Abstract
Novel polymerizable red and yellow dyes, consisting of anthraquinone chromophore, alkyl spacer, and acryloyl group, were first synthesized and then used as comonomers in the semicontinuous emulsion copolymerization of styrene, butyl acrylate, and methacrylic acid to fabricate polymer latexes. The influences of the dye monomers on the emulsion polymerization process, the latex particle size and its distribution, the molecular weight of the latex polymer, as well as the light fastness of the polymer latex films, were investigated. Results indicated that, despite of the inhibition effect of the polymerizable dyes on polymerization, stable colored polymer latexes could be prepared with high conversion of total monomers, whereas the conversion of the polymerizable dye decreased as increasing the amount of dye. The light fastness of the covalently colored polymer latex films was proved to be much better than that of the noncovalently colored polymer latex films due to the covalent bond of dye and polymer chains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colored polymer latexes, both visible color and fluorescent latexes, have a wide variety of traditional applications, such as in paint, ink, textile, and plastic industries [1–5]. Recently, due to the development of biomedical engineering and material engineering, the colored nano/microlatex particles attracted considerable attention and have been applied in various areas, such as cell markers [6, 7], enzyme immobilization [8], and chemical/biochemical sensors [9, 10]. Generally, colored polymer latex can be obtained by emulsion polymerization in the presence of inert oil-soluble dyes [11, 12] or by mixing colorless latex with colorant [13, 14]. However, the fading of the colored latex seems to be a big problem, which could be ascribed to the separation of the dye and the polymer latex. Meanwhile, the colored polymer film prepared from the colored latex exhibits poor migration fastness of dye, and also, its light fastness is usually unsatisfactory. It is believed that these defects originate from the weakly noncovalent interaction between dyes and polymer chains. Attempts have been made to improve the dye preservation in the polymer matrix, for example, Gu and coworkers prepared colored latex by encapsulation of hydrophilic dye with polystyrene using double miniemulsion polymerization [15]. Liu fabricated a set of nanocolorants by miniemulsion polymerization with hydrophobic dyes and proper amount of a cross-linker [16]. Although these efforts provided significant methods to obtain relatively stable colored latex, as suggested by Takasu [17], dye migration was still observed after long-term storage.
Covalent incorporation of chromophores with polymer is an effective solution for the promotion of dye fastness. In copolymerization, the chromophoric groups of polymerizable dyes can be integrated into the polymer chains, resulting in covalently colored polymers with enhanced migration fastness and light fastness [18, 19]. This technique has been used in stepwise polymerization for producing colored polyurethane [20], as well as, in addition polymerization for synthesizing fluorescent dye-labeled particles [21]. However, because of the inhibition effect of chromophores on free radical polymerization, the introduction of polymerizable dyes, such as anthraquinone derivatives, often fails in emulsion copolymerization processes.
In order to improve the performance of colored polymer latex, in this paper, two new polymerizable anthraquinone dyes, consisting of anthraquinone chromophore (AQ), alkyl spacer, and acryloyl group, were first synthesized and then used as comonomers in the semicontinuous emulsion copolymerization of styrene (St), butyl acrylate (BA), and methacrylic acid (MAA) to fabricate red and yellow polymer latexes. The chemical structure of the polymerizable dyes and the properties of latexes were characterized by 1H NMR, Fourier transform-infrared (FT-IR) spectroscopy, gel permeation chromatography (GPC), and transmission electron microscopy (TEM). Moreover, the influence of the polymerizable dyes on the emulsion copolymerization was investigated, and the light fastness of the covalently colored polymer latex films was studied in comparison with the noncovalently colored polymer latex films.
Experimental
Materials
BA, St, and MAA (First Chemical Reagent Factory, Tianjin) were purified by distillation under reduced pressure. Ammonium persulfate (APS) (Shanghai Aijian Modern Reagent Co.) was purified by recrystallization in water before use. Dichloromethane, toluene, and n-hexane (Beijing Chemicals) were dried with anhydrous MgSO4 and stored with 4A molecular sieve. Acryloyl chloride, terephthaloyl dichloride, 2-hydroxyethyl acrylate (HEA), and 4-dimethylaminopyridine (DMAP) were purchased from Alfa Aesar and used as received. Sodium dodecyl sulfate (SDS), octylphenol polyoxyethylene (10) ether (OP-10) (Beijing Chemicals), 1,6-diaminohexane (First Chemical Reagent Factory, Tianjin), 1-aminoanthraquinone (Chongqing Kuayue Co.), 1-nitroanthraquinone (Wuqiao Chemical Co.), and the other reagents were used without further purification.
