1.7 mV or less, and the error in the pH was estimated to be 0.03 pH units or lower.
Elemental analysis (carbon, hydrogen, nitrogen) of the samples was performed with an Vario EL Cube III instrument made by Elementar Analysensysteme GmbH. The degree of TiO2-SiO2 surface modification was estimated on the basis of FTIR spectra taken by EQUINOX 55 made by Bruker. The structure of the TiO2-SiO2 oxide composite was resolved by the wide-angle X-ray scattering method (WAXS).
Results and discussion
Adsorption properties
The adsorption properties of the commercial titania and TiO2-SiO2 oxide composite obtained by precipitation are characterised by the results presented in Table 2. The surface area of TiO2-SiO2 oxide composites precipitated from emulsion systems is 54 m2/g, which is much larger than that of commercial TiO2 RS, namely 9 m2/g. This result is a consequence of the fact that a stoichiometric addition of silica causes an increase in the surface area of the composite. The mean diameter of pores in TiO2 RS is 9.8 nm and their volume is 0.020 cm3/g, while for the TiO2-SiO2 oxide composite, the corresponding values are 5.2 nm and 0.070 cm3/g.
It was found that subjecting the TiO2-SiO2 oxide composite to a process of modification significantly reduces the basic adsorption properties. For samples modified with the silane U-511, the surface area lies in the range 2.9–5.5 m2/g. There is a noticeable drop in the value of the Brunauer, Emmett and Teller (BET) surface area as the amount of modifier used for functionalisation of the composite surface increases. A similar relationship can be observed for pore volume (0.002–0.004 cm3/g) and for average pore diameter (except for the sample modified with 5 parts by mass of 3-methacryloxypropyltrimethoxysilane), where as the quantity of silane used for surface functionalisation increases, there is a visible reduction in pore diameter from 5.2 nm (for unmodified TiO2-SiO2) to 2.9 nm for composite modified with 10 parts by mass of silane U-511. The largest value of pore diameter is found for the system modified with 5 parts by mass of 3-methacryloxypropyltrimethoxysilane, while for samples modified with 1 and 3 parts by mass of that silane, the respective values are 3.1 and 3.0 nm.
Analysis of the results obtained for TiO2-SiO2 composite modified with vinyltrimethoxysilane shows that these samples have the highest values for BET surface area among all the modified samples. The TiO2-SiO2 sample modified with 1 part by mass of silane U-611 has the highest value of surface area, equal to 8.5 m2/g. Only slightly lower values are recorded for the composite modified with the greatest quantity of silane U-611 (the surface area is 7.5 m2/g). Slightly poorer adsorption properties are found for the samples modified with 3 and 5 parts by mass of vinyltrimethoxysilane, which have surface areas of 5.8 and 5.2 m2/g, respectively, and a pore volume value of 0.004 cm3/g. The pore diameter ranges from 1.4 nm for the TiO2-SiO2 sample modified with 10 parts by mass of silane U-611 to 3.1 nm for the oxide composite modified with 5 parts by mass of vinyltrimethoxysilane.
Modification with N-2-(aminoethyl)-3-aminopropyltrimethoxysilane, similarly to the previous silanes, also caused blocking of the active centres present on the composite surface, thus reducing the surface area. The value of the BET surface area of samples modified with the aminosilane U-15D ranges from 5.8 m2/g for the product modified with the smallest amount of the modifier to 3.7 m2/g for the TiO2-SiO2 sample functionalised using 5 parts by mass of U-15D. Values for pore volume and diameter are close to those obtained for the silanes U-511 and U-611.
Measurement of the BET surface area showed clearly the significance of the changes that take place on the surface of TiO2-SiO2 oxide composite subjected to the modification process. The process leads to blocking of the active centres present on the surface of the composite.