Synthesis of the polymerizable dyes and the unreactive dyes
Synthesis of 1-(6-aminohexylamino)anthraquinone
To a suspension containing dimethoxyethane (50 mL), 1-nitroanthraquinone (2.53 g, 10 mmol), and Na2CO3 (1.06 g, 10 mmol), 4.64 g (40 mmol) of 1,6-diaminohexane was added. The resulting mixture was stirred and refluxed at 85 °C for 2 h. It was cooled to room temperature and then poured into an aqueous NaOH solution (0.2 wt.%). The solid precipitate was filtered and washed with deionized water for three times. After evaporation of water at reduced pressure, the product was purified by recrystallization from ethyl acetate to give 3.06 g of dark red solid (95 % yield).
1H NMR (600 MHz, [D6]DMSO, TMS, δ/ppm): 8.22 (d, 1H, AQ 5-H), 8.14 (d, 1H, AQ 8-H), 7.91 (t, 1H, AQ 7-H), 7.85 (t, 1H, AQ 6-H), 7.67 (t, 1H, AQ 3-H), 7.45 (d, 1H, AQ 4-H ), 7.27 (d, 1H, AQ 2-H), 3.37 (t, 2H, CH 2NH-AQ), 1.68 (t, 2H, CH 2NH2), 1.33–1.46 (m, 8H, alkyl H).
FT-IR (KBr, cm−1): 2,929, 2,852 (alkyl C–H); 1,674 (C = O stretch); 1,632, 1,593, 1,574, 1,510 (AQ C = C); 1,269 (C–N stretch and N–H bend).
Synthesis of 1-(6-acrylamidohexylamino)anthraquinone (AHAQ)
To a solution containing dichloromethane (40 mL), 1-(6-aminohexylamino)anthraquinone (1) (2.58 g, 8 mmol), and triethylamine (1.2 g, 12 mmol), 0.905 g of acryloyl chloride (10 mmol), dissolved in dichloromethane (10 mL), was added slowly over 15 min. The round-bottomed flask was cooled in an ice–water bath and stirred for 2 h. After removal of solvent by rotary evaporation, the dark red solid was purified by silica column chromatography using a mixture of dichloromethane/ethyl acetate (3:1) as eluent to give 2.44 g of product (81 % yield).
1H NMR (600 MHz, [D6]DMSO, TMS, δ/ppm): 8.15 (d, 1H, AQ 5-H), 8.10 (d, 1H, AQ 8-H), 7.88 (t, 1H, AQ 7-H), 7.82 (t, 1H, AQ 6-H), 7.60 (t, 1H, AQ 3-H), 7.39 (d, 1H, AQ 4-H), 7.18 (d, 1H, AQ 2-H), 6.23 (d × d, 1H, 2-acryloyl H), 6.09 (d, 1H, 3-acryloyl cis H), 5.60 (d, 1H, 3-acryloyl trans H), 3.30 (t, 2H, CH 2NH-AQ), 3.15 (t, 2H, CH 2NHCO), 1.31–1.70 (m, 8H, alkyl H).
FT-IR (KBr, cm−1): 3,286 (N–H stretch); 2,935, 2,856 (alkyl C–H); 1,674 (AQ C = O stretch); 1,655 (acryloyl C = O); 1,541 (amide N–H bend); 1,267 (AQ C–N stretch and N–H bend).
Synthesis of 4-(anthraquinone-1-ylcarbamoyl)benzoyl chloride
Of terephthaloyl dichloride, 10.15 g (50 mmol) was added into a suspension containing toluene (80 mL) and 1-aminoanthraquinone (2.23 g, 10 mmol), and the resulting mixture was stirred and refluxed at 115 °C for 3 h. It was cooled to room temperature and then poured into n-hexane (400 mL). The solid precipitate was filtered and washed with n-hexane for three times and dried in vacuum to give 3.86 g of bright yellow product (99 % yield).