Dispersive and morphological properties
The particle size distribution according to volume contribution estimated for the TiO2 sample Tytanpol® RS shows two bands (see Fig. 1a). The first corresponds to particles of diameters in the range 164–1,480 nm (the maximum volume contribution of 10.5 % comes from particles and aggregates 342 nm in diameter). The second one covers the range of particle diameters from 2,300 to 6,440 nm (the maximum volume contribution of 4.5 % comes from agglomerates 4,800 nm in diameter). It is shown by SEM and TEM analyses that the particles are spherical in shape (see Fig. 1b, c).
Figure 2a presents the analogous data for TiO2-SiO2 oxide composite. The particle size distribution according to volume contribution shows a single band covering particle diameters from 615 to 6,440 nm. The maximum volume contribution of 12.4 % comes from particles 1,990 nm in diameter. As is shown in the SEM and TEM images (Fig. 2c), the particles have spherical shape and exhibit a small tendency towards agglomerate formation.
Table 3 presents dispersion data for TiO2-SiO2 oxide composite samples modified with the silanes U-15D, U-511 and U-611. The results of particle size distribution measurements indicate that the surface modification has changed the dispersion characteristics of the oxide composites. The modification with 3 and 5 parts by mass of the modifier gives products with particles of smaller diameters.
Electrokinetic characteristics
At the next stage of the study, the influence of ionic strength and type of electrolyte on the zeta potential dependence on pH was analysed for the commercial titania (Fig. 3a, b). Change in ionic strength and the type of monovalent electrolyte used (NaCl, NaNO3) have only a small effect on the values obtained for zeta potential and consequently also on the stability of the dispersion. Analysing literature reports, Roy et al. [22], who studied zeta potential of aluminium oxide and zirconia, have proved that an increase in the ionic strength of the electrolyte from 0.01 to 0.1 M has practically no effect on the positive value of zeta potential, while such an increase at a negative potential value causes its weakening. Similar relations can be observed for the sample of TiO2 RS. The zeta potential values for the commercial titanium white sample take negative values over a wide range of pH (5–11). The described rutile variety of titanium dioxide achieves IEP at a pH of around 5, which is also confirmed in previous observations reported in the literature [17]. The character of the electrokinetic curves for this sample can be explained by the presence of a very small amount of Al2O3 used for the surface modification of TiO2 RS. Ntalikwa et al. found that Al2O3 takes IEP values in the pH range of 3.3 to 9.2. These large differences in the isoelectric point values may result from (1) the choice of type of method used to determine the zeta potential, (2) the size and shape of the particles analysed, (3) the chemical and mineralogical composition of the analysed compound, or (4) the method for obtaining it [25]. The author also confirmed that the IEP value is independent of the ionic strength of the electrolyte solution used. An analogous relationship was also observed in the case of the electrokinetic properties of titanium dioxide of RS type.
Figure 4 illustrates the influence of the type of electrolyte on zeta potential at a constant ionic strength of 0.01 M. On analysing the obtained electrokinetic curves, it may be concluded that only small changes occurred in the obtained zeta potential values over the range of pH greater than the IEP. The influence of background electrolyte on the position of the isoelectric point has been theoretically studied in [26]. The relationships obtained regarding the electrokinetic curves are analogous to the literature data described in [27], which show that the electrokinetic curves for Al2O3 in the presence of NaCl and NaClO4 were subject to only small differences over the acidic pH range. In the alkaline range, the zeta potential in the presence of KBr and KNO3 was independent of the anion, while only small differences in the curves were observed over the alkaline pH range.
As follows from the electrokinetic curves, TiO2-SiO2 oxide composite is highly stable for pH values from 7 to 11, which is manifested by zeta potential values from −20 to −40 mV.
The values obtained for zeta potential also confirm the particular adsorption, in the range of pH greater than the IEP, of ions from monovalent electrolyte solutions. Studies on the stability of colloidal systems have shown that the presence of monovalent ions in the system can affect the tendency towards coagulation. The effect of monovalent ions has been expressed in the so-called Hofmeister series or lyotropic series (Cs+ > Rb+ > K+ > Na+ > Li+) [23].