1H NMR (600 MHz, [D6]DMSO, TMS, δ/ppm): 9.16 (d, 1H, AQ 2-H); 8.31 (d, 1H, AQ 8-H); 8.22 (d, 1H, AQ 5-H); 8.20 (d, 2H, phenyl 2,6-H); 8, 19 (d, 2H, phenyl 3,5-H); 7.95–8.08 (m, 4H, AQ 3,4,6,7-H).
FT-IR (KBr, cm−1): 3,196, 3,113 (Ar C–H); 1,770 (chloride C = O); 1,682 (AQ C = O); 1,641, 1,591, 1,579, 1,527 (AQ and benzene ring).
Synthesis of 2-acroyloxyethyl-4-(anthraquinone-1-ylcarbamoyl)benzoate (AAQCB)
A mixture of 4-(anthraquinone-1-ylcarbamoyl)benzoyl chloride (3) (3.12 g, 8 mmol), triethylamine (1.2 g, 12 mmol), 2-hydroxyethyl acrylate (2.78 g, 24 mmol), and dichloromethane (40 mL) was stirred at room temperature for 4 h and then poured into n-hexane (400 mL). The bright yellow solid precipitate was filtered and purified by silica column chromatography using a mixture of dichloromethane/ethyl acetate (3:1) as eluent to give 2.93 g of product (78 % yield).
1H NMR (600 MHz, [D6]DMSO, TMS, δ/ppm): 9.05 (d, 1H, AQ 2-H); 8.22 (d, 1H, AQ 8-H); 8.15 (d, 2H, phenyl 3,5-H); 8, 13 (d, 2H, phenyl 2,6-H); 8.12 (d, 1H, AQ 5-H); 7.96 (d, 1H, AQ 4-H); 7.90–7.94 (m, 3H, AQ 3,6,7-H); 6.39 (d, 1H, 3-acryloyl cis H); 6.24 (d × d, 1H, 2-acryloyl H); 5.99 (d, 1H, 3-acryloyl trans H); 4.60 (t, 2H, CH 2OCO-benzene ring); 4.52 (t, 2H, CH 2OCOCH = CH2).
FT-IR (KBr, cm−1): 1,724 (acryloyl C = O); 1,689 (AQ C = O); 1,643, 1,581, 1,525 (AQ and benzene ring).
Synthesis of 2-hydroxyethyl-4-(anthraquinone-1-ylcarbamoyl)benzoate
A mixture of 4-(anthraquinone-1-ylcarbamoyl)benzoyl chloride (3) (1.56 g, 4 mmol), triethylamine (0.6 g, 6 mmol), ethylene glycol (2.48 g, 40 mmol), and dichloromethane (40 mL) was stirred at room temperature for 4 h and then poured into n-hexane (400 mL). The bright yellow precipitate was filtered and washed with deionized water for three times and then purified by recrystallization from ethyl acetate to give 1.32 g of product (80 % yield).
1H NMR (600 MHz, [D6]DMSO, TMS, δ/ppm): 9.13 (d, 1H, AQ 2-H); 8.28 (d, 1H, AQ 8-H); 8.25 (d, 2H, phenyl 3,5-H); 8, 20 (d, 2H, phenyl 2,6-H); 8.19 (d, 1H, AQ 5-H); 8.02 (d, 1H, AQ 4-H); 7.94–7.99 (m, 3H, AQ 3,6,7-H); 4.36 (t, 2H, CH 2OCO-benzene ring); 3.76 (t, 2H, CH 2OH).
FT-IR (KBr, cm−1): 3,410 (O–H stretch); 1,691 (AQ C = O); 1,645, 1,591, 1,525 (AQ and benzene ring).
Synthesis of 2-acetoxyethyl-4-(anthraquinone-1-ylcarbamoyl)benzoate
A mixture of 2-hydroxyethyl-4-(anthraquinone-1-ylcarbamoyl)benzoate (5) (1.41 g, 3.4 mmol), DMAP (0.06 g, 0.5 mmol), acetic anhydride (10 mL), and pyridine (10 mL) was stirred and heated at 100 °C for 1 h. It was cooled to room temperature and then dropped slowly into water. The solid precipitate was filtered and washed with deionized water for three times. After evaporation of water in vacuum, the product was purified by silica column chromatography using a mixture of dichloromethane/ethyl acetate (3:1) as eluent to give 1.17 g of product (75 % yield).
1H NMR (600 MHz, [D6]DMSO, TMS, δ/ppm): 9.12 (d, 1H, AQ 2-H); 8.27 (d, 1H, AQ 8-H); 8.21 (d, 2H, phenyl 3,5-H); 8, 19 (d, 2H, phenyl 2,6-H); 8.18 (d, 1H, AQ 5-H); 8.01 (d, 1H, AQ 4-H); 7.98 (t, 1H, AQ 3-H); 7.93–7.96 (m, 2H, AQ 6,7-H); 4.55 (t, 2H, CH 2OCO-benzene ring); 4.41 (t, 2H, CH 2OCOCH = CH2).
FT-IR (KBr, cm−1): 1,722 (acetyl C = O); 1,689 (AQ C = O); 1,643, 1,581, 1,529 (AQ and benzene ring).
Preparation of the covalently colored polymer latex
The preparation of covalently colored P(St-BA-MAA) latex, using red dye monomer AHAQ (2) or yellow dye monomer AAQCB (4) (abbreviated as PSBM-AHAQ and PSBM-AAQCB, respectively) as comonomer, was carried out by semicontinuous emulsion copolymerization with typical recipes listed in Table 1. The dye monomer was dissolved in a mixture of St, BA, and MAA, after that 25 mL of water, surfactants (SDS and OP-10), NH4HCO3, and 20 wt.% of the monomer mixture were charged into a four-necked 100 mL round-bottom flask equipped with a N2 inlet, a feeding inlet, a reflux condenser, and an electric mechanical stirrer. The mixture was stirred at around 200 rpm and heated in a water bath. APS was dissolved in 5 mL of water prior to use. When the temperature stabilized at 80 °C, 40 wt.% of APS solution was injected to initiate polymerization, and the residual monomer mixture was added dropwise into the flask for 1 h. The residual 60 wt.% of APS solution was introduced at different polymerization time as follows: 10 wt.% at an interval of 15 min in the monomer feeding process and 20 wt.% at 1.5 h. Subsequently, the system was maintained at 80 °C for an additional 1 h and cooled down to room temperature to obtain the colored latex. In control experiments, 1.0 wt.% of unreactive dye (1 or 6) to the total monomers was employed using the same method as polymerizable dye to produce the noncovalently colored latex.
Characterization
1 H NMR spectra were obtained on a JEOL JNM-ECA600 spectrometer. FT-IR spectroscopy experiments were performed using a Nicolet 560 instrument. UV-Vis absorption was studied using a UV-Vis spectrometer (T6, Pgeneral). The size (D p) and morphology of the latex particles were characterized by transmission electron microscopy (TEM, Hitachi H-7650B). The hydrodynamic diameter (D h), size polydispersity, and zeta potential of the latex particles were measured on Zetasizer 3000HS (Malvern). The molecular weight and its distribution were measured by gel permeation chromatography (GPC, Shimadzu). The GPC instrument was equipped with a refractive index detector (Wyatt Optilab rEX) and fitted with a PLgel 5 μm mixed-D column, which was calibrated using polystyrene standard. THF was used as the eluent, and the measurements were carried out at 35 °C with an eluent flow rate of 1.0 mL/min. The yield of the dye was measured by the ratio of its experimental and theoretical molar quantity.
Monomer conversion
Total monomer conversion (Conv wt.%) of the emulsion copolymerization was determined gravimetrically and calculated as below:
where S and S t denote, respectively, the experimental solid content and the theoretical solid content assuming all of the monomers were polymerized with 100 % conversion. The measurement of the dye monomer conversion, involving methanol extraction and absorptiometry, was carried out as follows: the PSBM-AHAQ or PSBM-AAQCB latex was coated on a glass plate and dried at 65 °C in an oven to form a latex film with the thickness about 140 μm; then the colored film was extracted with methanol in a Soxhlet apparatus for 5 h, and unreacted dye in extract solution was quantified by UV-Vis absorption method. The dye monomer conversion (Convdye, wt%) was calculated as below:
where, M E and M denote the mass of extracted dye and dye used in the recipe, respectively.