Figure 5 shows the zeta potential as a function of pH measured for TiO2-SiO2 oxide composite for different ionic strengths (0.001–0.01 M) and for different electrolyte (NaCl, NaNO3). The electrokinetic curves obtained are very similar. The analysed TiO2-SiO2 oxide composite had high electrokinetic stability over the pH ranges 2–4 and 7–11. The isoelectric point was attained at a pH value of around 5.2, which may reflect a higher content of titanium dioxide in the analysed oxide composite.
Figure 6a presents the zeta potential versus pH recorded for unmodified TiO2-SiO2 oxide composite and samples of this composite modified with 1, 3, 5 and 10 parts by mass of U-511. The main objective of the described experiments (Figs. 6a, b and 7) was to determine the influence of the quantity and type of pro-adhesive silane compound used for surface functionalisation of TiO2-SiO2 oxide composite; hence, the carrier electrolyte solution chosen was a 0.001 M solution of NaCl, which is the most frequently used in measurements of electrokinetic potential. The described modified oxide systems were also measured in other solutions (KCl and NaNO3) at different ionic strengths (0.001–0.1 M). Very similar zeta potential values were obtained (this may be an indication of wide possibilities for the application of oxide composites of this type); hence in the remainder of this work, we present results obtained with 0.001 M NaCl. For the unmodified TiO2-SiO2 sample, zeta potential takes positive values in the acidic pH range and reaches IEP at pH close to 5.2. Modification of the composite surface with the silanes U-511 or U-611 (Fig. 6a, b) results in a shift of the electrokinetic curve towards lower pH, which extends the pH range in which these samples show high stability within the alkaline pH range (7–11).
Figure 7 gives the zeta potential versus pH for TiO2-SiO2 samples unmodified and modified with U-15D, measured in a 0.001 M solution of NaCl. For the unmodified sample, the electrokinetic curve is the same as in Fig. 6, as it was recorded in the same solution. For the sample modified with 1 part by mass of silane U-15D, IEP was shifted to a pH close to 7.8. The greatest shift of the electrokinetic curve was noted for the sample modified with 10 parts by mass of U-15D. For this sample, IEP was reached at pH nearly 10.5 and the zeta potential took positive values in the entire pH range analysed, showing high stability for the pH range 1–9, manifested by potential values from 70 to 48 mV. Modification of the TiO2-SiO2 oxide composite surface with N-2-(aminoethyl)-3-aminopropyltrimethoxysilane results in a significant shift of the electrokinetic curve towards higher pH, relative to that for the unmodified sample. For the sample modified with the above silane, the ionisation of NH2 groups is important. A high density of H+ ions induces the formation of NH +3 groups, responsible for the positive charge on the composite surface. With an increasing concentration of H+ ions, the process of dissociation is restricted and the surface charge decreases [22].
Modification with amine compounds leads to high IEP values, which is related to the basic character of the composite surface. A scheme of aminosilane attachment to the surface of the TiO2-SiO2 oxide composite is presented in Fig. 8.
Effectiveness of surface modification
In order to check the effectiveness of the surface functionalisation of TiO2-SiO2 oxide composite, the elemental composition (C, H, N) of the samples studied and the degree of coverage were determined (see Table 4). The degree of surface coverage was calculated using the equation proposed by Berendsen and de Golan [24]. With an increasing quantity of silane used for the modification, an increase in the percentage content of the above elements was noted, along with a significant increase in the degree of coverage. For example, for the samples modified with U-15D, the degree of coverage increases from 0.621 μmol/m2 for the sample modified with 1 part by mass of U-15D to 5.648 μmol/m2 for the sample modified with 10 parts by mass of U-15D.