Measurement of the light fastness
The colored latex films with the thickness about 140 μm were irradiated using a Xenon lamp for 10 days under the condition, i.e., 180 W/m2 irradiation intensity, 40 °C, 50 % relative humidity. The color difference (ΔE) before and after light irradiation was obtained on a white printing paper by a colorimeter (HP-200, Chinaspec).
Results and discussion
Synthesis and characterization of polymerizable dyes
In order to obtain covalently colored polymer latex by free radical emulsion polymerization, two kinds of polymerizable AQ dyes were designed and synthesized. It is well known that AQ derivatives are free radical scavengers, which can inhibit the polymerization significantly, and steric hindrance of AQ structure also has great effect on monomer activity [22–24]. Therefore, in the synthesis of polymerizable dye molecules, a flexible spacer of alkyl chain was inserted between chromophore and unsaturated acryloyl group. In this way, the influence of AQ π-conjugated system on propagating radical can be minimized, and the steric hindrance of AQ structure on copolymerization is lowered. In addition, incorporation of the alkyl chain can improve the solubility of the polymerizable dyes in the mixture of St and BA, which is beneficial to the copolymerization of dye monomer with others.
The red dye monomer AHAQ (2) was prepared from 1-nitroanthraquinone as outlined in Scheme 1. First, aminolysis of 1-nitroanthraquinone was carried out with excessive 1,6-diaminohexane to produce a dark red dye (1), which was a nonpolymerizable dye and used for control experiment, and then acryloyl chloride was employed for amidation reaction to obtain monomer AHAQ (2).
The yellow dye monomer AAQCB (4) was designed in analogy to commercial C.I. Pigment Yellow 193. 1-Aminoanthraquinone was coupled with excessive terephthaloyl dichloride and then esterified by HEA to afford target dye monomer as illustrated in Scheme 2. Meanwhile, we also synthesized a yellow dye (6) for control experiment, which is not polymerizable.
The structures of AHAQ and AAQCB were confirmed by 1HNMR and IR. 1HNMR spectra of them showed particular signals of AQ ring protons at δ = 7.27–9.05 ppm. The acrylamide group in AHAQ exhibited two doublets, and one double-double split resonances at δ = 5.60, 6.09, and 6.23 ppm, respectively, whereas the acrylate group in AAQCB displayed the signal at δ = 5.99, 6.39, and 6.24 ppm.
Preparation and characterization of the covalently colored polymer latex
A series of the covalently colored polymer latexes were prepared via semicontinuous emulsion polymerization of St, BA, and MAA in the presence of polymerizable AHAQ/AAQCB using APS as initiator, and effect of the polymerizable dyes on the emulsion polymerization and the latex properties were investigated.
Influence of the polymerizable dye on the emulsion polymerization
Because of the inhibition effect of AQ structure on free radical polymerization, the monomer conversion was significantly influenced by the polymerizable dye in this work. For example, when 0.5 wt.% APS was used, the colorless latex could be fabricated with 99.9 % monomer conversion, whereas the polymerization with 1 wt.% AHAQ or AAQCB was finished with the total monomer conversion about 89.2 % (AHAQ) or 93.5 % (AAQCB) and the dye monomer conversion as low as 76.2 % (AHAQ) or 80.5 % (AAQCB). Also, it was noticed that the inhibiting ability of AHAQ was stronger than that of AAQCB, and this phenomenon might be ascribed to the inductive effect of substituent on AQ chromophore, since alkylamino group in AHAQ is a stronger electron-donating group comparing to amide group in AAQCB.
Apparently, sufficient amount of initiator must be utilized to promote the monomer conversion. It was found that, when the amount of APS was up to 1.5 and 1.0 wt.% for PSBM-AHAQ and PSBM-AAQCB latex preparation, respectively, the emulsion polymerization proceeded smoothly, and the stable latexes colored with 0.1–1.5 wt.% dye monomers could be prepared with high total monomer conversion. However, the dye concentration higher than 1.5 wt.% was not available because of their solubility limitation in the mixture of St and BA. The influences of the polymerizable dye on the monomer conversion, the molecular weight, and its distribution were given in Table 2.
The conversion of the dye monomer is of crucial importance for the preparation of covalently colored polymer latexes. Here, the dye monomer conversion was determined using solvent extraction followed by absorptiometry. Since methanol is a good solvent for the dye monomers, the unreacted dye can be extracted out from the colored polymer latex film, and its amount in the extraction solution can be quantified by UV-Vis absorptiometry, thus the dye monomer conversion can be figured out. It is clear that the dye monomer conversion decreased gradually with the increase of dye monomer amount, both in PSBM-AHAQ and in PSBM-AAQCB preparations (Table 2). This tendency might be due to a relatively lower reactivity of the dye monomers and, more importantly, their low diffusion rate in water [7, 15]. Since the dyes were water insoluble, diffusion of dye monomers from monomer phase into the growing particles through the aqueous phase was relatively difficult, and as a result, a part of dye monomers would precipitate as dyestuff aggregates if large amount of dye was employed in recipe, leading to a lower dye monomer conversion.
Although the conversion of AAQCB was slightly lower than that of AHAQ, more than 92 % of the dye monomers were copolymerized when their amounts were equal to or less than 1 wt.%. Even if the amount of the dye monomers was increased to 1.5 wt.%, the dye monomer conversion still reached 88.7 % for AHAQ and 87.0 % for AAQCB. Moreover, one factor should be taken into account, since low molecular weight polymer with dye units may have been extracted out during the Soxhlet extraction process, the dye monomer conversion in Table 2 may be slightly underestimated.
The molecular weight of the resulted polymer was also influenced by the amount of dye monomer. As shown in Table 2, the molecular weights of both PSBM-AHAQ and PSBM-AAQCB polymers increased with the increase of the dye monomer and were higher than that of the polymer without using dye monomer, which was consistent with the description in literature [25]. In general, the molecular weight in free radical polymerization increases with the decrease of initiator concentration. In this work, dye monomer AHAQ and AAQCB acted as inhibitors to consume a part of primary radicals decomposed from APS. When a constant amount of APS was used, the number of effective free radicals to initiate the polymerization was reduced by adding the dye, which caused the increase of the polymeric molecular weight.
Property of the covalently colored polymer latex
The colloidal properties of resultant colored polymer latexes were given in Table 3. For all of the samples, the hydrodynamic diameters (D h) were maintained 70–80 nm, and their zeta potential was distributed in the range of −60 to −67 mV, which were less dependent on the amount of dye monomers, but the polydispersity of particle size increased a little bit with addition of dyes. The obtained colored polymer latex performed good colloidal stability, and that is neither the precipitation in the latex nor the leakage of dye from the polymer particles was evidently observed within a storage time of 6 months. Typical TEM images of the latex particles (Fig. 1) showed that the morphology of colored polymer particles was uniform sphere, and the average diameter of the dried particles (D p) ranged from 60 to 70 nm, which was smaller than the hydrodynamic diameter D h as expected.
UV-Vis spectra of the polymerizable dye and the covalently colored polymer
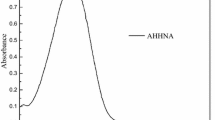
UV-Vis absorption spectra were obtained from the ethyl acetate solution of the covalently colored polymer and the corresponding dye monomer using ethyl acetate as reference solvent. Comparing with the maximum absorption wavelength (λ max) of the corresponding dye, λ max of each colored polymer showed no noticeable batho- or hypsochromic shift as shown in Fig. 2. Both the dye monomer AHAQ and the colored polymer PSBM-AHAQ displayed their λ max at 503 nm, while AAQCB and PSBM-AAQCB displayed λ max at 406 nm, indicating that the AQ dye chromophores did not change either during the copolymerization or as a result of the covalently bonding to the polymer chain [26]. Also, AQ chromophores had no apparent interaction with each other, thus excluding from π–π stacking, which would generate the bathochromic shift of λ max [27].