Figure 9 presents the FTIR spectra of the unmodified TiO2-SiO2 oxide composite and of the same composite modified with 3 and 10 parts by mass of N-2-(aminoethyl)-3-aminopropyltrimethoxysilane (Fig. 9a), 3-methacryloxypropyltrimethoxysilane (Fig. 9b) and vinyltrimethoxysilane (Fig. 9c) in the amounts of 3 and 10 parts by mass of TiO2-SiO2 oxide composite. Modification of the initial sample of the analysed oxide composite caused a decrease in the intensity of bands belonging to -OH groups (at a wave number of 3,500 cm−1) and -O-Ti-O- groups occurring at 650 cm−1. For the composite modified with the silanes U-511 and U-611, there can also be observed a slight increase in the intensity of the bands corresponding to C–H bonds (λ = 3,000 cm−1), most clearly in the case of samples modified with the aminosilane U-15D. Functionalisation of the surface with 10 parts by mass of the silane U-511 and the aminosilane U-15D caused an increase in the intensity of the band attributed to ≡Ti-O-Si≡ groups (occurring at λ = 1,650 cm−1). An increase in the amount of vinyltrimethoxysilane adsorbed on the composite surface causes the intensity of that band to decrease. Slight differences in intensity were also observed for a second band belonging to the ≡Ti-O-Si≡ bond (λ = 950 cm−1), in case of modification of the composite surface with 3 parts by mass of any of the silanes used and with 10 parts by mass of the silanes U-511 and U-611. However, in the case of the TiO2-SiO2 sample modified with 10 parts by mass of the silane U-15D, the intensity of that band increased slightly. Analysis of the spectra confirmed the effectiveness of functionalisation of the composite surface, which was manifested by changes in the intensity of selected characteristic bands.
Structural properties
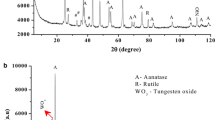
Diffractograms of TiO2 RS and TiO2-SiO2 oxide composite obtained by the WAXS method are presented in Fig. 10a, b. Titanium dioxide of a well-defined polymorphous variety gives maxima for a certain value of 2θ, so these signals permit identification of the corresponding variety. The rutile variety gives maxima for 2θ values of 27, 36, 39, 41, 45, 55 and 57, while the anatase variety gives maxima at 2θ values of 24, 37, 38, 49, 54 and 56. As the diffractograms show, TiO2-SiO2 oxide composite contains a mixture of anatase and rutile varieties of TiO2, with the latter dominant.
Conclusions
Surface modification of a TiO2-SiO2 oxide composite with selected alkoxysilanes was shown to significantly change the properties of the composite and hence extend the possible range of its application. Stability of the composite dispersion, evaluated on the basis of the measured zeta potential, was found to be insignificantly affected by the type of electrolyte used and its ionic strength. Modification of the TiO2-SiO2 oxide composite, performed in order to introduce specific functional groups on its surface, also significantly influenced the pH range of stability of a given colloidal system. This influence was particularly pronounced when the modifying agent was aminosilane.
The zeta potential values were confirmed to be determined by the chemism of the composite surface. Even a small amount of silane changes the surface charge of the composite and its zeta potential values.
References
Veronovski N, Andreozzi P, La Mesa C et al (2010) Stable TiO2 dispersions for nanocoating preparation. Surf Coat Technol 204:1445–1451
Panagiotou GD, Petsi T, Bourikas K et al (2008) Mapping the surface (hydr)oxo-groups of titanium oxide and its interface with an aqueous solution: the state of the art and a new approach. Adv Colloid Interface Sci 142:20–42
Ridley MK, Hackley VA, Machesky ML (2006) Characterization and surface-reactivity of nanocrystalline anatase in aqueous solutions. Langmuir 22:10972–10982
Chun H, Yizhong W, Hongxiao T (2001) Preparation and characterization of surface bond-conjugated TiO2/SiO2 and photocatalysis for azo dyes. Appl Catal, B 30:277–285