Light fastness of the colored polymer latex film
The light fastness of PSBM-AHAQ and PSBM-AAQCB latex films, both with 0.5 and 1 wt.% dye amount to the total monomers, was measured by an irradiation test and compared with the latex films colored by dye 1 and 6 (1 wt.%). Color difference (ΔE) was used to evaluate the light fastness of polymer latex film, and the ΔE of the colorless P(St-BA-MAA) latex film was deducted from each colored sample. As shown in Figs. 3 and 4, the light fastness of the covalently colored polymer latex films was much better than that of the noncovalently colored film with the unreactive dye, and the ΔE of the covalently colored polymer latex films was affected slightly by the amount of dye monomer. The enhancement of the photostability might be due to an electron transfer from the covalently bound chromophore to the main polymer chain during the transfer from excited state to ground state S 1 → S 0, and this process could prevent photodegradation of the dye structure [28]. In addition, the polymerized dye units were homogeneously distributed on the polymer chain and had better compatibility with the polymeric matrix which could insulate the ambient oxygen and other harmful chemicals.
Conclusion
In this study, based on anthraquinone structure, two novel polymerizable dyes (AHAQ and AAQCB) were first synthesized, and the covalently colored PSBM-AHAQ and PSBM-AAQCB polymer latexes were then successfully prepared via semicontinuous emulsion copolymerization of St, BA, MAA, and polymerizable dyes. It was found that with the increase of the dye monomer, the dye monomer conversion decreased, but the molecular weight of the colored polymer increased. When the amount of the dye monomer was in the range of 0.1–1.5 wt.% to the total monomers, a high total monomer conversion above 97 % could be achieved using proper amount of initiator APS, and most of the dye molecules were copolymerized. Meanwhile, the chromophores covalently bonded to polymer chains maintained unchanged UV-visible spectrum. In addition, the light fastness of the covalently colored latex films was proved to be much better than that of the noncovalently colored latex films. Accordingly, these kinds of colored polymer latex might have potential application in various fields, such as waterborne coating, ink, paint, and dyestuff for textile printing.
References
Lelu S, Novat C, Graillat C, Guyot A, Bourgeat-Lami E (2003) Encapsulation of an organic phthalocyanine blue pigment into polystyrene latex particles using a miniemulsion polymerization. Polym Int 52(4):542–547
Biry S, Sieber W (2004) Organic pigments for ink jet applications: key properties and impact on ink performance. Final Program and Proceedings of IS&T's NIP20: International Conference on Digital Printing Technologies 763-768
Koskinen M, Wilén CE (2009) Preparation of core-shell latexes for paper coatings. J Appl Polym Sci 112(3):1265–1270
Wang HH, Tzai GM, Chang CC (2005) Alkali reduction and reactive dye dyeing of T/N nonwoven fabrics dipped into silicon-containing, water-borne polyurethane. J Appl Polym Sci 96(6):2324–2335
Clemens T, Boehm AJ, Kielhorn-Bayer S, Rossmanith P (2000) Nanocolorants: pigments with dyestuff properties. Abstr Pap Am Chem S 219(Part 2):U364
Holzapfel V, Lorenz M, Weiss CK, Schrezenmeier H, Landfester K, Mailänder V (2006) Synthesis and biomedical applications of functionalized fluorescent and magnetic dual reporter nanoparticles as obtained in the miniemulsion process. J Phys-Condes Matter 18(38SI):S2581–S2594
Holzapfel V, Musyanovych A, Landfester K, Lorenz MR, Mailänder V (2005) Preparation of fluorescent carboxyl and amino functionalized polystyrene particles by miniemulsion polymerization as markers for cells. Macromol Chem Phys 206(24):2440–2449
Pichot C (2004) Surface-functionalized latexes for biotechnological applications. Curr Opin Colloid Interface Sci 9(3–4):213–221
Waich K, Sandholzer M, Mayr T, Slugovc C, Klimant I (2010) A highly flexible polymerization technique to prepare fluorescent nanospheres for trace ammonia detection. J Nanopart Res 12(4):1095–1100
Kalogianni DP, Litos IK, Christopoulos TK, Ioannou PC (2009) Dipstick-type biosensor for visual detection of DNA with oligonucleotide-decorated colored polystyrene microspheres as reporters. Biosens Bioelectron 24(6):1811–1815
Takasu S, Shiratani T, Takeshita K, Murayama T, Shimizu K (2002) Leaching-free colorant-containing polymer emulsions and method for their manufacture. JP 2002146156-A
Yu DG, An JH, Bae JY, Jung DJ, Kim S, Ahn SD, Kang SY, Suh KS (2004) Preparation and characterization of acrylic-based electronic inks by in situ emulsifier-free emulsion polymerization for electrophoretic displays. Chem Mater 16(23):4693–4698
Nistor CL, Donescu D, Purcar V, Petcu C, Serban S, Ghiurea M, Corobea MC (2008) Polymer-silica hybrids latexes dyed with Rhodamine B. e-Polymers no. 116
Liu YY, Lin HM, Chen H (2011) Preparation of monodisperse dye-doped copolymer spheres for photonic crystals. Mol Cryst Liq Cryst 534:124–133
Zhao XA, Zhou SX, Chen M, Wu LM, Gu GX (2011) Encapsulation of hydrophilic dyes with polystyrene using double miniemulsion technique. J Appl Polym Sci 119(6):3615–3622
Hu ZK, Xue MZ, Zhang Q, Sheng QR, Liu YG (2008) Nanocolorants: a novel class of colorants, the preparation and performance characterization. Dyes Pigments 76(1):173–178
Takasu M, Shiroya T, Takeshita K, Sakamoto M, Kawaguchi H (2004) Improvement of the storage stability and photostability of colored latex prepared by miniemulsion polymerization. Colloid Polym Sci 282(7):740–746
Grabchev I, Bojinov V (2001) Photophysical and photochemical properties of blue fluorescent polystyrene. J Photochem Photobiol A-Chem 139(2–3):157–160
Meng QH, Huang DY, Wei SH, Chen L (2002) Synthesis and application of polymeric dyes containing the anthraquinone structure. J Appl Polym Sci 83(6):1252–1257
Mallakpour S, Rafiemanzelat F, Faghihi K (2007) Synthesis and characterization of new self-colored thermally stable poly (amide-ether-urethane)s based on an azo dye and different diisocyanates. Dyes Pigments 74(3):713–722
Bardajee GR, Vancaeyzeele C, Haley JC, Li AY, Winnik MA (2007) Synthesis, characterization, and energy transfer studies of dye-labeled poly(butyl methacrylate) latex particles prepared by miniemulsion polymerization. Polymer 48(20):5839–5849
Huang SS, Yeh SF, Hong CY (1995) Effect of anthraquinone derivatives on lipid-peroxidation in rat heart mitochondria: structure-activity relationship. J Nat Prod 58(9):1365–1371
Yen GC, Duh PD, Chuang DY (2000) Antioxidant activity of anthraquinones and anthrone. Food Chem 70(4):437–441
Odian G (2004) Principles of polymerization (Chapter 3: Radical chain polymerization), 4th edn. Wiley, Hoboken, pp 255–279
Marechal E (1982) Polymeric dyes—synthesis, properties and uses. Prog Org Coat 10(3):251–287
Polpanich D, Asawapirom U, Thiramanas R, Piyakulawat P (2011) Self-colored nanoparticles containing naphthalene-bisimide derivatives: synthesis and protein adsorption study. Mater Chem Phys 129(1–2):495–500
Kim JH, Matsuoka M, Fukunishi K (1999) Three dimensional molecular stacking acid functionalities of aminonaphthoquinone by intermolecular hydrogen bondings and interlayer pi-pi interactions. Dyes Pigments 40(1):53–57
Grabchev I, Bojinov V (2000) Synthesis and characterisation of fluorescent polyacrylonitrile copolymers with 1,8-naphthalimide side chains. Polym Degrad Stabil 70(2):147–153
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 3421 kb)
Rights and permissions
About this article
Cite this article
Li, B., Shen, J., Liang, R. et al. Synthesis and characterization of covalently colored polymer latex based on new polymerizable anthraquinone dyes. Colloid Polym Sci 290, 1893–1900 (2012). https://doi.org/10.1007/s00396-012-2718-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-012-2718-7