Xu Y, Langford CH (1997) Photoactivity of titanium dioxide supported on MCM41, zeolite X, and zeolite Y. J Phys Chem B 101:3115–3125
Murashkevich AN, Lavitskaya AS, Alisienok OA et al (2009) Fabrication and properties of SiO2/TiO2 composites. Inorg Mater 45:1146–1152
Bellardita M, Addamo M, Di Paola A et al (2010) Photocatalytic activity of TiO2/SiO2 systems. J Hazard Mater 174:707–713
Nilchi A, Janitabar-Darzi S, Mahjoub AR et al (2010) New TiO2/SiO2 nanocomposites—phase transformations and photocatalytic studies. Colloids Surf A Physicochem Eng Aspects 361:25–30
Zhan S, Chen D, Jiao X et al (2007) Mesoporous TiO2/SiO2 composite nanofibers with selective photocatalytic properties. Chem Commun 20:2043–2045
Ren S, Zhao X, Zhao L et al (2009) Preparation of porous TiO2/silica composites without any surfactants. J Solid State Chem 182:312–316
Yaremko ZM, Tkachenko NH, Hellmann C et al (2006) Redispergation of TiO2 particles in aqueous solutions. J Colloid Interface Sci 296:565–571
Hunter RJ (1981) Zeta potential in colloid science. Academic, New York
Kosmulski M, Rosenholm JB (2004) High ionic strength electrokinetics of anatase in the presence of multivalent inorganic ions. Colloids Surf A Physicochem Eng Aspects 248:121–126
Kosmulski M, Gustafsson J, Rosenholm JB (1999) Ion specificity and viscosity of rutile dispersions. Colloid Polym Sci 277:550–556
Kosmulski M, Dukhin AS, Priester T et al (2003) Multilaboratory study of the shifts in the IEP of anatase at high ionic strengths. J Colloid Interface Sci 263:152–155
Kosmulski M (2002) The significance of the difference in the point of zero charge between rutile and anatase. Adv Colloid Interface Sci 99:255–264
Kosmulski M (2001) Chemical properties of material surfaces. Marcel Dekker, New York
Kosmulski M, Durand-Vidal S, Gustafsson J et al (1999) Charge interactions in semi-concentrated titania suspensions at very high ionic strengths. Colloids Surf A Physicochem Eng Aspects 157:245–259
Gustafsson J, Nordenswan E, Rosenholm JB (2003) Consolidation behaviour in sedimentation of TiO2 suspensions in the presence of electrolytes. J Colloid Interface Sci 258:235–243
Jesionowski T, Ciesielczyk F, Krysztafkiewicz A (2010) Influence of selected alkoxysilanes on dispersive properties and surface chemistry of spherical silica precipitated in emulsion media. Mater Chem Phys 119:65–74
Jesionowski T, Krysztafkiewicz A (2000) Comparison of the techniques used to modify amorphous hydrated silicas. J Non-Cryst Solids 277:45–57
Roy SK, Sengupta PK (1988) Electrical properties and the surface characteristics of lanthanum oxide/water interface. J Colloid Interface Sci 125:340–343
Janusz W, Gałgan A (2001) Electrical double layer at manganese oxides/1:1 electrolyte solution interface. Physicochem Probl Miner Process 35:31–41
Berendsen GE, de Golan L (1978) Preparation and chromatographic properties of some chemically bonded phases for reversed-phase liquid chromatography. J Liq Chromatogr 1:561–586
Ntalikwa JW, Bryant R, Zunzu JSM (2001) Electrophoresis of colloidal α-alumina. Colloid Polym Sci 279:843–849
Sonnefeld J (2001) On the influence of background electrolyte concentration on the position of the isoelectric point and the point of zero charge. Colloids Surf A Physicochem Eng Aspects 190:179–183
Johnson SB, Scales PJ, Healy TW (1999) The binding of monovalent electrolyte ions on a-alumina. I. Electroacoustic studies at high electrolyte concentrations. Langmuir 15:2836–2843
Acknowledgments
This work was supported by the Polish National Centre of Science research grant no. 2011/01/B/ST8/03961.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nowacka, M., Siwińska-Stefańska, K. & Jesionowski, T. Structural characterisation of titania or silane-grafted TiO2-SiO2 oxide composite and influence of ionic strength or electrolyte type on their electrokinetic properties. Colloid Polym Sci 291, 603–612 (2013). https://doi.org/10.1007/s00396-012-2762-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-012-2762-3